Abstract
Antibiotic efficacy is greatly enhanced the earlier it is administered following infection with a bacterial pathogen. However, in a clinical setting antibiotic treatment usually commences following the onset of symptoms, which in some cases (e.g., biothreat agents) may be too late. In a BALB/c murine intranasal model of infection for Francisella tularensis SCHU S4 infection, we demonstrate during a time course experiment that proinflammatory cytokines and the damage-associated molecular pattern HMGB1 were not significantly elevated above naive levels in tissue or sera until 72 h postinfection. HMGB1 was identified as a potential therapeutic target that could extend the window of opportunity for the treatment of tularemia with antibiotics. Antibodies to HMGB1 were administered in conjunction with a delayed/suboptimal levofloxacin treatment of F. tularensis. We found in the intranasal model of infection that treatment with anti-HMGB1 antibody, compared to an isotype IgY control antibody, conferred a significant survival benefit and decreased bacterial loads in the spleen and liver but not the lung (primary loci of infection) 4 days into infection. We also observed an increase in the production of gamma interferon in all tested organs. These data demonstrate that treatment with anti-HMGB1 antibody is beneficial in enhancing the effectiveness of current antibiotics in treating tularemia. Strategies of this type, involving antibiotics in combination with immunomodulatory drugs, are likely to be essential for the development of a postexposure therapeutic for intracellular pathogens.
INTRODUCTION
Francisella tularensis is a Gram-negative intracellular bacterium that has the ability to infect several different types of cell and is the causative agent of the disease tularemia (1, 2). Four subspecies of F. tularensis have been identified, all with different degrees of pathogenicity and virulence. F. tularensis subsp. tularensis strains (type A) are considered to be the most virulent in humans and have a low infectious dose of between 1 to 10 CFU in humans (3). F. tularensis SCHU S4 is a type A strain with a high mortality rate of between 30 and 40% if left untreated and has been listed as a category A biothreat agent as a consequence (4–7).
Current treatments for tularemia are limited. A live vaccine strain (LVS), which is an attenuated form of F. tularensis subsp. holarctica (or type B strain), has proved to be an effective vaccine. However, as the nature of the attenuation has yet to be defined, LVS remains unlicensed, meaning there is no licensed vaccine available to protect against F. tularensis (8, 9). Therefore, at present, antibiotics represent the only clinically available treatment for tularemia. The levels of protection afforded by antibiotics are highly dependent upon timely administration following infection (10–12). This is exemplified by the intranasal mouse model of infection for F. tularensis, in which it was found that the administration of the fluoroquinolone levofloxacin at 72 h postinfection (p.i.) confers 100% survival but this protection is lost when antibiotic administration is delayed to 120 h p.i. (0%) (13). Therefore, a strategy that could increase the antibiotic therapeutic window may be of use for the treatment of F. tularensis infections and those caused by other intracellular pathogens.
The ability of F. tularensis to subvert the host immune response within murine models of infection has also been previously reported. The organism appears to block the production of proinflammatory cytokines up to 72 h p.i., and immediately following this immune modulatory event there is a rapid increase in cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-6 (IL-6), and the chemokine CCL2, that is reminiscent of a “cytokine storm” (14, 15). This has led to the theory that a cytokine release of this type and scale is damaging to the infected host and is in fact “too much, too late” (16). In addition, it has been proposed that the resulting hypercytokinemia and bacteremia are indicative of sepsis (16). Recent studies have focused on markers of sepsis during the course of an infection with F. tularensis. High-mobility group protein B1 (HMGB1), a late marker of sepsis, has been found to be released during infection with F. tularensis (15, 17). The protein acts as a “damage-associated molecular pattern” (DAMP) secreted by immune cells in response to tissue damage caused by injury, necrosis, or infection (18). Its role as a mediator of inflammation means that the protein plays a pivotal role in the generation of a cytokine storm and therefore represents a potential target for immunotherapy. In this study, we explored the hypothesis that targeting HMGB1 represents a useful therapeutic strategy for the treatment of tularemia. By modulating the levels of HMGB1 present in the host, we aimed to dampen the host immune response following infection with F. tularensis and potentially increase the efficacy of antibiotics to treat the disease. Here, we report the effects of administering a polyclonal antibody (Ab) raised against HMGB1, in combination with a delayed (suboptimal) administration of the antibiotic levofloxacin, within a BALB/c mouse model of infection for F. tularensis SCHU S4.
