Abstract
Erwinia amylovora causes a devastating disease called fire blight in rosaceous plants. The type III secretion system (T3SS) is one of the important virulence factors utilized by E. amylovora in order to successfully infect its hosts. By using a green fluorescent protein (GFP) reporter construct combined with a high-throughput flow cytometry assay, a library of phenolic compounds and their derivatives was studied for their ability to alter the expression of the T3SS. Based on the effectiveness of the compounds on the expression of the T3SS pilus, the T3SS inhibitors 4-methoxy-cinnamic acid (TMCA) and benzoic acid (BA) and one T3SS inducer, trans-2-(4-hydroxyphenyl)-ethenylsulfonate (EHPES), were chosen for further study. Both the T3SS inhibitors (TMCA and BA) and the T3SS inducer (EHPES) were found to alter the expression of T3SS through the HrpS-HrpL pathway. Additionally, TMCA altered T3SS expression through the rsmBEa-RsmAEa system. Finally, we found that TMCA and BA weakened the hypersensitive response (HR) in tobacco by suppressing the T3SS of E. amylovora. In our study, we identified phenolic compounds that specifically targeted the T3SS. The T3SS inhibitor may offer an alternative approach to antimicrobial therapy by targeting virulence factors of bacterial pathogens.
INTRODUCTION
Erwinia amylovora is a Gram-negative bacterial plant pathogen that belongs to the family Enterobacteriaceae. It is the causal agent of fire blight in rosaceous plant species such as apple, raspberry, cotoneaster, and pear. It can infect blossoms, leaves, succulent shoots, and immature fruits of these host plants (1). The pathogen can enter the plant naturally through wounds created by wind, hail, or rain. Pollinating agents like insects and splashing rain help spread the infection. After establishing itself in the plant, the bacterium moves through the vascular system and accumulates in the xylem. Symptoms observed in the infected plant parts, such as twigs and leaves, include water soaking, discoloration, and wilt, followed by necrosis, which gives the plant a fire-scorched appearance, hence the name fire blight (2).
During infection, E. amylovora utilizes different virulence factors to cause disease in a susceptible host. These include the type III secretion system (T3SS); exopolysaccharides, such as amylovoran and levan (3); the metalloprotease PrtA (4); the iron-scavenging siderophore desferrioxamine (5); the multidrug efflux pump AcrAB (6); and others (7, 8). The T3SS is encoded by hypersensitive response and pathogenicity (hrp) genes and is considered to be one of the major virulence determinants in E. amylovora. The T3SS forms a proteinaceous, syringe-like structure which secretes and translocates effector proteins from bacteria to the plant apoplast or cytoplasm (9, 10). These effectors manipulate the host cellular activities in order to ensure survival of the bacteria and cause disease in the host (11). In the case of resistant host plants and nonhost plants, such as tobacco, these effectors elicit the hypersensitive response (HR) (12). Since the T3SS is a conserved virulence factor in many Gram-negative bacteria, it is an attractive target for designing antimicrobial compounds.
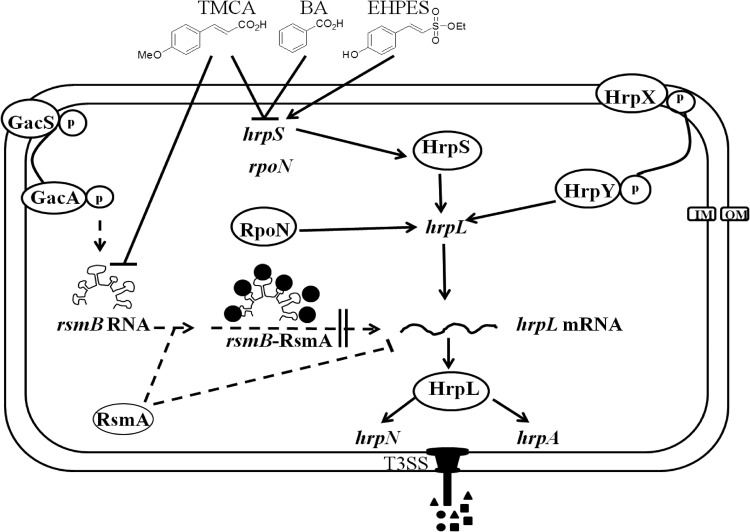
The expression of E. amylovora hrp genes is environmentally regulated in response to nutritional conditions, including carbon and nitrogen sources, pH, and temperature (13). Under appropriate environmental conditions, HrpX/HrpY, a two-component signal transduction system (14), regulates the expression of hrpL, which encodes an alternative sigma factor that binds at the promoter region of the hrp genes encoding T3SS effectors, harpin proteins, chaperones, and the type III pilus (15, 16) (Fig. 1). A σ54 consensus sequence has been found in the promoter region of hrpL (14), and expression of hrpL is partially controlled by HrpS (17), which belongs to the NtrC family of σ54 enhancer binding proteins. Unlike other plant pathogens, such as Pantoea stewartii or Dickeya dadantii, HrpS is independent of the HrpXY signal in E. amylovora (14) (Fig. 1). In soft-rot pathogens such as Pectobacterium carotovorum and D. dadantii, the hrp genes are subject to transcriptional and posttranscriptional regulation. RsmA, which is a global small RNA-binding regulatory protein, acts by reducing the half-life of hrpL mRNA (18, 19). rsmB is a noncoding regulatory small RNA (18) that sequesters multiple units of RsmA and neutralizes its degradation effect on various mRNA species. A functional homolog of rsmB has been identified in E. amylovora, and it has been shown that rsmBEa is able to reverse the negative effect of RsmAEa (20). In P. carotovorum, it has been shown that the GacS/GacA system upregulates rsmB production, which in turn positively regulates extracellular polysaccharide production, motility, and pathogenicity (20, 21). GacS/GacA responds to acidic pH conditions, and a previous study of E. amylovora (22) along with other plant pathogens, such as Pseudomonas syringae (23) indicated that it can positively regulate the T3SS, which is also activated under conditions such as acidic pH.
Fig 1.
Schematic of the effect of compounds on T3SS regulation in E. amylovora. Solid lines indicate direct regulation (protein-protein interaction or direct binding to the promoter region), and dashed lines indicate indirect regulation or hypothetical regulatory links based on evidence shown in E. amylovora or other plant-pathogenic bacteria. IM and OM, inner and outer membrane, respectively. Based on genetic analysis in E. amylovora and biochemical evidence obtained with P. stewartii (65), HrpX/Y forms a two-component system that activates the transcription of hrpL (14). HrpL activates the expression of the T3SS genes such as hrpA and hrpN (16, 52). HrpS is a σ54 enhancer-binding protein that activates the transcription of hrpL, independent of HrpXY (14, 17). Based on the two-component system study in E. amylovora and the evidences in P. syringae (22, 23), it is suggested that in E. amylovora, the GacAS two-component system may activate transcription of hrpS. Also, based on previous work in P. carotovorum (54) and the evidence of the functional homolog of rsmB found in E. amylovora E9 (20), the rsmB-RsmA system may regulate the T3SS by affecting the stability of hrpL mRNA in E. amylovora. In this study, we observed that TMCA, BA, and EHPES significantly alter the hrpA promoter activity through HrpS-HrpL pathway. In addition, TMCA alters the T3SS expression through the rsmBEa-RsmAEa pathway.
Phenolics are one of the secondary metabolites synthesized by plants. Apart from their role in pigmentation, growth, and reproduction, it also plays a key role in disease resistance (24). Plants have developed a systemic acquired resistance (SAR) mechanism to protect themselves from pathogen invasion. SAR occurs at sites distant from the initial site of pathogen infection, and salicylic acid (SA) has been identified as a signaling molecule that acts during SAR development (25, 26). During this process, many natural compounds—for example, stilbenes, coumarins, and isoflavonoids—are produced in response to microbial attack and are effective against a broad spectrum of pathogens (27–29). Many of the above compounds induced in response to pathogen attack are derived from multiple branches of the phenylpropanoid biosynthesis pathway. Phenylpropanoids are a group of secondary metabolites exclusively produced by plants from l-phenylalanine (30–35). Although natural products occupy an important position in the area of plant disease management, researchers have encountered difficulty in isolating specific active components from plant extracts, which usually consists of a mixture of a large number of structurally related compounds with various degrees of bioactivity, or even opposing effects (growth inhibitors versus growth stimulants), and even some with cytotoxicity (36).
Recently, we reported that the plant phenolic compounds p-coumaric acid (PCA), o-coumaric acid (OCA), and t-cinnamic acid (TCA) were able to either inhibit or induce the expression of T3SS genes of a bacterial phytopathogen D. dadantii 3937 (37, 38). OCA and TCA induced the expression of the T3SS through the rsmB-RsmA pathway, and the inhibition of T3SS expression by PCA was found to be moderated through the HrpS-HrpL pathway. Given that the T3SS is conserved in many phytopathogenic bacteria, we hypothesized that some of the plant phenolic compounds found in plants and/or their derivatives may affect the T3SS of E. amylovora. In this report, an inventory of phenolic compounds, including newly synthesized derivatives was screened for their effect on T3SS expression of E. amylovora. Several of these compounds showed either induction or repression of the T3SS. Two T3SS inhibitors, 4-methoxy-cinnamic acid (TMCA) and benzoic acid (BA), and one inducer, trans-2-(4-hydroxyphenyl)-ethenylsulfonate (EHPES), were chosen, and their effects on T3SS regulatory components were further investigated. In addition, the effectiveness of the T3SS inhibitors TMCA and BA on suppression of the HR of E. amylovora in tobacco was examined.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. All bacterial strains were stored at −80°C in 20% glycerol. Wild-type Erwinia amylovora 273, Escherichia coli, and Agrobacterium strains were routinely grown in Luria-Bertani (LB) medium at 28°C, 37°C, and 30°C, respectively. For induction of T3SS genes, E. amylovora 273 was grown in HIM (hrp-inducing minimal medium) supplemented with 10 mM mannitol as previously described (39). When necessary, antibiotics were added to growth medium at the following concentrations: kanamycin (50 μg/ml), ampicillin (100 μg/ml), chloramphenicol (40 μg/ml), gentamicin (15 μg/ml), and rifampin (50 μg/ml).
Recombinant DNA techniques.
Preparation of genomic or plasmid DNA, PCR, restriction digestion, ligation, DNA electrophoresis, and transformations were performed as described by Sambrook and Russell (40). Primers used for PCR in this report are listed in Table S1 in the supplemental material.
Sources of the screened compounds.
Compounds TS1 to TS35, TS108 to TS113, TS134 to TS136, and TS144 to TS145 were purchased from the commercial sources Aldrich (St. Louis, MO), Alfa Aesar (Ward Hill, MA), and TCI (Tokyo, Japan). TS100 to TS107 and TS114 to TS133 were synthesized via the routes described in our recent publication (41). The remaining compounds were synthesized by the following methods.
(i) Synthesis of cinnamyl hydroxamates and amides from the corresponding esters.
To an ice-cold solution of methyl cinnamate (5 mmol) dissolved in anhydrous MeOH (5 ml) and THF (5 ml) was added hydroxylamine hydrochloride or amine hydrochloride (15 mmol, 3 equivalents) followed by 25% sodium methoxide in methanol solution (5.25 ml, 22.5 mmol, 4.5 equivalents). The reaction mixture was stirred under argon at 0°C for 2 h and then allowed to warm to an ambient temperature with continuous stirring overnight. The resulting yellow suspension was condensed to dryness with a rotary evaporator, and the residue was treated with a 1 N HCl aqueous solution (20 ml). The mixture was extracted with ethyl acetate (EtOAc) (3 times, 30 ml each time) and dried (anhydrous Na2SO4). Evaporation of the solvent afforded the crude products, which was purified by flash silica gel chromatography (elution with 5 to 15% MeOH in dichloromethane [DCM]) to yield the corresponding hydroxamic acid or amide. TS137: white solid; 1H nuclear magnetic resonance (NMR) (300 MHz, deuterated methanol [CD3OD]): δ2.82 (s, 3H), 6.38 (d, J = 15.9 Hz, 1H), 6.78 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 15.9 Hz, 1H); mass spectrometry (MS) (electrospray ionization [ESI]): m/z 176 (M − 1). TS138: white solid; 1H NMR (300 MHz, CD3OD): δ3.41 (t, J = 5.4 Hz, 2H), 3.65 (t, J = 5.4 Hz, 2H), 6.43 (d, J = 15.9 Hz, 1H), 6.77 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 15.9 Hz, 1H); MS (ESI): m/z 206 (M − 1). TS139: light brown solid; 1H NMR (300 MHz, dimethyl sulfoxide [DMSO]-d6): δ2.24 (t, J = 8.1 Hz, 2H), 2.78 (t, J = 8.1 Hz, 2H), 7.16-7.25 (m, 5H), 8.71 (s, 1H), 10.20 (s 1H); MS (ESI): m/z 164 (M − 1). TS 140: yellow solid; 1H NMR (300 MHz, DMSO-d6): δ6.53 (d, J = 15.9 Hz, 1H), 7.32-9.92 (m, 9H), 7.51 (d, J = 15.9 Hz, 1H); MS (ESI): m/z 238 (M − 1). TS141: brown sold; 1H NMR (300 MHz, DMSO-d6): δ6.39 (d, J = 15.9 Hz, 1H), 7.24 (m, 2H), 7.43 (d, J = 15.9 Hz, 1H), 7.57 (m, 2H), 9.05 (broad singlet [brs], 1H), 10.74 (brs 1H); MS (ESI): m/z 180 (M − 1). TS142: light-brown solid; 1H NMR (300 MHz, CD3OD): δ2.40 (s, 3H), 6.22 (d, J = 15.6 Hz, 1H), 6.87 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.52 (d, J = 15.6 Hz, 1H); MS (ESI): m/z 176 (M − 1). TS143: white sold; 1H NMR (300 MHz, DMSO-d6): δ4.43 (s, 2H), 6.91-7.30 (m, 5H), 8.90 (brs, 1H), 10.77 (brs, 1H); MS (ESI): m/z 166 (M − 1).
(ii) Synthesis of cinnamyl hydroxamates from the corresponding carboxylic acids.
To a stirred mixture of cinnamic acid (7 mmol) and diisopropylethylamine (DIEA) (2.45 ml, 17 mmol) in anhydrous dimethylformamide (DMF) (20 ml) was added O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) (2.92 g, 7.7 mmol) at room temperature, followed by a solution of hydroxylamine hydrochloride (0.98 g, 14 mmol) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (2.13 g, 14 mmol) in anhydrous DMF (8 ml), and then the whole reaction mixture was stirred at room temperature for 1 h. After most of the DMF was removed with a rotary evaporator, the residue was dissolved in EtOAc (150 ml), then washed with H2O (50 ml) and brine (50 ml), and dried (anhydrous Na2SO4). Evaporation of the solvent yielded the crude product, which was purified by flash silica gel chromatography (eluting with 5 to 15% MeOH in DCM) to yield hydroxamic acid. TS146: yellow solid; 1H NMR (300 MHz, DMSO-d6): δ6.48 (d, J = 15.6 Hz, 1H), 7.44 (d, J = 15.6 Hz, 1H), 7.58 (d, J = 7.2 Hz, 2H), 7.61 (d, J = 7.2 Hz, 2H), 9.05 (brs, 1H), 10.76 (brs 1H), 11.29 (s, 1H); MS (ESI): m/z 189 (M − 1). TS147: yellow solid; 1H NMR (300 MHz, DMSO-d6): δ4.49 (s, 2H), 6.42 (d, J = 15.9 Hz, 1H), 7.32 (d, J = 7.2 Hz, 2H), 7.42 (d, J = 15.9 Hz, 1H), 7.49 (d, J = 7.2 Hz, 2H); MS (ESI): m/z 192 (M − 1). TS160: light-yellow solid; 1H NMR (300 MHz, DMSO-d6): δ2.35 (s, 3H), 6.34 (d, J = 15.6 Hz, 1H), 7.23 (m, 3H), 7.49 (d, J = 7.5 Hz, 1H), 7.66 (d, J = 15.6 Hz, 1H), 9.05 (brs, 1H), 10.78 (brs 1H); MS (ESI): m/z 176 (M − 1). TS161: light-brown solid; 1H NMR (300 MHz, DMSO-d6): δ6.24 (d, J = 15.3 Hz, 1H), 7.06 (m, 1H), 7.34 (m, 1H), 7.60 (m, 1H), 7.66 (d, J = 15.3 Hz, 1H), 9.10 (brs, 1H), 10.80 (brs 1H); MS (ESI): m/z 168 (M − 1).
(iii) Synthetic method for TS165.
A 100-ml round-bottom flask, equipped with a condenser and a stirring bar, was charged with benzyl-4-bromophenyl ketone (7.43 g, 27 mmol), sodium acetate NaOAc (2.66 g, 32.4 mmol, 1.2 equivalent), tert-butyl acrylate (4.70 ml, 32.4 mmol, 1.2 equivalents), and N-methylpyrrolidinone (NMP) (35 ml) at room temperature. In a separate 100-ml flask, palladium(II) acetate [Pd(OAc)2] (22.5 mg, 0.1 mmol) was dissolved in NMP (50 ml) with stirring, resulting in a 0.002 M solution. At room temperature and under N2, 6.75 ml (0.0135 mmol) of this solution was added in one portion to the reaction flask via a syringe. The reaction mixture was heated at 135°C with stirring in an oil bath for 40 min, resulting in a gray mixture. After being cooled to room temperature, the reaction mixture was quenched by adding water (200 ml), and the resulting suspension was extracted with EtOAc (3 times with 100 ml each). The combined organic layers were washed with water (twice with 100 ml each) and brine (100 ml) and dried over anhydrous Na2SO4. The solution was filtered through a pad of Celite to remove the catalyst, the Celite was rinsed with EtOAc (twice with 40 ml each), and the solvents were evaporated with a rotary evaporator to dryness, yielding trans-4-(benzylcarbonyl)cinnamic acid tert-butyl ester as an oil (about 10 g), which was used for the next step without further purification.
To a mixture of trimethylsulfoxonium iodide (15.9 g, 72 mmol, 3.75 equivalents) and 60% NaH (2.54 g, 63.5 mmol, 3.3 equivalent) was added anhydrous DMSO (85 ml) in one portion at room temperature (RT), and the mixture was stirred under nitrogen for 1.5 h. A solution of trans-4-(benzylcarbonyl)cinnamic acid tert-butyl ester obtained as described above (6.2 g, 19.2 mmol) in anhydrous DMSO (40 ml) was added dropwise within 12 min to the resulting suspension at RT. After the whole mixture was stirred at RT for 3 h, the reaction was quenched with brine (160 ml). The resulting mixture was extracted with ether (4 times for 100 ml each), washed with brine (twice for 50 ml), and dried. The solvent was evaporated with a rotary evaporator to dryness, and the crude product was purified by flash column chromatography (eluting with 95:5 to 90:10 hexane-EtOAc) to yield trans-2-[(4′-benzylcarbonyl]phenylcyclo-propane-1-carboxylic acid tert-butyl ester (4.0 g, 62% yield) as a white solid.
To an ice-cold stirred solution of trans-2-[(4′-Benzylcarbonyl]phenylcyclopropane-1-carboxylic acid tert-butyl ester (1.1 g, 3.2 mmol) dissolved in dichloromethane (12 ml) was added trifluoroacetic acid (TFA) (3.5 ml) in one portion, and the reaction mixture was stirred at 0°C for 1 h and then allowed to warm to the ambient temperature, and the stirring was continued for additional 2 h. The solvents were evaporated to dryness with a rotary evaporator, and the solid residue was subjected to azeotropic distillation with toluene (10 ml, twice) to form trans-2-[(4′-benzylcarbonyl]phenyl-cyclopropane-1-carboxylic acid (TS165) (700 mg, 78% yield) as an off-white solid. 1H NMR (300 MHz, CD3OD): δ1.35 (m, 1H), 1.50 (m, 1H), 1.80 (m, 1H), 2.58 (m, 1H), 3.60 (s, 2H), 6.86 (d, J = 8.4 Hz, 2H), 7.08 (m, 5H), 7.14 (d, J = 8.4 Hz, 2H); MS (ESI): m/z 279 (M − 1).
GFP transcriptional reporter screening.
To screen compounds that induce or inhibit the expression of the E. amylovora 273 T3SS, a 260-bp fragment containing the promoter region of hrpA was PCR amplified using the primer set phrpA-F (5′-ATATGGATCCCGATAAAGAGCAGCGTAG) and phrpA-R (5′-ATTAGAATTCTTAGACGCCTGAGCATTG). Underlined letters in the forward and reverse primers represent the recognition sites for BamHI and EcoRI, respectively. The amplified fragment and pPROBE-gfp[AAV], a broad-host-range vector carrying promoterless gfp (42), were digested with EcoRI and BamHI, gel purified, and ligated to create a promoter-probe construct called phrpA. This constructed plasmid was then transferred to E. amylovora 273 by electroporation. Expression of hrpA was analyzed using a FACSCalibur flow cytometer as previously described (BD Biosciences, San Jose, CA) (43). Wild-type E. amylovora 273 carrying the promoter-probe phrpA or pPROBE-gfp[AAV] (vector control) was grown in LB broth overnight and transferred to hrp-inducing minimal medium (HIM) or HIM supplemented with 100 μM concentrations of the compounds as described previously (38). HIM was used in order to induce the hrp genes, which encode the proteins that form the T3SS structure, and also to express the virulence proteins secreted through it. Kanamycin was added to the medium whenever a promoter-probe construct was examined. The promoter activity of hrpA in E. amylovora 273 was monitored by measuring intensity of the green fluorescence protein (GFP) using flow cytometry. Two independent experiments were performed, and three replicates were used in each experiment. The 50% inhibitory concentration (IC50) was measured by diluting the selected compounds and measuring the fluorescence at 9 h of bacterial growth in HIM. Due to the low expression levels of hrpS and hrpL, promoter regions of hrpS and hrpL of E. amylovora 273 were cloned into pPROBE-AT at BamHI and EcoRI sites. The pPROBE-AT contains a wild-type gfp reporter and has been routinely used in D. dadantii for measuring promoter activity of the bacterium (37).