MATERIALS AND METHODS
Bacterial culture.
F. tularensis strain SCHU S4 was cultured from frozen stock for 2 days on blood cysteine glucose agar (BCGA) with cysteine at 37°C. Subsequently, bacteria were harvested to inoculate 50 ml of modified cysteine partial hydrolysate (MCPH) broth with cysteine and glucose and incubated at 37°C overnight on a rotary shaker (150 rpm). The suspension was then adjusted using phosphate-buffered saline (PBS) until the optical density at 590 nm was 0.10, where the estimated bacterial density would be 5 × 108 CFU per ml. Bacterial numbers for challenge were determined on agar following serial dilution (1:10) of samples.
Animal husbandry, challenge, and monitoring.
Six- to 8-week-old male BALB/c mice (Charles River, United Kingdom) were transferred to a high-containment class III rigid isolator, where they were given unlimited access to food and water. Mice were challenged with F. tularensis strain SCHU S4 by the intranasal route via pipette and under light anesthesia with 2-bromo-2-chloro-1,1,1-trifluoroethane (halothane). Mice were checked twice daily and scored for clinical symptoms. Mice were culled at predetermined humane endpoints. Survival times were recorded for some mice, and others were culled for analysis of tissues at different time points. All procedures and housing were in accordance with the United Kingdom Animal (Scientific Procedures) Act (1986).
Time course of infection.
BALB/c mice were challenged in two separate experiments (n = 7 and 5) with F. tularensis SCHU S4 (99 and 89 CFU, respectively), and animals were culled at 1, 2, 3, 4, or 5 days postinfection. The bacterial burden, HMGB1 protein levels in the serum, and also the proinflammatory cytokine response within the lung, liver, and spleen were measured at each time point. In addition, tissue samples from naive mice were used to establish the background levels of each parameter.
Measurement of HMGB1 in serum.
Whole blood was microcentrifuged at 7,000 rpm for 20 min, and the serum was removed and stored at −80°C. Serum HMGB1 was measured by an enzyme-linked immunosorbent assay (ELISA) kit (Shinotest, supplied through Oxford Biosystems Cadama, United Kingdom) in accordance with the manufacturer's instructions. In brief, serum samples and standards were diluted and placed on an antibody-coated plate and incubated at 37°C for 20 to 24 h. The plate was washed 5 times and then coated with a peroxidase-linked anti-HMGB1 monoclonal antibody (supplied with the kit and the same antibody used in treatment) for 2 h at 25°C. Plates were washed prior to the addition of the substrate solution (3, 3′, 5, 5′-tetramethyl-benzidine and buffer containing 0.005 mol/liter hydrogen peroxide) for 30 min. The reaction was stopped with 0.35 mol/liter sulfuric acid, and plates were read at 450 nm (microplate reader).
Administration of anti-HMGB1 Ab in a suboptimal antibiotic model.
Three separate experiments were conducted in which BALB/c mice were challenged via the intranasal route. Each animal received either anti-HMGB1 polyclonal Ab or the isotype control IgY via the intraperitoneal route (600 μg; Shinotest, supplied through Oxford Biosystems Cadama, United Kingdom) at 48 and 72 h p.i. Groups of mice receiving either the anti-HMGB1 or the isotype control Ab were culled at 96 h to examine the effects of the treatment alone. Additionally, a second group of mice received a combination therapy of the antibody and the antibiotic levofloxacin (Table 1). In these groups, the antibiotic was administered once daily via the intraperitoneal route at 40 mg/kg of body weight commencing 96 h p.i. and continuing for 7 days. When mice were culled, they were exsanguinated using cardiac puncture following terminal anesthesia. Blood was extracted as above and placed into 1.5-ml Eppendorf tubes. Lungs, spleen, and liver were removed and placed into universal tubes filled with 2 ml of PBS.
Table 1.
Experimental setup
| Expt no. | Challenge dose (CFU F. tularensis) | No. of mice (no. culleda) receiving: |
|
|---|---|---|---|
| Anti-HMGB1 Ab | Isotype control Ab | ||
| 1 | 87 | 11 (4) | 24 (7) |
| 2 | 77 | 15 (5) | 15 (5) |
| 3 | 103 | 15 (5) | 15 (5) |
Number of animals culled for analysis 96 h postinfection, prior to the administration of levofloxacin.
Measurement of bacterial burdens and proinflammatory cytokines.
The lung, liver, spleen, and blood were all processed at less than 1 h postmortem. All organs were weighed and collected in 2 ml PBS and then disrupted through a 40-μm cell sieve, and the resulting homogenate was collected. Subsequently, 100-μl aliquots of the cell suspension were used for enumeration of bacteria on agar following serial dilution in PBS. For cytokine analysis, 200-μl aliquots of cell suspension were centrifuged for 5 min at 2,000 rpm. Supernatants were removed for cytokine analysis and stored at −80°C. The levels of cytokines IL-6, monocyte chemoattractant protein 1 (MCP-1), TNF-α, IL-10, IFN-γ, and IL-12p70 in the lung, liver, and spleen were measured via the Cytometric Bead Array (CBA; Becton, Dickinson) according to the manufacturer's instructions, with the additional step of fixing the samples in 4% paraformaldehyde in PBS for at least 24 h at 4°C. Briefly, prior to fixing, samples were incubated with the combined capture bead cocktail and phycoerythrin (PE) detection antibodies for 2 h. Samples were then washed in fluorescence-activated cell sorter (FACS) buffer and resuspended in the 4% paraformaldehyde in PBS. Cytokine concentrations were measured via quantification of PE fluorescence of samples in reference to a standard curve using a BD FACS Canto flow cytometer. The cytokine standards were treated in the same manner and simultaneously with the test samples, negating any effects of fixation.
Statistical analysis.
All graphs were generated using Graphpad Prism V4.0. All statistical comparisons were made using PASW (SPSS release 18.0); some data manipulation and basic calculations were performed using Microsoft Excel 2007. Data from cytometric bead arrays were analyzed by fitting a quadratic regression to the standard curves and reading the samples as unknowns. Cytokine and bacterial burden data were converted to “per gram of tissue” for comparison. Analyses included stratified log rank tests and univariate repeated-measures or multivariate linear models. When necessary, data were transformed to the logarithm of 10 for data to better fit the requirement for equal variance between groups in these tests (tested by Levene's tests of equal variance).
RESULTS
Rapid increases in serum levels of HMGB1 precede death.
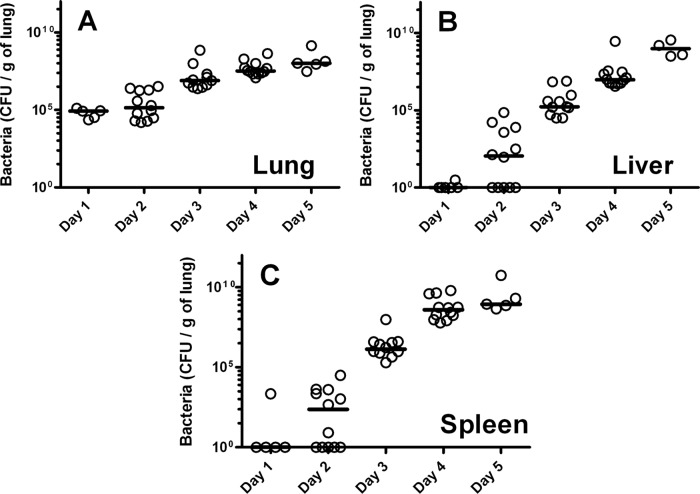
To determine the inflammatory kinetics of intranasal F. tularensis strain SCHU S4 infection, BALB/c mice were infected with 99 CFU (experiment 1) and 89 CFU (experiment 2). Mice were culled at 1, 2, 3, 4, or 5 days p.i., with the bacterial load enumerated and the proinflammatory cytokine response assessed (Fig. 1 and 2, respectively) in the lung, liver, and spleen. The lung, being the primary site of infection, showed the highest bacterial burden at days 1 and 2 p.i. (Fig. 1A), with a steady increase over time. The infection spread to the liver and spleen, with detectable bacteria at day 2 p.i. (Fig. 1B and C) followed by more-rapid increases in bacterial load between days 3 and 5 as the infection progressed (P < 0.001 for all organs). At day 5 p.i., only 4/7 (experiment 1) and 1/5 (experiment 2) mice were still alive; however, all surviving mice had high loads (109 to 1010 CFU/g of tissue) of bacteria in all organs.