RNA extraction and quantitative PCR analysis.
E. amylovora cells were cultured in LB medium overnight at 28°C and subcultured in HIM supplemented with DMSO or with 100 μM TMCA, BA, or EHPES for 6 h. Total bacterial RNA was isolated using an RNeasy minikit (Qiagen, Valencia, CA) and treated with a Turbo DNA-free DNase kit (Ambion, Austin, TX). cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) from 0.6 μg of total RNA. The Real Master Mix SYBR ROX (5 Prime, Gaithersburg, MD) was used for quantitative PCR (qPCR) reactions to quantify the cDNA level of target genes in different samples. Data were collected by the Opticon 2 system (Bio-Rad, Hercules, CA) and analyzed using the Relative Expression software tool as described by Pfaffl and coworkers (44). A housekeeping gene rplU was used as an endogenous control for data analysis (45). The primer pairs used in this study are listed in Table S1 in the supplemental material.
Western blot analysis.
The HrpN protein level was determined by immunoblot using anti-HrpN antibody prepared by Proteintech. Sample preparation was performed as previously described with modifications (46). Wild-type E. amylovora 273 was grown overnight in LB medium at 28°C. The culture was washed with hrp inducing medium (HIM) and resuspended in 40 ml of HIM to an optical density at 600 nm (OD600) of 0.2. To determine the effect of inhibitors or inducers on the HrpN protein level, the compounds TMCA, BA, and EHPES dissolved in DMSO were added to the culture at a final concentration of 100 μM, and the cultures were grown with moderate shaking at 28°C. After 24 h, the cells were collected by centrifugation at 3,500 × g for 10 min and resuspended in 20 ml phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.47 mM KH2PO4 [pH 7.4]). After the cells were washed with PBS, cells were centrifuged and the pelleted cells were resuspended in 4 ml B-PER bacterial protein extraction reagent (Pierce Biotechnology Inc., Rockford, IL). The resuspended cells were incubated in the shaker at 200 rpm for 15 min at 28°C. Thereafter, the cells were centrifuged, and the supernatant was collected as the soluble cell fraction protein. The optical density of the bacterial cells treated with different compounds was measured at 590 nm and normalized before the protein extraction (47). Protein concentration was further normalized for all the treatments with the help of the Bradford assay (Bio-Rad protein assay for microtiter plates) (48). Bovine serum albumin (BSA) was used as the standard. A 35-μl portion of 1× sample buffer (2% [wt/vol] sodium dodecyl sulfate, 2% [vol/vol] glycerol, 2 mM β-mercaptoethanol, 50 mM Tris-HCl [pH 6.8], and 0.01% [wt/vol] bromophenol blue) was added to 100 μl of normalized sample protein and heat treated at 95°C for 10 min using a dry bath incubator (Fisher Scientific, Pittsburgh, PA). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted to a polyvinylidene fluoride (PVDF) membrane using a Trans-Blot SD semidry transfer cell (Bio-Rad Laboratories, Hercules, CA), and subjected to immunodetection. The blotted membrane was then washed three times with PBS and incubated in blocking solution (5% [wt/vol] skim milk in PBS) overnight. After being washed three times with washing buffer (PBS containing 0.3% [vol/vol] Triton X-100), the membrane was incubated in washing buffer supplemented with an anti-HrpN polyclonal antibody (Proteintech) for 1.5 h and then in washing buffer supplemented with an anti-rabbit IgG conjugated with alkaline phosphatase (AP) (Southern Biotech, Birmingham, AL) for another 1.5 h. Between the incubations membrane was washed three times with washing buffer. The membrane was then incubated in AP reaction buffer (100 mM Tris base [pH 9.5], 100 mM NaCl, and 50 mM MgCl2) for 5 min, and HrpN proteins were detected by the chromogenic detection method. The results were analyzed using ImageJ (http://imagej.nih.gov/ij/index.html) to obtain the relative intensity of the HrpN protein level.
RNA isolation and Northern blot analysis.
E. amylovora cells grown in HIM supplemented with DMSO or 100 μM TMCA, BA, or EHPES for 6 h were harvested, and total RNA was isolated using the Tri reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions, followed by DNase treatment with a Turbo DNA-free DNase kit (Ambion, Austin, TX). Each RNA sample was analyzed by Northern blotting using a NorthernMax kit (Ambion, Austin, TX) according to the manufacturer's instructions. Hybridization probes used to detect the mRNA of target genes were PCR amplified and labeled with biotin using a BrightStar psoralen-biotin kit (Ambion, Austin, TX). Signals were developed using the Bright-Star BioDetect kit (Ambion, Austin, TX). 16S rRNA was visualized under a UV transilluminator (Syngene, Frederick, MD) and used as an internal control for normalization of RNA.
Measurement of growth rate.
E. amylovora cells were grown overnight in LB at 28°C. The cells were then resuspended in M9 medium supplemented with DMSO or 100 μM concentrations of the selected 56 compounds to have an OD590 of 0.05, and their growth rates were monitored for 24 h. The cultures were grown in 15-ml glass tubes, and 200 μl of the cultures was transferred to 96-well flat-bottom plates (Corning, NY) in order to measure the absorbance at different time points by using an Infinite M200 Pro plate reader (Tecan, Crailsheim, Germany). Two independent experiments were performed, and three replicate samples were used in each experiment.
Hypersensitive response assay.
E. amylovora cells from overnight cultures grown in LB broth were resuspended in sterile distilled water (dH2O). Cell suspensions were mixed with compounds and incubated for 30 min before plant inoculation. Nicotiana tabacum cv. Xanthi plants were used for HR assays. Tobacco leaves were pressure infiltrated by using a needleless syringe (49) with 8 × 105 cells/ml of E. amylovora and 100 μM TMCA or BA. Bacterial cell concentration was determined using a serial dilution plating method. Plants were photographed and assessed for macroscopic tissue collapse indicative of an HR 18 h postinoculation. For transient-gene-expression studies, tobacco leaves were first infiltrated with Agrobacterium tumefaciens strain GV3101 carrying pCPP5247. After that, 100 μM TMCA and BA were used to infiltrate the different sets of tobacco leaves at 0, 3, 6, 9, 12, and 24 h after infiltration by the A. tumefaciens strain.
Agrobacterium-mediated transient expression.
A. tumefaciens strain GV3101 carrying pCPP5247 was grown at 30°C in LB supplemented with appropriate antibiotics to stationary phase. Bacteria were sedimented by centrifugation at 3,500 × g for 10 min at room temperature and washed and resuspended in 10 mM morpholinoethanesulfonic acid (MES) (pH 5.5) and 100 μM acetosyringone to a final optical density at 590 nm (OD590) of 0.5 (50). Cells were infiltrated into abaxial air spaces of N. tabacum cv. Xanthi using a 1-ml blunt syringe. Plants inoculated with bacteria were incubated at 24°C with 12 h of daily illumination.
Statistical analysis.
Means and standard deviations of experimental results were calculated using Excel (Microsoft, Redmond, WA), and the statistical analysis was performed using a two-tailed t test.
RESULTS
Various compounds either inhibit or induce the hrpA promoter activity.
In order to screen the compounds that would affect T3SS expression, a promoter-reporter fusion plasmid was constructed. The promoter of hrpA, which encodes the type III pilus (16, 51) was cloned into the promoter-probe vector pPROBE-gfp[AAV] (42). This vector contains the promoterless green fluorescent protein gene (gfp), whose expression is driven by the promoter cloned immediately upstream. This construct was referred to as phrpA. E. amylovora 273 containing phrpA was cultured in a hrp-inducing medium (HIM) (39) supplemented with dimethyl sulfoxide (DMSO; solvent of phenolic compounds) or with 100 μM concentrations of each phenolic compound. The promoter activity of hrpA was measured at 6 and 9 h of induction by measuring GFP intensity using flow cytometry (Table 1) (43). Several of the compounds tested in the initial screen had a strong inhibitory effect on hrpA promoter activity, such as trans-cinnamic acid (TCA), benzoic acid (BA), salicylic acid (SA), and ortho-cinnamic acid (OCA). Many were found to induce hrpA promoter activity when added to HIM, such as diethyl trans-2-(4-hydroxyphenyl)-vinylphosphonate (TS114), 2,4-dihydroxycinnamic acid (TS2), trans-3-(4-hydroxyphenyl) acrylohydrazide (TS133), ethyl trans-2-(4-hydroxyphenyl)-ethenylsulfonate (EHPES), and methyl-para-coumate (TS101). The mean GFP intensity (MFI) of the total bacterial population is shown in Table 1. This method of screening indicated that several compounds have an effect on the T3SS of E. amylovora. To eliminate the possibility that the decline in phrpA reporter fluorescence signal was due to the cell death or stasis of cells in HIM, the E. amylovora 273 cells carrying phrpA were grown in HIM supplemented with phenolic compounds, TMCA, BA, and EHPES for 9 h. Similar numbers of bacterial cells were observed in HIM supplemented with DMSO and HIM supplemented with EHPES (see Fig. S1 in the supplemental material). Interestingly, compared with numbers seen with DMSO, a slight increase in bacterial numbers was observed in HIM supplemented with TMCA and BA (see Fig. S1). Also, no plasmid loss was observed in E. amylovora 273 cells carrying phrpA grown in HIM supplemented with phenolic compounds (TMCA, BA, and EHPES) for 9 h (data not shown).
Table 1.