Fig 1.
Bacterial colonization in organs following intranasal infection with F. tularensis strain SCHU S4. Bacterial numbers were enumerated on supplemented BCGA plates for three organs: lung (A), liver (B), and spleen (C). Each data point represents a single mouse, and lines indicate the median. Data shown are for two independent experiments. The calculated doses for experiments 1 and 2 were 99 and 89 CFU, respectively: 7 mice (experiment 1) and 5 mice (experiment 2) were culled at days 1 to 4 p.i., and 4 mice (experiment 1) and 1 mouse (experiment 2) were culled at day 5 p.i. (remaining survivors). An additional 5 mice were used to establish a baseline/naive concentration (day 0).
Fig 2.
Organ cytokine profile following intranasal infection with F. tularensis strain SCHU S4. Levels of IL-6, MCP-1, IFN-γ, TNF-α, IL-10, and IL-12p70 were measured via Cytometric Bead Array (CBA) plate counts. Cytokine concentrations are displayed as pg/g per organ (lung, liver, and spleen). Each data point represents a single mouse, and the lines indicate the medians. Data shown are for two independent experiments. The calculated doses for experiments 1 and 2 were 99 and 89 CFU, respectively. Seven mice (experiment 1) and 5 mice (experiment 2) were culled at days 1 to 4 p.i. Four mice (experiment 1) and 1 mouse (experiment 2) were culled at day 5 p.i. (remaining survivors). An additional 5 mice were used to establish a baseline/naive concentration (day 0).
The ability of F. tularensis to suppress the host inflammatory response was evident within the BALB/c model of infection. Proinflammatory cytokines IL-6, MCP-1, TNF-α, IL-10, IFN-γ, and IL-12p70 were measured in all three organ types across all five time points. IL-6, MCP-1, TNF-α, and IFN-γ were found to be below the limits of detection at days 0, 1, and 2 p.i. despite the presence of bacteria (Fig. 2). However, it was found that the amounts of IL-6, MCP-1, TNF-α, and IFN-γ present in the lung, liver, and spleen then increased rapidly and significantly across days 3 and 5 p.i. (P < 0.001 for all four cytokines). In contrast, the amount of IL-10 and IL-12p70 present remained low and relatively constant throughout the time course of infection, with no obvious correlation with bacterial load (P = 0.052 for IL-10; P = 0.298 for IL-12p70).
The quantities of the “sepsis marker” HMGB1 present in the blood were also examined. The amount of HMGB1 in the sera of infected animals remained constant from days 1 to 3 p.i. and comparable to that for naive blood samples (P > 0.05). However, significant increases in HMGB1 levels were detected at day 4 p.i. (P < 0.001), with a further rapid increase at day 5 p.i. in the remaining survivors (P < 0.001) (Fig. 3).
Fig 3.
Serum HMGB1 release in mice following intranasal infection with F. tularensis strain SCHU S4. Levels of serum HMGB1 (ng/ml) were assessed via ELISA. Each data point represents a single mouse, and the lines indicate the medians. Data shown are for two independent experiments. The calculated doses for experiments 1 and 2 were 99 and 89 CFU, respectively. Seven mice (experiment 1) and 5 mice (experiment 2) were culled at days 1 to 4 p.i.; 4 mice (experiment 1) and 1 mouse (experiment 2) were culled at day 5 p.i. (remaining survivors). An additional 13 mice were used to establish a baseline/naive concentration (day 0).
Treatment of intranasal F. tularensis infection with HMGB1 neutralizing antibody increases host survival and correlates with increased IFN-γ.
Three experiments were performed in which mice were infected with estimated challenge doses of 87 CFU (experiment 1), 77 CFU (experiment 2), and 103 CFU (experiment 3) by the intranasal route. Mice were treated with two doses of either 600 μg of anti-HMGB1 Ab or 600 μg matched isotype (IgY) control Ab at days 2 and 3 p.i. by the intraperitoneal route. Preliminary data indicated that effective suppression of HMGB1 levels required once-daily dosing (data not shown). At day 4 p.i., a subset of animals were culled for bacterial and cytokine analysis. The remaining mice received 7 daily intraperitoneal doses of levofloxacin, a suboptimal regimen compared to the 14 days of treatment required for complete clearance of the organism (13). We observed significantly increased median time to death of mice treated with the anti-HMGB1 Ab compared to their isotype-treated counterparts (P = 0.004) and found a suitable level of repeatability between experimental runs (P = 0.263) (Fig. 4).