Promoter activity of E. amylovora 273 hrpA measured by the GFP promoter-reporter fusion plasmid (phrpA) in HIM and HIM supplemented with phenolic compounds and their analogs
| Strain and phenolic compounda | MFI (avg ±SD)b at: |
|
|---|---|---|
| 6 h | 9 h | |
| 273(pPROBE-gfp[AAV]) | 1.4 ± 0.2 | 1.2 ± 0.1 |
| 273(phrpA) | ||
| DMSO | 1,229.4 ± 39.2 | 1,024.3 ± 52.9 |
| TS24, trans-2-carboxycinnamic acid | 361.1 ± 76.1* | 332.2 ± 19.6* |
| TS30, methyl trans-cinnamate | 674.8 ± 252.1 | 668.5 ± 31.2* |
| TS32, cinnamyl alcohol | 681.6 ± 217.9 | 784.0 ± 53.3* |
| TS27, trans-4-aminocinnamic acid | 255.6 ± 7.4* | 132.1 ± 8.5* |
| TS26, trans-4-mercaptocinnamic acid | 159.7 ± 22.0* | 44.7 ± 2.7* |
| TS29, trans-4-formlycinnamic acid | 285.1 ± 40.8* | 218.3 ± 9.9* |
| TS28, trans-4-nitrocinnamic acid | 14.4 ± 16.9* | 4.0 ± 0.3* |
| TS101, methyl para-coumate | 1,443.4 ± 49.4* | 1,227.8 ± 41.6* |
| TS102, trans-4-hydroxycinnamaide | 1,134.0 ± 36.7 | 766.4 ± 94.8 |
| TS103, trans-4-hydroxycinnamohydroxamic acid | 206.9 ± 3.9* | 168.2 ± 24.9* |
| TS104, para-coumaryl alcohol | 814.7 ± 55.2* | 556.9 ± 40.3* |
| TS105, trans-2-(4-methoxyphenyl)-1-cyclopropanecarboxylic acid | 345.3 ± 11.9* | 200.2 ± 11.0* |
| TS106, ethyl trans-2-(4-hydroxyphenyl)-1-cyclopropanecarboxylate | 1,289.3 ± 46.5 | 1,110.9 ± 67.5 |
| TS107, trans-2-(4-hydroxyphenyl)-1-cyclopropanecarboxylic acid | 1,464.3 ± 76.5* | 1,348.0 ± 82.6* |
| TS108, trans-4-phenylcinnamic acid | 173.7 ± 7.9* | 79.4 ± 1.8* |
| TS109, trans-4-chlorocinnamide | 172.9 ± 20.5* | 90.6 ± 12.0* |
| TS110, trans-4-fluorocinnamic acid | 279.2 ± 5.3* | 152.4 ± 0.7* |
| TS111, trans-4-bromocinnamic acid | 102.8 ± 23.5* | 61.8 ± 4.2* |
| TS112, trans-4-dimethylaminocinnamic acid | 87.0 ± 4.0* | 37.3 ± 3.7* |
| DMSO | 1,476.7 ± 63.8 | 1,649.1 ± 96.9 |
| TS6, ortho-coumaric acid | 443 ± 16.9* | 437.8 ± 21.1* |
| TS1, trans-cinnamic acid | 471.2 ± 12.6* | 432.6 ± 17.8* |
| TS4, para-coumaric acid | 920.6 ± 29.1* | 993.8 ± 43.3* |
| TS5, meta-coumaric acid | 1,212 ± 47.6 | 1,439.1 ± 97.9 |
| TS33, salicylic acid | 49.8 ± 13.9* | 34.3 ± 4.2* |
| TS34, benzoic acid | 48.1 ± 5.4* | 32.6 ± 1.9* |
| DMSO | 1,242.1 ± 27.0 | 1,672.5 ± 126.0 |
| TS122, ethyl trans-2-(4-hydroxyphenyl)-ethenylsulfonate | 2,042.8 ± 20.7* | 2,900.6 ± 120.1* |
| TS123, trans-2-(4-hydroxyphenyl)ethenylsulfonic acid tetra(n-butyl)ammonium salt | 1,073.4 ± 107.7 | 1,377.3 ± 81.1 |
| TS124, trans-4-hydroxymethylcinnamic acid | 419.8 ± 12.2* | 444.3 ± 11.4* |
| TS125, trans-4-methoxycinnamohydroxamic acid | 215.5 ± 6.5* | 230.0 ± 4.4* |
| TS126, trans-4-methoxycinnamyl alcohol | 386.9 ± 19.4* | 401.4 ± 10.8* |
| TS127, trans-3-indoleacrylohydroxamic acid | 91.4 ± 4.9* | 102.7 ± 0.5* |
| TS128, trans-4-bromocinnamohydroxamic acid | 17.6 ± 2.8* | 5.7 ± 2.2* |
| TS129, trans-2-hydroxycinnamohydroxamic acid | 187.9 ± 1.2* | 181.0 ± 0.7* |
| TS130, trans-3-hydroxycinnamohydroxamic acid | 431.4 ± 4.1* | 491.0 ± 10.2* |
| TS131, trans-3,4-dihydroxycinnamohydroxamic acid | 1,057.5 ± 41.2* | 1,515.1 ± 39.1 |
| TS132, trans-cinnamohydroxamic acid | 145.7 ± 1.2* | 147.8 ± 4.2* |
| TS133, trans-3-(4-hydroxyphenyl)acrylohydrazide | 1,716.5 ± 33.1* | 2,780.9 ± 58.5* |
| TS136, phenylpropiolic acid | 261.7 ± 1.0* | 307.0 ± 9.3* |
| TS134, benzhydroxamic acid | 679.0 ± 12.7* | 921.6 ± 10.3* |
| TS135, salicylhydroxamic acid | 124.7 ± 7.7* | 147.8 ± 4.3* |
| DMSO | 1,068.6 ± 170.9 | 1,157.8 ± 58.7 |
| TS8, hydrocinnamic acid | 395.9 ± 16.0* | 342.0 ± 11.7* |
| TS9, phenoxyacetic acid | 342.7 ± 24.8* | 269.9 ± 15.2* |
| TS10, trans-2-phenylcyclopropane-1-carboxylic acid | 336.7 ± 28.1* | 293.0 ± 4.7* |
| TS13, trans-3-(3-pyridyl)acrylic acid | 468.3 ± 7.7* | 245.7 ± 3.1* |
| TS35, 3-(2-naphthyl)acrylic acid | 178.9 ± 9.7* | 89.0 ± 6.2* |
| TS12, trans-3-indoleacrylic acid | 33.7 ± 8.9* | 47.9 ± 6.9* |
| TS11, trans-3-(2-thienyl)acrylic acid | 230.1 ± 5.8* | 167.3 ± 8.2* |
| TS15, trans-2-methoxycinnamic acid | 342.6 ± 7.0* | 255.1 ± 1.7* |
| TS18, trans-2-methylcinnamic acid | 303.8 ± 8.7* | 262.5 ± 9.3* |
| TS21, trans-2-chlorocinnamic acid | 225.7 ± 35.8* | 192.7 ± 8.6* |
| TS14, trans-3-(4-imidazolyl)acrylic acid | 874.6 ± 23.1* | 839.2 ± 17.6* |
| TS2, 2,4-dihydroxycinnamic acid | 1,365.3 ± 104.9 | 1,479.8 ± 72.9* |
| TS7, 3-(4-hydroxyphenyl)propionic acid | 1,093.1 ± 62.8 | 1,211.0 ± 58.7 |
| TS23, trans-4-chlorocinnamic acid | 171.8 ± 7.7* | 125.8 ± 3.9* |
| TS17, trans-4-methoxycinnamic acid | 132.5 ± 5.5* | 76.4 ± 2.7* |
| TS20, trans-4-methylcinnamic acid | 208.5 ± 2.7* | 145.4 ± 3.7* |
| TS3, 3,4-dihydroxycinnamic acid | 783.4 ± 80.2* | 787.3 ± 25.2* |
| TS22, trans-3-chlorocinnamic acid | 332.9 ± 16.9* | 238.3 ± 8.7* |
| TS16, trans-3-methoxycinnamic acid | 265.3 ± 24.4* | 198.5 ± 20.0* |
| TS19, trans-3-methylcinnamic acid | 311.5 ± 18.6* | 251.6 ± 13.3* |
| DMSO | 1,120.4 ± 44.4 | 1,499.8 ± 30.9 |
| TS25, trans-cinnamamide | 1,034.7 ± 12.5 | 1,378.1 ± 14.6 |
| TS31, trans-4-carboxycinnamic acid | 257.7 ± 14.3* | 187.1 ± 8.4* |
| TS100, ethyl trans-2-(4-methoxyphenyl)-1-cyclopropanecarboxylate | 1,090.5 ± 20.6 | 1,406.4 ± 9.0 |
| TS113, trans-4-trifluoromethylcinnamic acid | 172.1 ± 4.7* | 199.2 ± 11.1* |
| TS114, diethyl trans-2-(4-hydroxyphenyl)-vinylphosphonate | 1,371.3 ± 16.1* | 2,023.3 ± 28.9* |
| TS115, trans-2-(4-hydroxyphenyl)-vinylphosphonic acid | 1,176.6 ± 56.6 | 1,636.9 ± 53.3 |
| TS117, para-coumarylamine | 897.6 ± 48.9* | 1,372.6 ± 48.6 |
| TS118, N-(4-methoxycinnamyl)phthalimide | 919.8 ± 34.4 | 1,240.0 ± 29.7 |
| TS119, trans-4-methoxycinnamylamine | 285.9 ± 44.2* | 570.0 ± 68.4* |
| TS120, ethyl trans-2-(4-methoxyphenyl)-ethenylsulfonate | 982.1 ± 12.8 | 1,338.3 ± 75.4 |
| TS121, trans-2-(4-methoxyphenyl)ethenylsulfonic acid tetra(n-butyl)ammonium salt | 977.1 ± 20.4 | 1,336.3 ± 79.2 |
| DMSO | 1,100.1 ± 30.8 | 1,351.9 ± 62.4 |
| TS160, trans-2-methylcinnamohydroxamic acid | 122.7 ± 1.9* | 95.7 ± 3.7* |
| TS161, trans-3-(2-thienyl)acrylhydroxamic acid | 132.4 ± 13.2* | 145.0 ± 11.7* |
| TS165, trans-2-[(4′-benzylcarbonyl]phenylcyclopropane-1-carboxylic acid | 292.8 ± 17.1* | 229.4 ± 15.0* |
| DMSO | 1,132.3 ± 93.6 | 1,295.0 ± 86.5 |
| TS137, N-methyl-4-hydroxycinnamamide | 1,064.1 ± 99.3 | 1,121.1 ± 52.1 |
| TS138, N-(2-hydroxyethyl)-4-hydroxycinnamamide | 1,474.5 ± 30.4* | 1,907.0 ± 39.8* |
| TS139, 3-phenylpropionohydroxamic acid | 306.3 ± 11.9* | 286.3 ± 10.0* |
| TS140, trans-4-phenylcinnamohydroxamic acid | 101.7 ± 17.1* | 82.8 ± 4.3* |
| TS141, trans-4-fluorocinnamohydroxamic acid | 167.6 ± 4.7* | 119.0 ± 7.1* |
| TS142, trans-4-methylcinnamohydroxamic acid | 126.2 ± 5.6* | 106.0 ± 13.6* |
| TS143, 2-phenoxyacetohydroxamic acid | 228.7 ± 4.6* | 178.5 ± 27.1* |
| TS144, 4-hydroxybenzoic acid | 434.4 ± 22.1* | 424.7 ± 24.8* |
| TS145, 3-hydroxybenzoic acid | 559.4 ± 23.6* | 529.9 ± 32.0* |
| TS146, trans-4-formylcinnamohydroxamic acid | 414.3 ± 31.6* | 435.2 ± 36.2* |
| TS147, trans-4-hydroxymethylcinnamohydroxamic acid | 688.6 ± 39.2* | 746.8 ± 96.3* |
HIM (hrp-inducing minimal medium) was supplemented with 100 μM concentrations of the indicated compounds. The compounds were assayed two different times, with HIM supplemented with DMSO as the control treatment for each set of experiments.