Fig 4.
Survival of mice following anti-HMGB1 Ab treatment in combination with levofloxacin. Male BALB/c mice were challenged with 87 CFU (experiment 1), 77 (experiment 2), or 103 CFU (experiment 3) F. tularensis SCHU S4 via the intranasal route. Groups were treated with either 600 μg of anti-HMGB1 Ab or 600 μg of an isotype control (IgY) at 48 and 72 h p.i. Levofloxacin (40 mg/kg) was administered via the intraperitoneal route at 96 h p.i. and continued once daily for 7 days for both groups. Each data point represents the percentage of survivors in each group at the corresponding time point. In the first experiment, 7 animals received anti-HMGB1 Ab and 17 animals received isotype control IgY; in the second and third experiments, 10 animals received anti-HMGB1 Ab and 10 animals received isotype control IgY. Data were analyzed by stratified log rank test.
In the subset of mice culled at day 4 p.i., ELISA was used to identify whether levels of HMGB1 had been reduced. We found that the amount of circulating HMGB1 was significantly lower in the treatment group than in the control (P = 0.003). Overall, the serum HMGB1 levels were approximately 4-fold (95% confidence interval [CI], 1.54 to 6.42) lower in the anti-HMGB1 Ab-treated mice than in the IgY-treated mice.
The bacterial burden and the quantity of cytokines present were also assessed on day 4 p.i. (Fig. 5 and 6, respectively) in the lung, liver, and spleen of these animals. We subjected these data to repeated-measures (by organ) univariate analysis and found that the treatment had a significant effect on bacterial numbers overall (P = 0.010). Further independent analysis of each organ showed significantly reduced bacterial loads in the livers (P = 0.005) and spleens (P = 0.006) in the mice receiving anti-HMGB1 Ab treatment; however, this was not observed in the lung (P = 0.312). This decrease in bacterial burden between groups correlated with a significant change in levels of IFN-γ. Overall, it was consistently found that the treatment groups receiving the anti-HMGB1 polyclonal Ab had significantly increased amounts of IFN-γ (P < 0.001) present within the infected organs compared to those receiving the isotype control Ab. Despite monitoring a range of proinflammatory cytokines, no other significant differences were found. To further determine where differences in cytokine production occurred during infection, the data were further split by organ and reanalyzed using univariate linear models. We found that IFN-γ was increased in all three organs of anti-HMGB1 Ab-treated mice (lung, P < 0.001; liver, P < 0.001; spleen, P = 0.022) (Fig. 6). The quantities of the chemokine MCP-1 present were not affected by anti-HMGB1 Ab treatment in the lung and spleen (P = 0.674 and P = 0.069, respectively), but a reduction in the liver was noted when compared with equivalent data from the isotype control group (P = 0.035). No significant differences were seen in the levels of IL-6, IL-10, TNF-α, and IL-12p70 cytokines at the organ level.
Fig 5.
Bacterial colonization in anti-HMGB1- and IgY Ab-treated mice. Male BALB/c mice were challenged with 87 CFU (experiment 1), 77 CFU (experiment 2), and 103 CFU (experiment 3) F. tularensis SCHU S4 via the intranasal route. Mice were culled at 96 h p.i. and bacteria enumerated on supplemented BCGA for the lung, liver, and spleen. Groups were treated with either 600 μg of anti-HMGB1 Ab or 600 μg of an isotype control Ab (IgY) at 48 and 72 h p.i. Each data point represents a mouse, and the lines represent the medians. In the first experiment, 4 animals received anti-HMGB1 Ab and 7 animals received isotype control IgY Ab; in the second and third experiments, 5 animals received anti-HMGB1 Ab and 5 animals received isotype control IgY Ab. Data were analyzed by univariate linear models of the log10 transformed data.
Fig 6.
Cytokine production in anti-HMGB1- and IgY Ab-treated mice. Male BALB/c mice were challenged with 87 CFU (experiment 1), 77 CFU (experiment 2), and 103 CFU (experiment 3) F. tularensis SCHU S4 via the intranasal route. Mice were culled at 96 h p.i. and IFN-γ and MCP-1 cytokines assayed for the lung, liver, and spleen. Groups were treated with either 600 μg of anti-HMGB1 Ab or 600 μg of an isotype control (IgY) Ab at 48 and 72 h p.i. Each data point represents a mouse, and the lines represent the medians. In the first experiment, 4 animals received anti-HMGB1 Ab and 7 animals received isotype control IgY Ab; in the second and third experiments, 5 animals received anti-HMGB1 Ab and 5 animals received isotype control IgY Ab. Data were analyzed by the univariate linear model of the log10 transformed data. Significance markers have been included to show differences associated with the different treatments. Levels of IL-6, IL-10, TNF-α, and IL-12p70 were also determined but are not shown.
DISCUSSION
The control of acute bacterial infections by the host requires the activation of the innate immune system and the subsequent initiation of an inflammatory response. However, during lethal infectious disease, the induction of inflammation can be the principal cause of mortality due to immunopathology. There is a fine line between the host mounting an appropriate immune response to clear infection and mounting an overactive immune response that can lead to host tissue damage and mortality (19). Evidence indicates that F. tularensis has evolved to disrupt this balance. The initial delay in the inflammatory response allows the pathogen to colonize and grow within the host. This ultimately results in a hypercytokinemia and a bacteremia that is characteristic of sepsis (14, 15). Therefore, the targeting of therapeutics toward key immunomodulators involved in the inflammatory response may represent an effective strategy for the treatment of an established, symptomatic infection.
In these studies, we demonstrate that during an intranasal BALB/c mouse model of F. tularensis SCHU S4 there is an absence of proinflammatory cytokines during the first 2 days of infection. Thereafter, there is a rapid increase of IL-6, TNF-α, IFN-γ, and MCP-1 within the lungs, liver, and spleen and an increase in the DAMP HMGB1 within the blood. This is the first time this has been demonstrated within the BALB/c murine model. Importantly, we also provide evidence that targeting HMGB1 with an antibody in combination with an antibiotic, levofloxacin, can increase host survival time and this survival correlates with an increase in secretion of IFN-γ.
During two detailed time course studies, we show that, despite there being detectable bacteria at the site of infection (lung) at day 1 p.i. and in the spleen and liver on day 2 p.i., the levels of proinflammatory cytokines are not measurable until day 3 p.i. in all organs. Following this initial release, levels rapidly increase, indicative of a cytokine storm (18, 20). These findings are supported by previously published research with different strains of mice and/or F. tularensis (21–23).
In addition to monitoring the amount of cytokine released in the tissues, the quantities of the DAMP HMGB1 were also assessed in the blood during the whole time course. It was found that HMGB1 was detected in the sera of infected mice only between 72 and 96 h p.i. Previous studies using different models of F. tularensis infection again validate our data with release of this protein reported by other methods, such as Western blotting (14, 15).
Once HMGB1 is secreted into the extracellular milieu, it acts in a manner similar to that of other proinflammatory cytokines. The protein binds several receptors, most notably the receptor for advanced glycation end products (RAGE), which is expressed on several different cell types, including macrophages and endothelial cells (24). Following an intracellular signaling cascade, NF-κB is activated and release of other proinflammatory cytokines occurs, leading to a positive-feedback loop, which contributes to a cytokine storm (25). What separates HMGB1 from other proteins is that it is a late mediator of sepsis (26, 27). While strategies targeting TNF-α (previously believed to be the key cytokine) have failed to treat sepsis (28), targeting HMGB1 has shown some encouraging results. Preliminary studies have demonstrated that anti-HMGB1 antibodies could protect the host following lethal lipopolysaccharide (LPS) exposure and that the efficacy of the therapy was dose dependent (29).
In these studies, we sought to determine whether controlling/dampening down the overactive inflammatory response by targeting HMGB1 was a suitable strategy to increase the effectiveness of antibiotic treatments. Our study utilized an adapted version of a previously published F. tularensis levofloxacin antibiotic model (13) to test our hypothesis of whether anti-HMGB1 Ab therapy could extend the window for successful antibiotic treatment and thus increase overall host survival. We used a suboptimal antibiotic dosing regimen (commencing antibiotic treatment at 96 h for a total of 7 days) to establish, first, whether anti-HMGB1 Ab therapy could increase the antibiotic window during acute disease and, second, whether the therapy could aid in bacterial clearance.