E. amylovora 273 cells carrying the GFP reporter phrpA were used in this study. The promoter activities at 6 and 9 h of bacterial growth were determined. GFP mean fluorescence intensity (MFI) was determined for gated populations of bacterial cells by flow cytometry. Values are representative of two experiments, and three replicates were used for each experiment. *, the value is statistically significantly different from that for the DMSO control (P < 0.05, Student's t test).
Growth rate of E. amylovora is not affected by a majority of inhibitory or inducing compounds tested.
Of 85 compounds that were screened, 56 exhibited 50% or greater inhibition of hrpA promoter activity (Table 1). To exclude the possibility that the reduced GFP intensity was due to the effect on bacterial growth, these compounds were then tested for their effect on the growth rate of E. amylovora. Since HIM was not able to support the growth of E. amylovora beyond an OD590 of 0.15, M9 medium was chosen to test the effect of the compounds on growth rate. Out of 56 compounds, 49 did not cause a significant inhibition of the growth rate of the bacterium when the medium was supplemented with 100 μM concentrations of the compounds (see Table S2 in the supplemental material). All the inducers found in the screening did not affect the growth rate.
Minimum T3SS-inhibitory concentrations of TMCA, SA, BA, MCHA, and TAHA.
Out of 49 T3SS inhibitors, the 4 compounds, TMCA, SA, BA, and TS161 [trans-3-(2-thienyl)acrylhydroxamic acid (TAHA)] that showed the greatest inhibition of hrpA promoter activity were chosen for further study. TS160 (trans-2-methylcinnamohydroxamic acid [MCHA]) is a novel compound that has not been reported to be synthesized elsewhere. Therefore, although MCHA reduced the growth rate of the E. amylovora at 100 μM, we were interested in testing its effect on bacterial growth as well as the T3SS expression at lower concentrations. To compare the inhibitory effects of these compounds, we tested their half-maximal inhibitory concentrations (IC50) for T3SS expression. Here, IC50 is defined as the concentration of compound that is required for the inhibition of 50% of the hrpA promoter activity compared to the DMSO control. No growth inhibition was observed with MCHA at concentrations of 12.5 and 25 μM (data not shown). At lower concentrations, such as 12.5 μM, MCHA and TAHA did not inhibit the hrpA promoter activity to the extent shown by TMCA, SA, or BA (Fig. 2). TMCA and BA were further chosen for the experiments described below.
Fig 2.
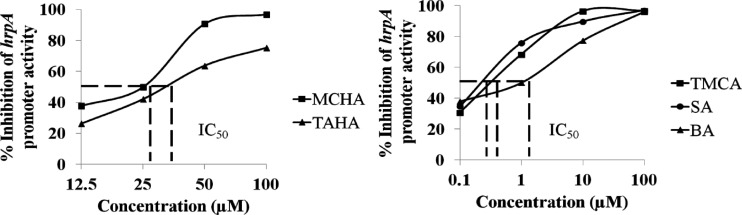
Effectiveness of selected compounds that inhibit E. amylovora hrpA promoter activity. A promoter-probe reporter fusion plasmid (phrpA) was examined by measuring GFP in the presence of the selected inhibitory compounds at various concentrations. Dashed lines show the IC50 of these compounds, i.e., the concentrations required for the inhibition of 50% of the promoter activity of hrpA compared to the DMSO control.
hrpA mRNA levels of E. amylovora are affected by inhibitors and inducers.
TMCA and BA showed an inhibitory effect, whereas EHPES induced hrpA promoter activity (Table 2). To confirm this finding, Northern blot analyses were performed to examine the effect of the compounds on hrpA mRNA levels. Compared to the DMSO control, lower levels of hrpA mRNA were observed with the addition of 100 μM TMCA and BA, whereas higher levels of hrpA mRNA were observed with the addition of 100 μM EHPES (Fig. 3). This result confirms the promoter activity result that the T3SS of E. amylovora 273 is affected by TMCA and BA (T3SS inhibitors) as well as EHPES (a T3SS inducer).
Table 2.
Expression of the T3SS genes hrpA, hrpL, and hrpS of E. amylovora 273 in HIM and HIM supplemented with DMSO or 100 μM TMCA, BA, or EHPESa
| Strain and compound | MFI (avg ± SD) for growth in HIM at: |
||
|---|---|---|---|
| 3 h | 6 h | 9 h | |
| 273(pNT-phrpA) | |||
| Vector only | 2.9 ± 1.0 | 2.7 ± 0.9 | 2.1 ± 0.3 |
| DMSO | 263.7 ± 8.3 | 1,242.1 ± 27 | 1,672.5 ± 126 |
| TMCA | 56.2 ± 4.1* | 154 ± 5.5* | 110.4 ± 3.5* |
| BA | 10.1 ± 0.2* | 40.5 ± 5.4* | 33.1 ± 2* |
| EHPES | 406.2 ± 7.8* | 2,042.8 ± 21* | 2,900 ± 120* |
| 273(pAT-phrpL) | |||
| Vector only | 2.9 ± 0.1 | 4.9 ± 0.1 | 4.5 ± 0.8 |
| DMSO | 47.2 ± 8.2 | 325.0 ± 33.2 | 491.6 ± 19.5 |
| TMCA | 7.4 ± 0.2* | 48.9 ± 3.4* | 78.9 ± 12.8* |
| BA | 9.7 ± 1.1* | 11.2 ± 0.4* | 14.0 ± 0.4* |
| EHPES | 181.1 ± 16* | 743.3 ± 8* | 1,101.5 ± 30* |
| 273(pAT-phrpS) | |||
| Vector only | 3.8 ± 0.2 | 3.9 ± 0.2 | 5.1 ± 0.1 |
| DMSO | 57.2 ± 1.5 | 93.1 ± 6.6 | 163.3 ± 8.1 |
| TMCA | 35.1 ± 0.2* | 19.22 ± 0.2* | 25.9 ± 0.3* |
| BA | 15.2 ± 0.9* | 14.2 ± 1.6* | 15.1 ± 0.2* |
| EHPES | 108.3 ± 4* | 223.0 ± 27* | 342.2 ± 11.6* |
hrpA promoter was cloned in pPROBE-gfp[AAV] (pNT), whereas the promoters of hrpL and hrpS were cloned in pPROBE-AT (pAT). The promoter activities were compared at 3, 6, and 9 h of bacterial growth in TMCA, BA, and EHPES. GFP mean fluorescence intensity (MFI) was determined for gated populations of bacterial cells by flow cytometry. Values are representative of two experiments, and three replicates were used for each experiment. Empty vector controls (pNT and pAT) were also included for all the promoter activities tested. *, the value is statistically significantly different from that for the DMSO control (P < 0.01, Student's t test).
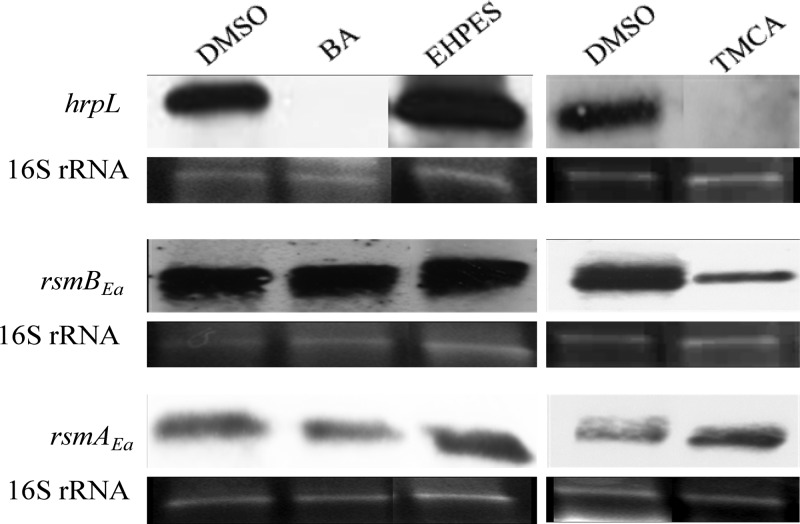
Fig 3.
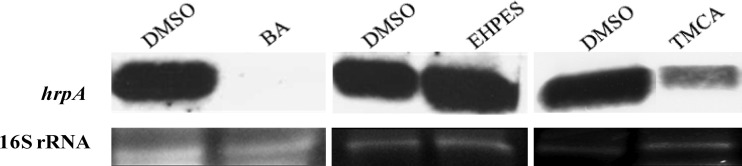
Northern blot analysis of hrpA transcripts of E. amylovora 273. RNA was isolated from cells harvested after 6 h of bacterial growth in HIM supplemented with DMSO and 100 μM BA, TMCA, and EHPES. 16S rRNA was used as an RNA loading control. The experiment was repeated three times with similar results. The image was spliced to conserve space.
Inhibitors and inducers alter T3SS expression through the HrpS-HrpL pathway.
Based on the initial experiments, we found that T3SS inhibitors and inducers altered the hrpA expression levels. hrpA is a HrpL regulon gene; in order to understand the effect of these inhibitors and inducers on the regulatory components of the T3SS, we measured both the promoter activity and mRNA levels of hrpL. Our result showed that hrpL levels were also reduced by TMCA and BA, whereas they were induced by EHPES (Table 2 and Fig. 4).
Fig 4.
Northern blot analysis of hrpL, rsmBEa, and rsmAEa transcripts of E. amylovora 273. RNA was isolated from cells harvested after 6 h of bacterial growth in HIM supplemented with DMSO control and 100 μM TMCA, BA, and EHPES. 16S rRNA was used as an RNA loading control. The experiment was repeated three times with similar results. The image was spliced to conserve space.
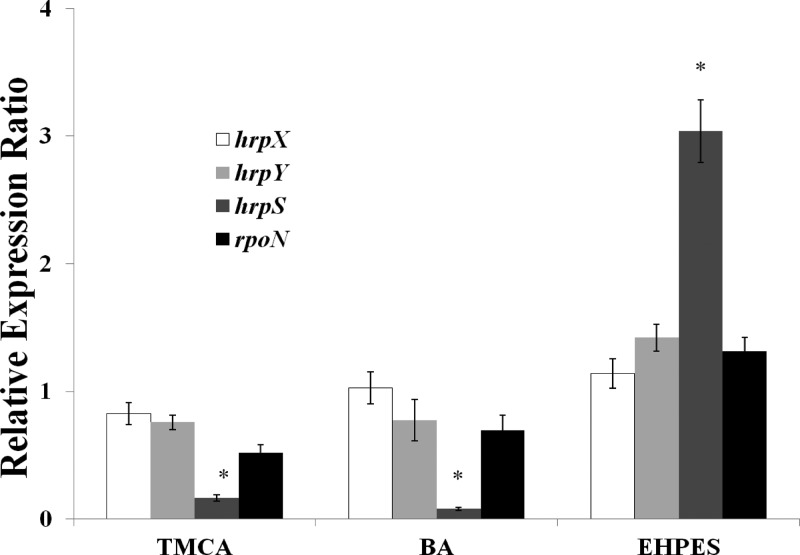
Previously, it was shown that HrpS and RpoN (σ54) are required for transcription of hrp genes in P. syringae pathovars (52, 53) and P. carotovorum (54). In the case of E. amylovora, it is known that hrpL transcription is positively regulated by HrpS (17). In order to find out whether the T3SS inhibitors and the inducer affect the expression of hrpL through hrpS and rpoN, expression of hrpS and rpoN were compared in the presence and absence of T3SS inhibitors and the inducer. We found that both the promoter activity and the mRNA levels of hrpS were decreased with inhibitor treatment and were elevated with the inducer treatment (Table 2 and Fig. 5). rpoN mRNA levels were not altered significantly by either inhibitors or the inducer (Fig. 5). This result suggests that the T3SS inhibitors (TMCA and BA) and the inducer (EHPES) alter T3SS expression through the HrpS-HrpL pathway.