Since HMGB1 levels are raised between 72 h and 96 h p.i., we concluded that administration of anti-HMGB1 Ab should commence at 48 h p.i., as HMGB1 release was most likely commencing in the tissue at this time point and would allow time for the antibody to take effect. An additional administration of anti-HMGB1 Ab was given at 72 h in order to reduce the quantities of HMGB1 present prior to antibiotic administration at 96 h. Previous studies in our lab have shown that the effect of a single antibody dose of anti-HMGB1 Ab lasted 24 h (unpublished data). We found that anti-HMGB1 Ab treatment was not detrimental to the host and provided a statistically significant survival benefit, reducing the bacterial burden in the liver and spleen. This reduction in bacterial burden following treatment with anti-HMGB1 Ab may be due to the increased phagocytic ability of macrophages that has been previously reported during Pseudomonas aeruginosa infection (30).
The most surprising and interesting result generated in these studies was the increase in IFN-γ in anti-HMGB1 Ab-treated groups compared to isotype control-treated mice. In all three independent experiments, anti-HMGB1 Ab caused an increase in IFN-γ in all of the organs analyzed. This increase is unlikely to be solely due to changes in bacterial load, as the bacterial burden in the lung was not found to be significantly different between treatment groups. In addition, the levels of proinflammatory cytokines tend to correlate with bacterial load. Therefore, an increased bacterial load would also be expected in the anti-HMGB1 Ab-treated mice, and this is certainly not the case, since decreases in bacterial load were observed in the spleen and liver following therapeutic treatment.
Indeed, the increase in cytokines seems to be specific to IFN-γ, as quantities of MCP-1 (another proinflammatory marker) decrease in the liver following treatment with anti-HMGB1 Ab and the other cytokines measured remain unchanged between groups.
The effect on IFN-γ in these studies may indicate a further mechanism of action by the therapeutic antibody, in addition to the previously mentioned increased phagocytic ability of macrophages. Indeed, it is well reported that IFN-γ is critical for protection against tularemia (31–33). Our data suggest that treatment with anti-HMGB1 Ab leads to a more appropriate immune response by increasing levels of IFN-γ and decreasing MCP-1. The exact role of the increase of IFN-γ in this model is yet to be determined but is most likely due to increased nitric oxide production in macrophages, resulting in enhanced bacterial killing.
In our experiments, administration of anti-HMGB1 Ab in combination with suboptimal antibiotic dosing did not lead to bacterial clearance, with mice succumbing to infection several days following the 7-day antibiotic treatment regime. Clearly, in order to make these findings clinically relevant, it would be advantageous to conduct further work examining the efficacy of this therapeutic strategy by administering anti-HMGB1 Ab at the same time as antibiotics (i.e., from 96 h postinfection). Nonetheless, this study demonstrates proof of concept that targeting an immunostimulatory DAMP (such as the HMGB1 protein) with an appropriate antibody therapy can increase the window of opportunity of antibiotics within an acute model of infection. In addition, the apparent increase in IFN-γ, known to be important for the clearance of a range of infectious diseases, following the modulation of the amounts of HMGB1 present in vivo, highlights the potential for this antibody-antibiotic combination therapeutic regimen for the treatment of diseases caused by other intracellular pathogens.
ACKNOWLEDGMENTS
This work was funded by the United Kingdom Ministry of Defense.