Fig 5.
mRNA levels of hrpX, hrpY, hrpS, and rpoN E. amylovora 273 in HIM supplemented with 100 μM TMCA, BA, and EHPES compared to levels in HIM with DMSO, as determined by qPCR. RNA was collected at 6 h of bacterial growth. There is no significant difference between HIM supplemented with DMSO and HIM supplemented with TMCA, BA, or EHPES for hrpX, hrpY, and rpoN. Levels of gene expression of hrpS are significantly different between HIM supplemented with DMSO and HIM supplemented with 100 μM TMCA, BA, and EHPES (⁎, P < 0.001). Five replicates were used in this experiment. The P value was calculated with the Relative Expression software tool (44). The experiment was repeated three times with similar results.
TMCA alters the T3SS expression through the rsmBEa-RsmAEa system.
Previous work has identified rsmB in E. amylovora. It has also been shown that rsmBEa in E. amylovora positively regulates extracellular polysaccharide production, motility, and pathogenicity (20). Based on the sequence of rsmB in E. amylovora E9 (20), an rsmB sequence was located in the genome of E. amylovora 273, and the expression level of rsmB was determined in the presence of the inhibitors or the inducer. Northern blot results showed that rsmBEa levels were not affected by BA and EHPES (Fig. 4). A reduced level of rsmBEa was found upon treatment with the T3SS inhibitor TMCA. Similar rsmAEa (csrA homolog) expression levels were observed among bacterial cells treated with TMCA, BA, EHPES, and the DMSO control (Fig. 4). This result suggests that TMCA may also alter the T3SS expression through the rsmBEa-RsmAEa system. Similar levels of rsmB and rsmA RNAs were observed in bacterial cells treated with BA and EHPES.
Inhibitors and inducers do not alter the expression of hrpX/hrpY.
In E. amylovora, hrpX and hrpY encode a functional two-component system where HrpX acts as a sensor kinase and HrpY acts as a response regulator. This two-component system is known to regulate the T3SS expression in response to appropriate environmental conditions (14). In order to determine whether the phenolic compounds affect T3SS expression through the HrpX/Y system, mRNA levels of hrpX and hrpY were measured in the presence of the T3SS inhibitors and the inducer. We observed that the inhibitors TMCA and BA and the inducer EHPES did not alter the hrpX and hrpY mRNA levels (Fig. 5). This result suggests that the phenolic compounds do not affect the expression of hrpXY.
HrpN levels are altered in the presence of the T3SS inhibitors and T3SS inducer.
We found that the T3SS inhibitors and the inducer affected hrpL expression. hrpN of E. amylovora is a HrpL regulon gene, and its encoding protein is characterized as a major elicitor of the HR in plants. The hrpN was examined for its expression at both mRNA and protein levels. First, Northern blot analysis of E. amylovora cells treated with TMCA or BA or with EHPES showed that the mRNA levels of hrpN were either significantly reduced or increased, respectively, compared to the level in the DMSO control (Fig. 6A). In addition, Western blot analysis was performed using an anti-HrpN polyclonal antibody against the total cell fraction collected after 24 h of bacterial growth in HIM supplemented with the inhibitor TMCA or BA or the inducer EHPES. The amount of HrpN protein in the total cell fraction of E. amylovora treated with TMCA and BA was reduced, whereas the amount of HrpN protein in EHPES-treated cells was higher than that in cells receiving the control treatment (DMSO) (Fig. 6B). These results confirm the inhibitory and inducing effects of TMCA and BA and of EHPES on hrpL, respectively. Also, they indicate that the T3SS inhibitors and the inducer alter the HrpN protein levels. HrpN protein is secreted via the T3SS. To determine the effect of BA and TMCA on HrpN protein secretion, we performed Western blot analysis on the supernatant fraction of the cell cultures treated with BA and TMCA. The results demonstrated that the secreted levels of HrpN are also reduced by the T3SS inhibitors BA and TMCA (Fig. 6C).
Fig 6.
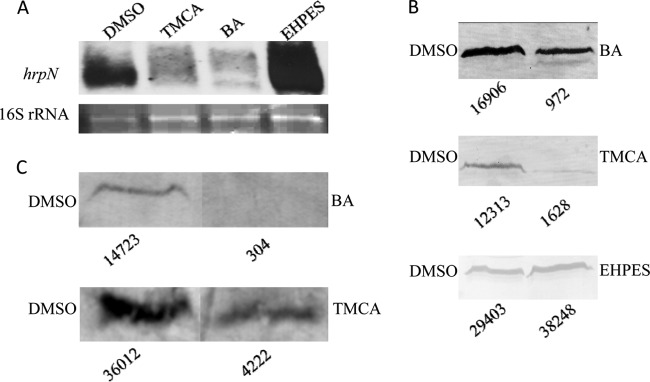
Alteration of the T3SS effector hrpN by T3SS inhibitors and inducers in E. amylovora. (A) Northern blot analysis of hrpN. RNA was isolated from cells harvested after 6 h of bacterial growth in HIM supplemented with DMSO control and 100 μM TMCA, BA, and EHPES. 16S rRNA was used as RNA loading control. The experiment was repeated twice with similar results. (B and C) E. amylovora cells were grown in HIM supplemented with DMSO (all blots) or 100 μM BA, TMCA, or EHPES. Western blot analyses of total cell fractions and supernatant fractions were performed using an anti-HrpN polyclonal antibody. Numbers below the blots are relative intensities of secreted HrpN provided by ImageJ. The experiment was repeated three times with similar results. The image in panel C was spliced to conserve space.
TMCA and BA weakened the HR in tobacco by targeting the T3SS of E. amylovora.
Based on the effects of inhibitors on the T3SS, TMCA and BA were evaluated for their ability to suppress the HR in the nonhost plant tobacco induced by E. amylovora. Compared to the DMSO treatment, a reduced HR phenotype was observed in tobacco leaves when TMCA and BA were coinfiltrated with E. amylovora (Fig. 7A). To rule out the possibility that TMCA and BA suppressed the plant defense response, leading to suppression of the HR, a tobacco plant transiently expressing the hopQ1 effector gene of P. syringae DC3000 was used in this study. HopQ1 is an effector known to elicit the HR in tobacco (55). Agrobacterium tumefaciens GV3101 carrying the hopQ1 effector gene of P. syringae DC3000(pCPP5247) was used to infiltrate tobacco leaves, which allowed the transient expression of hopQ1 in the plants (50). TMCA and BA were applied at different time points after the leaves were infiltrated with Agrobacterium, and the HR symptoms were monitored at 18 h postinfiltration. The results showed that the development of the HR in the tobacco plants transiently expressing HopQ1 was not affected in the presence of TMCA or BA (Fig. 7B).
Fig 7.
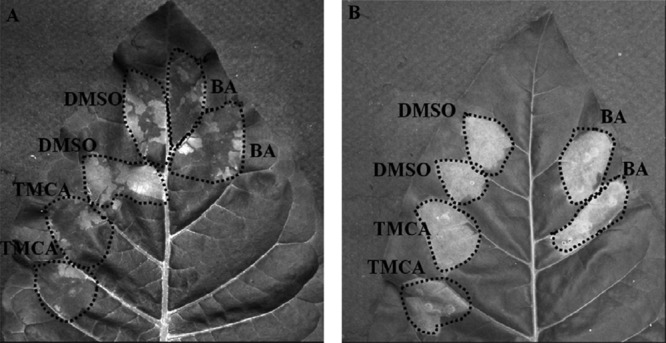
(A) Effect on HR development when BA and TMCA were coinfiltrated with E. amylovora 273 cells in Nicotiana tabacum cv. Xanthi leaves. (B) Effect on HR development in tobacco leaves transiently expressing hopQ1. TMCA and BA (100 μM) were injected at different time points after the tobacco leaves had been infiltrated with Agrobacterium tumefaciens GV3101 carrying the effector gene hopQ1 of P. syringae DC3000. In this particular picture, the compounds were injected at 6 h after the infiltration of Agrobacterium. The infiltrated areas are outlined. Both the experiments were repeated three times with similar results.
DISCUSSION
The T3SS is a crucial virulence determinant among many Gram-negative bacteria. Since it is well conserved, this makes it a good candidate target for drug development. In animal pathogens such as P. aeruginosa, Salmonella enterica serovar Typhimurium, and Yersinia pestis, large libraries of synthetic small molecules have been screened to identify potential candidates that can target the T3SS of the pathogens (56–59). Phenolic compounds are one of the major classes of secondary metabolites found in the plant. About 10,000 structures of phenolics have been identified to date (60). These compounds are usually involved in pigmentation, growth, resistance to pathogens, and reproductive functions of the plant. Our previous work showed that phenolic compounds such as TCA, OCA, and PCA specifically alter the T3SS expression of D. dadantii 3937 (37, 38). In addition, phenolic compound derivatives were found to either induce or inhibit the T3SS expression of P. aeruginosa (41). In this study, a library consisting of 85 compounds, including several synthesized phenolic derivatives, was screened in an effort to identify compounds that alter the T3SS expression in E. amylovora.
Out of 85 compounds, several showed a spectrum of inhibition or induction of the hrpA promoter activity (Table 1). Previous research has shown that low nutrient levels, pH, and temperatures can induce the T3SS (13). However, it is largely unknown whether there is any specific component that has the ability to trigger the T3SS of E. amylovora. It is interesting that several phenolic compounds that are able to induce T3SS expression were identified. Among the tested compounds, EHPES has exhibited the strongest inducing activity. TMCA and BA showed significant reduction of hrpA promoter activity, and did not have any impact on the growth rate of the bacteria (Table 1; also, see Table S2 in the supplemental material). Besides TMCA and BA, several other phenolic derivatives also show potent inhibitory effect against the T3SS of E. amylovora, such as SA (TS33), trans-4-mercaptocinnamic acid (TS26), and trans-4-dimethylaminocinnamic acid (TS112). There are electron-donating groups in TMCA (4-MeO group), TS26 (4-mercapto group), and TS112 (4-Me2N group). When an electron-withdrawing group, such as the nitro group, is presented in the 4 position, the corresponding trans-4-nitrocinnamic acid (TS28) affects the growth of the bacteria (Table 1). When the carboxylic acid group is replaced with N-hydroxylcarbamide, the resulting hydroxamic acids can maintain the inhibitory activity. For example, salicylhydroxamic acid (TS135) has demonstrated activity similar to that of TS33. However, some hydroxamic acids dramatically affect the bacterial growth, such as trans-4-bromocinnamohydroxamic acid (TS128) and trans-4-fluorocinnamohydroxamic acid (TS141).