We thank Michelle Nelson, Sophie Smither, Sarah Harding, and Rachel Dean for experimental help and advice.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.D'Elia R, Jenner DC, Laws TR, Stokes MGM, Jackson MC, Essex-Lopresti AE, Atkins HS. 2011. Inhibition of Francisella tularensis LVS infection of macrophages results in a reduced inflammatory response: evaluation of a therapeutic strategy for intracellular bacteria. FEMS Immunol. Med. Microbiol. 62:348–361 [DOI] [PubMed] [Google Scholar]
- 2.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76:5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis J, Oyston PCF, Green M, Titball RW. 2002. Tularemia. Clin. Microbiol. Rev. 15:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, Mcdade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. 2001. Tularemia as a biological weapon—medical and public health management. JAMA 285:2763–2773 [DOI] [PubMed] [Google Scholar]
- 5.Kortepeter MG, Parker GW. 1999. Potential biological weapons threats. Emerg. Infect. Dis. 5:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLendon MK, Apicella MA, Allen LAH. 2006. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 60:167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Blackwell Publishing, Oxford, England: [DOI] [PubMed] [Google Scholar]
- 8.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis—infection and immunity in mice. Infect. Immun. 59:2922–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyston PCF. 2009. Francisella tularensis vaccines. Vaccine 27(Suppl 4):D48–D51 [DOI] [PubMed] [Google Scholar]
- 10.Piercy T, Steward J, Lever MS, Brooks TJG. 2005. In vivo efficacy of fluoroquinolones against systemic tularaemia infection in mice. J. Antimicrob. Chemother. 56:1069–1073 [DOI] [PubMed] [Google Scholar]
- 11.Russell P, Eley SM, Fulop MJ, Bell DL, Titball RW. 1998. The efficacy of ciprofloxacin and doxycycline against experimental tularaemia. J. Antimicrob. Chemother. 41:461–465 [DOI] [PubMed] [Google Scholar]
- 12.Steward J, Piercy I, Lever MS, Simpson AJH, Brooks TJG. 2006. Treatment of murine pneumonic Francisella tularensis infection with gatifloxacin, moxifloxacin or ciprofloxacin. Int. J. Antimicrob. Agents 27:439–443 [DOI] [PubMed] [Google Scholar]
- 13.Klimpel GR, Eaves-Pyles T, Moen ST, Taormina J, Peterson JW, Chopra AK, Niesel DW, Carness P, Haithcoat JL, Kirtley M, Ben Nasr A. 2008. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine 26:6874–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mares CA, Ojeda SS, Morris EG, Li Q, Teale JM. 2008. Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect. Immun. 76:3001–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma J, Mares CA, Li Q., Morris EG, Teale JM. 2011. Features of sepsis caused by pulmonary infection with Francisella tularensis type A strain. Microb. Pathog. 51:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowley SC. 2009. Proinflammatory cytokines in pneumonic tularemia: too much too late? J. Leukoc. Biol. 86:469–470 [DOI] [PubMed] [Google Scholar]
- 17.Sharma J, Li Q., Mishra BB, Pena C, Teale JM. 2009. Lethal pulmonary infection with Francisella novicida is associated with severe sepsis. J. Leukoc. Biol. 86:491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. 2013. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 20:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. 2012. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76:16–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanford MM, McFadden G, Karupiah G, Chaudhri G. 2007. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 85:93–102 [DOI] [PubMed] [Google Scholar]
- 21.Elkins KL, Cowley SC, Bosio CM. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105:284–324 [DOI] [PubMed] [Google Scholar]
- 22.Conlan JW, Zhao XG, Harris G, Shen H, Bolanowski M, Rietz C, Sjostedt A, Chen WX. 2008. Molecular immunology of experimental primary tularemia in mice infected by respiratory or intradermal routes with type A Francisella tularensis. Mol. Immunol. 45:2962–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538–4547 [DOI] [PubMed] [Google Scholar]
- 24.Stern D, Yan SD, Yan SF, Schmidt AM. 2002. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev. 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Wang HC, Czura CJ, Tracey KJ. 2005. The cytokine activity of HMGB1. J. Leukoc. Biol. 78:1–8 [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Czura CJ, Tracey KJ. 2005. HMGB1 as a late mediator and therapeutic target in sepsis. Shock 23:29 [Google Scholar]
- 27.Wang HC, Bloom O, Zhang MH, Vishnubhakat JM, Ombrellino M, Che JT, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251 [DOI] [PubMed] [Google Scholar]
- 28.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R, Pennington JE, Wherry JC. 1995. Efficacy and safety of monoclonal-antibody to human tumor-necrosis-factor-alpha in patients with sepsis syndrome—a randomized, controlled, double-blind, multicenter clinical trial. JAMA 273:934–941 [PubMed] [Google Scholar]
- 29.Yang H, Ochani M, Li JH, Qiang XL, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang HC, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. 2004. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. U. S. A. 101:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li JH, Wang HC, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, Mantell LL. 2012. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 18:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7:421–428 [DOI] [PubMed] [Google Scholar]
- 32.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjöstedt A, North RJ, Conlan JW. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369–1374 [DOI] [PubMed] [Google Scholar]