It is known that SA can reduce the expression of virulence factors, such as flagella (required for motility) and fimbriae (capsule development, required for biofilm formation) (61). SA also plays a critical signaling role in the activation of plant defense responses against pathogen infection (62). It is worth noting that SA was found to be able to suppress the T3SS expression of E. amylovora in this study. It is also worth mentioning that the SA suppression of T3SS expression of E. amylovora is specific; no effect of SA on the T3SS expression in D. dadantii was observed (37). Earlier studies on phenylpropanoid metabolism in cell suspension cultures has reported that TMCA (4-methoxy-cinnamic acid) is one of the intermediates in the biosynthetic conversion of cinnamic acid to benzoic acid in the cells of Vanilla planifolia (63). Also, the conversion of TCA to SA has been proposed to proceed via chain shortening to produce benzoic acid (BA), followed by hydroxylation at the C-2 position to derive SA (64). It is interesting that the intermediates of the phenylpropanoid pathways such as TMCA and BA are capable of lowering the expression of the T3SS of E. amylovora.
In several plant pathogens mentioned above, HrpX phosphorylates HrpY, changing it into a transcriptional activator that initiates the expression of the T3SS. Measurement of mRNA levels of hrpXY suggested that both the T3SS inhibitors (TMCA and BA) and the T3SS inducer (EHPES) do not affect the expression of hrpXY (Fig. 5). Since HrpX/HrpY is activated by phosphorylation, the effect of these T3SS inhibitors and inducer on this two-component regulatory system is unclear at this stage. Consistent with the phenotype that we observed for hrpA expression, the T3SS inhibitors (TMCA and BA) and the T3SS inducer (EHPES) also exhibited inhibitory and inducing effects on the expression of both hrpL and hrpS, respectively (Table 2 and Fig. 5). These results indicated that the compounds channel their inducing or inhibitory effects on the expression of HrpL via HrpS. Unlike in other plant pathogens, such as D. dadantii 3937, Pantoea stewartii, and Erwinia herbicola (65–67), hrpS expression is not regulated by the two-component system HrpXY in E. amylovora. From the sequence analysis of HrpS, it was observed that HrpS of E. amylovora possesses a helix-turn-helix (HTH) domain but a very short N-terminal A domain and hence possibly lacks the phosphorylation receiver domain (14). In the case of P. syringae pv. tomato, expression of hrpS is modulated by the GacAS two-component regulatory system (23). Little is known about the regulatory effect of GacAS on hrpS expression of E. amylovora. Together, our results suggest that the inhibitors and the inducer affect the expression of hrpA through the HrpS-HrpL regulatory pathway, possibly by targeting GacSA (Fig. 1).
Studies in P. carotovorum and P. syringae have revealed that the regulatory role of GacS/A is mediated through the regulator of secondary metabolism system (68). Further research in P. carotovorum and D. dadantii 3937 suggests that hrpL is also subject to posttranscriptional regulation mediated by RsmA and rsmB RNA (37, 69). Also, it has been reported that phenolic compounds such as OCA and TCA induce the T3SS through the rsmB-RsmA pathway (37). In this study, since mRNA levels of hrpL were affected in the presence of T3SS inhibitors or the inducer, we determined the rsmBEa and rsmAEa mRNA levels in E. amylovora. The rsmBEa and rsmAEa mRNA levels were not affected by the T3SS inhibitor BA or the T3SS inducer EHPES (Fig. 4). However, compared to the DMSO treatment (control), the mRNA levels of rsmBEa was altered in E. amylovora 273 cultures supplemented with TMCA. Although the regulatory mechanism of RsmA-rsmB on T3SS of E. amylovora is unclear, from other related bacterial pathogens reported, our result indicated that TMCA may exert an effect on the T3SS through rsmBEa-RsmAEa pathway (Fig. 1). Finally, in D. dadantii, new T3SS regulatory components were found such as PecS, PecT (70), SlyA (71), polynucleotide phosphorylase (PNPase) (72), and cyclic di-GMP (c-di-GMP) phosphodiesterase (73). In addition, HexA (a PecT homolog) and KdgR of P. carotovorum subsp. carotovorum regulate harpin production through rsmB (74). Although the roles of these regulators on T3SS of E. amylovora have not been studied, it is possible that the phenolic compounds may exhibit an effect on the T3SS through some of these regulators.
It is known that HrpN is a harpin protein secreted by E. amylovora via its T3SS into the intercellular spaces of the plant (75) and it triggers the HR, i.e., programmed cell death, when inoculated in a nonhost plant like tobacco (76, 77). Therefore, we performed the HR assay in the nonhost plant, tobacco, by coinfiltrating E. amylovora with the inhibitors TMCA and BA. We found that both these inhibitors reduced the HR response (Fig. 7A). Further, plant assays showed that development of the HR in the tobacco plants that transiently expressed HopQ1 was not affected in the presence of BA as well as TMCA (Fig. 7B). These results demonstrate that the reduced HR in tobacco is due to suppression of the T3SS of E. amylovora by BA and TMCA but not by suppression of the defense response of tobacco plants by these compounds. Consistent with the tobacco HR assay, Western blot analysis showed that the T3SS inhibitors TMCA and BA suppressed the production and the secretion of the HrpN protein (Fig. 6B and C).
In summary, this study identified small molecule phenolic compounds that affect the T3SS of E. amylovora. Their effect on regulatory components of T3SS was elucidated. Based on the results, the structure-activity relationships of these compounds on T3SS expression were examined. T3SS inhibition effect exhibited by TMCA or BA and several other compounds indicate that phenolic compounds may provide an alternative strategy in bacterial disease control. In our future work, we are in the process of formulating T3SS inhibitors using the lead compounds identified in this study. Their effect on disease management will be further evaluated.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alan Collmer for providing pCPP5247 and Dazhong Zhao for Agrobacterium tumefaciens GV3101. We are grateful to Thomas Shuck and Julia N. Keyes, who assisted us with plant assays and phenolic compound screening, respectively.
This project was supported by grants from the National Science Foundation (award no. EF-0332163), the National Science Foundation of China (grant 21272029), the Research Growth Initiative of the University of Wisconsin—Milwaukee, the UWM Research Foundation (Catalyst Grant in Advanced Automation), Support for Undergraduate Research Fellows (SURF) of UWM (awarded to Julia N. Keyes), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Footnotes
Published ahead of print 14 June 2013
This work is dedicated to Noel T. Keen.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00845-13.
REFERENCES
- 1.Norelli JL, Jones AL, Aldwinckle HS. 2003. Fire blight management in the twenty-first century: using new technologies that enhance host resistance in apple. Plant Dis. 87:756–765 [DOI] [PubMed] [Google Scholar]
- 2.Koczan JM, McGrath MJ, Zhao Y, Sundin GW. 2009. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathology 99:1237–1244 [DOI] [PubMed] [Google Scholar]
- 3.Metzger M, Bellemann P, Bugert P, Geider K. 1994. Genetics of galactose metabolism of Erwinia amylovora and its influence on polysaccharide synthesis and virulence of the fire blight pathogen. J. Bacteriol. 176:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Bak DD, Heid H, Geider K. 1999. Molecular characterization of a protease secreted by Erwinia amylovora. J. Mol. Biol. 289:1239–1251 [DOI] [PubMed] [Google Scholar]
- 5.Dellagi A, Brisset MN, Paulin JP, Expert D. 1998. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol. Plant Microbe Interact. 11:734–742 [DOI] [PubMed] [Google Scholar]
- 6.Burse A, Weingart H, Ullrich MS. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant Microbe Interact. 17:43–54 [DOI] [PubMed] [Google Scholar]
- 7.Aldridge P, Metzger M, Geider K. 1997. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Mol. Gen. Genet. 256:611–619 [DOI] [PubMed] [Google Scholar]
- 8.Bogs J, Geider K. 2000. Molecular analysis of sucrose metabolism of Erwinia amylovora and influence on bacterial virulence. J. Bacteriol. 182:5351–5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He SY, Nomura K, Whittam TS. 2004. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta 1694:181–206 [DOI] [PubMed] [Google Scholar]
- 10.Mota LJ, Cornelis GR. 2005. The bacterial injection kit: type III secretion systems. Ann. Med. 37:234–249 [DOI] [PubMed] [Google Scholar]
- 11.Nimchuk Z, Rohmer L, Chang JH, Dangl JL. 2001. Knowing the dancer from the dance: R-gene products and their interactions with other proteins from host and pathogen. Curr. Opin. Plant Biol. 4:288–294 [DOI] [PubMed] [Google Scholar]
- 12.Dangl JL, Jones JD. 2001. Plant pathogens and integrated defence responses to infection. Nature 411:826–833 [DOI] [PubMed] [Google Scholar]
- 13.Wei ZM, Sneath BJ, Beer SV. 1992. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J. Bacteriol. 174:1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Z, Kim JF, Beer SV. 2000. Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two-component system, and HrpS. Mol. Plant Microbe Interact. 13:1251–1262 [DOI] [PubMed] [Google Scholar]
- 15.Xiao Y, Hutcheson SW. 1994. A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants in Pseudomonas syringae. J. Bacteriol. 176:3089–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JF, Wei ZM, Beer SV. 1997. The hrpA and hrpC operons of Erwinia amylovora encode components of a type III pathway that secretes harpin. J. Bacteriol. 179:1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei ZM, Beer SV. 1995. hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of sigma factors. J. Bacteriol. 177:6201–6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Cui Y, Mukherjee A, Chatterjee AK. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219–234 [DOI] [PubMed] [Google Scholar]
- 19.Romeo T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321–1330 [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Cui Y, Liu Y, Dumenyo CK, Mukherjee A, Chatterjee AK. 2001. Molecular characterization of global regulatory RNA species that control pathogenicity factors in Erwinia amylovora and Erwinia herbicola pv. gypsophilae. J. Bacteriol. 183:1870–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Chatterjee A, Chatterjee AK. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpin (Ecc). Mol. Plant Microbe Interact. 14:516–526 [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Wang D, Nakka S, Sundin GW, Korban SS. 2009. Systems level analysis of two-component signal transduction systems in Erwinia amylovora: role in virulence, regulation of amylovoran biosynthesis and swarming motility. BMC Genomics 10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee A, Cui Y, Yang H, Collmer A, Alfano JR, Chatterjee AK. 2003. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol. Plant Microbe Interact. 16:1106–1117 [DOI] [PubMed] [Google Scholar]
- 24.Aoki T, Akashi T, Ayabe S-i. 2000. Flavonoids of leguminous plants: structure, biological activity, and biosynthesis. J. Plant Res. 113:475–488 [Google Scholar]
- 25.Durrant WE, Dong X. 2004. Systemic acquired resistance. Annu. Rev. Phytopathol. 42:185–209 [DOI] [PubMed] [Google Scholar]
- 26.Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, Nakashita H. 2008. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20:1678–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matern U. 1991. Coumarins and other phenylpropanoid compounds in the defense response of plant cells. Planta Med. 57:S15–20 [DOI] [PubMed] [Google Scholar]
- 28.Hammerschmidt R, Dann EK. 1999. The role of phytoalexins in plant protection. Novartis Found. Symp. 223:175–190 [DOI] [PubMed] [Google Scholar]
- 29.Liu CJ, Deavours BE, Richard SB, Ferrer JL, Blount JW, Huhman D, Dixon RA, Noel JP. 2006. Structural basis for dual functionality of isoflavonoid O-methyltransferases in the evolution of plant defense responses. Plant Cell 18:3656–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. 1994. A central role of salicylic acid in plant disease resistance. Science 266:1247–1250 [DOI] [PubMed] [Google Scholar]
- 31.Glazebrook J, Ausubel FM. 1994. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 91:8955–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nawrath C, Metraux JP. 1999. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11:1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achnine L, Blancaflor EB, Rasmussen S, Dixon RA. 2004. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16:3098–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagard M, Dellagi A, Roux C, Perino C, Rigault M, Boucher V, Shevchik VE, Expert D. 2007. Arabidopsis thaliana expresses multiple lines of defense to counterattack Erwinia chrysanthemi. Mol. Plant Microbe Interact. 20:794–805 [DOI] [PubMed] [Google Scholar]
- 35.Ravirala RS, Barabote RD, Wheeler DM, Reverchon S, Tatum O, Malouf J, Liu H, Pritchard L, Hedley PE, Birch PR, Toth IK, Payton P, San Francisco MJ. 2007. Efflux pump gene expression in Erwinia chrysanthemi is induced by exposure to phenolic acids. Mol. Plant Microbe Interact. 20:313–320 [DOI] [PubMed] [Google Scholar]
- 36.Jaki BU, Franzblau SG, Chadwick LR, Lankin DC, Zhang F, Wang Y, Pauli GF. 2008. Purity-activity relationships of natural products: the case of anti-TB active ursolic acid. J. Nat. Prod. 71:1742–1748 [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Peng Q, San Francisco M, Wang Y, Zeng Q, Yang CH. 2008. Type III secretion system genes of Dickeya dadantii 3937 are induced by plant phenolic acids. PLoS One 3:e2973. 10.1371/journal.pone.0002973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Peng Q, Selimi D, Wang Q, Charkowski AO, Chen X, Yang CH. 2009. The plant phenolic compound p-coumaric acid represses gene expression in the Dickeya dadantii type III secretion system. Appl. Environ. Microbiol. 75:1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh TV, Dahlbeck D, Staskawicz BJ. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245:1374–1377 [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Russell DW. 2006. Directional cloning into plasmid vectors. Cold Spring Harbor Protoc. 10.1101/pdb.prot3919 [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki A, Li J, Zeng Q, Khokhani D, Hutchins WC, Yost AC, Biddle E, Toone EJ, Chen X, Yang CH. 2012. Derivatives of plant phenolic compound affect the type III secretion system of Pseudomonas aeruginosa via a GacS-GacA two-component signal transduction system. Antimicrob. Agents Chemother. 56:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller WG, Leveau JH, Lindow SE. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant Microbe Interact. 13:1243–1250 [DOI] [PubMed] [Google Scholar]
- 43.Peng Q, Yang S, Charkowski AO, Yap MN, Steeber DA, Keen NT, Yang CH. 2006. Population behavior analysis of dspE and pelD regulation in Erwinia chrysanthemi 3937. Mol. Plant Microbe Interact. 19:451–457 [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O'Toole GA. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 [DOI] [PubMed] [Google Scholar]
- 46.Ham JH, Bauer DW, Fouts DE, Collmer A. 1998. A cloned Erwinia chrysanthemi Hrp (type III protein secretion) system functions in Escherichia coli to deliver Pseudomonas syringae Avr signals to plant cells and to secrete Avr proteins in culture. Proc. Natl. Acad. Sci. U. S. A. 95:10206–10211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolaus T, Deiwick J, Rappl C, Freeman JA, Schroder W, Miller SI, Hensel M. 2001. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J. Bacteriol. 183:6036–6045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLisa MP, Tullman D, Georgiou G. 2003. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. U. S. A. 100:6115–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu GN, He YZ, Wang L, Wang XK. 2006. Determination of amino acids in tobacco samples by capillary electrophoresis/indirect absorbance detection with isolation of the electrolysis compartment and p-aminobenzoic acid as a background electrolyte. Anal. Sci. 22:883–887 [DOI] [PubMed] [Google Scholar]
- 50.Badel JL, Shimizu R, Oh HS, Collmer A. 2006. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant Microbe Interact. 19:99–111 [DOI] [PubMed] [Google Scholar]
- 51.Jin Q, Hu W, Brown I, McGhee G, Hart P, Jones AL, He SY. 2001. Visualization of secreted Hrp and Avr proteins along the Hrp pilus during type III secretion in Erwinia amylovora and Pseudomonas syringae. Mol. Microbiol. 40:1129–1139 [DOI] [PubMed] [Google Scholar]
- 52.Xiao Y, Heu S, Yi J, Lu Y, Hutcheson SW. 1994. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol. 176:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grimm C, Aufsatz W, Panopoulos NJ. 1995. The hrpRS locus of Pseudomonas syringae pv. phaseolicola constitutes a complex regulatory unit. Mol. Microbiol. 15:155–165 [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee A, Cui Y, Chaudhuri S, Chatterjee AK. 2002. Identification of regulators of hrp/hop genes of Erwinia carotovora ssp. carotovora and characterization of HrpLEcc (SigmaLEcc), an alternative sigma factor. Mol. Plant Pathol. 3:359–370 [DOI] [PubMed] [Google Scholar]
- 55.Schechter LM, Roberts KA, Jamir Y, Alfano JR, Collmer A. 2004. Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186:543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aiello D, Williams JD, Majgier-Baranowska H, Patel I, Peet NP, Huang J, Lory S, Bowlin TL, Moir DT. 2010. Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion. Antimicrob. Agents Chemother. 54:1988–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keyser P, Elofsson M, Rosell S, Wolf-Watz H. 2008. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 264:17–29 [DOI] [PubMed] [Google Scholar]
- 58.Clatworthy AE, Pierson E, Hung DT. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3:541–548 [DOI] [PubMed] [Google Scholar]
- 59.Muschiol S, Bailey L, Gylfe A, Sundin C, Hultenby K, Bergstrom S, Elofsson M, Wolf-Watz H, Normark S, Henriques-Normark B. 2006. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. U. S. A. 103:14566–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy DO, Wightman EL. 2011. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2:32–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price CT, Lee IR, Gustafson JE. 2000. The effects of salicylate on bacteria. Int. J. Biochem. Cell Biol. 32:1029–1043 [DOI] [PubMed] [Google Scholar]
- 62.Loake G, Grant M. 2007. Salicylic acid in plant defence—the players and protagonists. Curr. Opin. Plant Biol. 10:466–472 [DOI] [PubMed] [Google Scholar]
- 63.Funk C, Brodelius PE. 1990. Phenylpropanoid metabolism in suspension cultures of Vanilla planifolia Andr.: III. Conversion of 4-methoxycinnamic acids into 4-hydroxybenzoic acids. Plant Physiol. 94:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yalpani N, Raskin I. 1993. Salicylic acid: a systemic signal in induced plant disease resistance. Trends Microbiol. 1:88–92 [DOI] [PubMed] [Google Scholar]
- 65.Merighi M, Majerczak DR, Stover EH, Coplin DL. 2003. The HrpX/HrpY two-component system activates hrpS expression, the first step in the regulatory cascade controlling the Hrp regulon in Pantoea stewartii subsp. stewartii. Mol. Plant Microbe Interact. 16:238–248 [DOI] [PubMed] [Google Scholar]
- 66.Nizan-Koren R, Manulis S, Mor H, Iraki NM, Barash I. 2003. The regulatory cascade that activates the Hrp regulon in Erwinia herbicola pv. gypsophilae. Mol. Plant Microbe Interact. 16:249–260 [DOI] [PubMed] [Google Scholar]
- 67.Yap MN, Yang CH, Barak JD, Jahn CE, Charkowski AO. 2005. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J. Bacteriol. 187:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heeb S, Haas D. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant Microbe Interact. 14:1351–1363 [DOI] [PubMed] [Google Scholar]
- 69.Chatterjee A, Cui Y, Chatterjee AK. 2002. Regulation of Erwinia carotovora hrpLEcc (sigma-LEcc), which encodes an extracytoplasmic function subfamily of sigma factor required for expression of the HRP regulon. Mol. Plant Microbe Interact. 15:971–980 [DOI] [PubMed] [Google Scholar]
- 70.Nasser W, Reverchon S, Vedel R, Boccara M. 2005. PecS and PecT coregulate the synthesis of HrpN and pectate lyases, two virulence determinants in Erwinia chrysanthemi 3937. Mol. Plant Microbe Interact. 18:1205–1214 [DOI] [PubMed] [Google Scholar]
- 71.Zou L, Zeng Q, Lin H, Gyaneshwar P, Chen G, Yang CH. 2012. SlyA regulates type III secretion system (T3SS) genes in parallel with the T3SS master regulator HrpL in Dickeya dadantii 3937. Appl. Environ. Microbiol. 78:2888–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng Q, Ibekwe AM, Biddle E, Yang CH. 2010. Regulatory mechanisms of exoribonuclease PNPase and regulatory small RNA on T3SS of Dickeya dadantii. Mol. Plant Microbe Interact. 23:1345–1355 [DOI] [PubMed] [Google Scholar]
- 73.Yi X, Yamazaki A, Biddle E, Zeng Q, Yang CH. 2010. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol. Microbiol. 77:787–800 [DOI] [PubMed] [Google Scholar]
- 74.Cui Y, Chatterjee A, Yang H, Chatterjee AK. 2008. Regulatory network controlling extracellular proteins in Erwinia carotovora subsp. carotovora: FlhDC, the master regulator of flagellar genes, activates rsmB regulatory RNA production by affecting gacA and hexA (lrhA) expression. J. Bacteriol. 190:4610–4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perino C, Gaudriault S, Vian B, Barny MA. 1999. Visualization of harpin secretion in planta during infection of apple seedlings by Erwinia amylovora. Cell. Microbiol. 1:131–141 [DOI] [PubMed] [Google Scholar]
- 76.Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. 1992. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257:85–88 [DOI] [PubMed] [Google Scholar]
- 77.Barny M-A. 1995. Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur. J. Plant Pathol. 101:333–340 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.