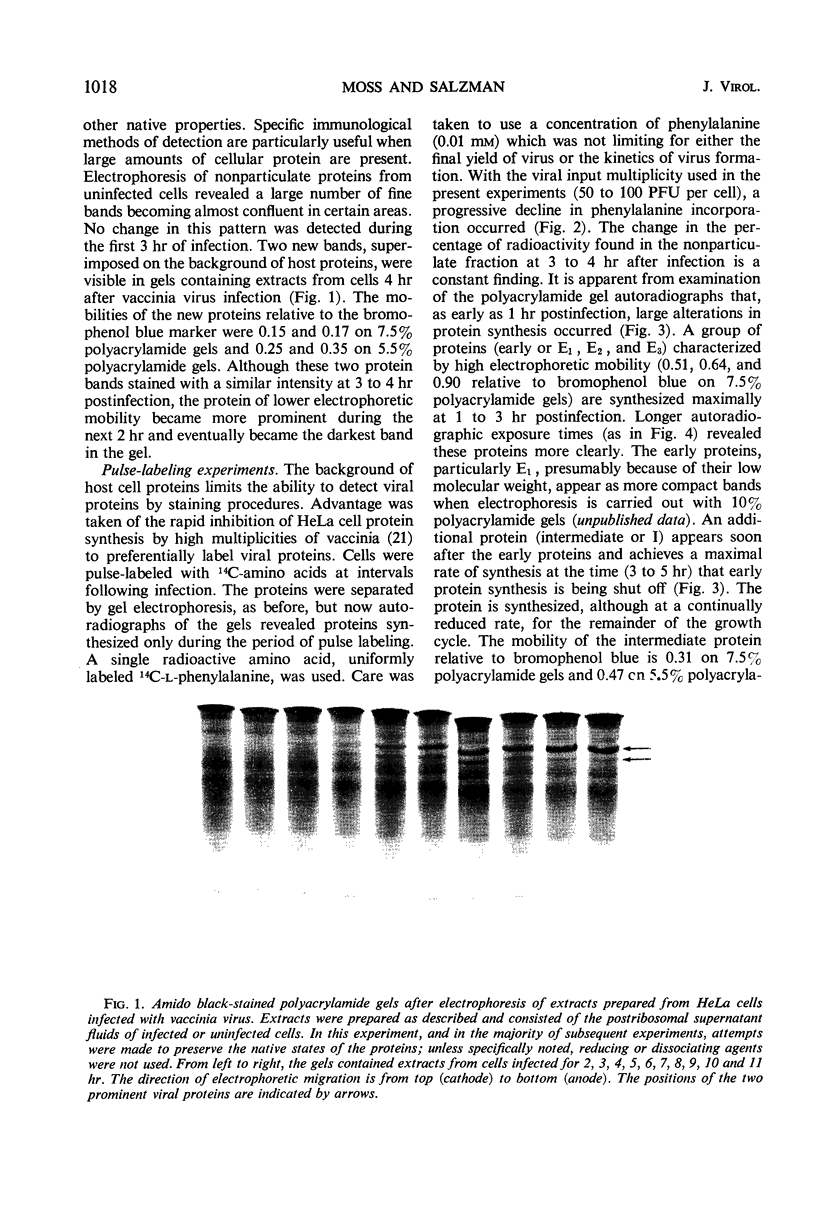
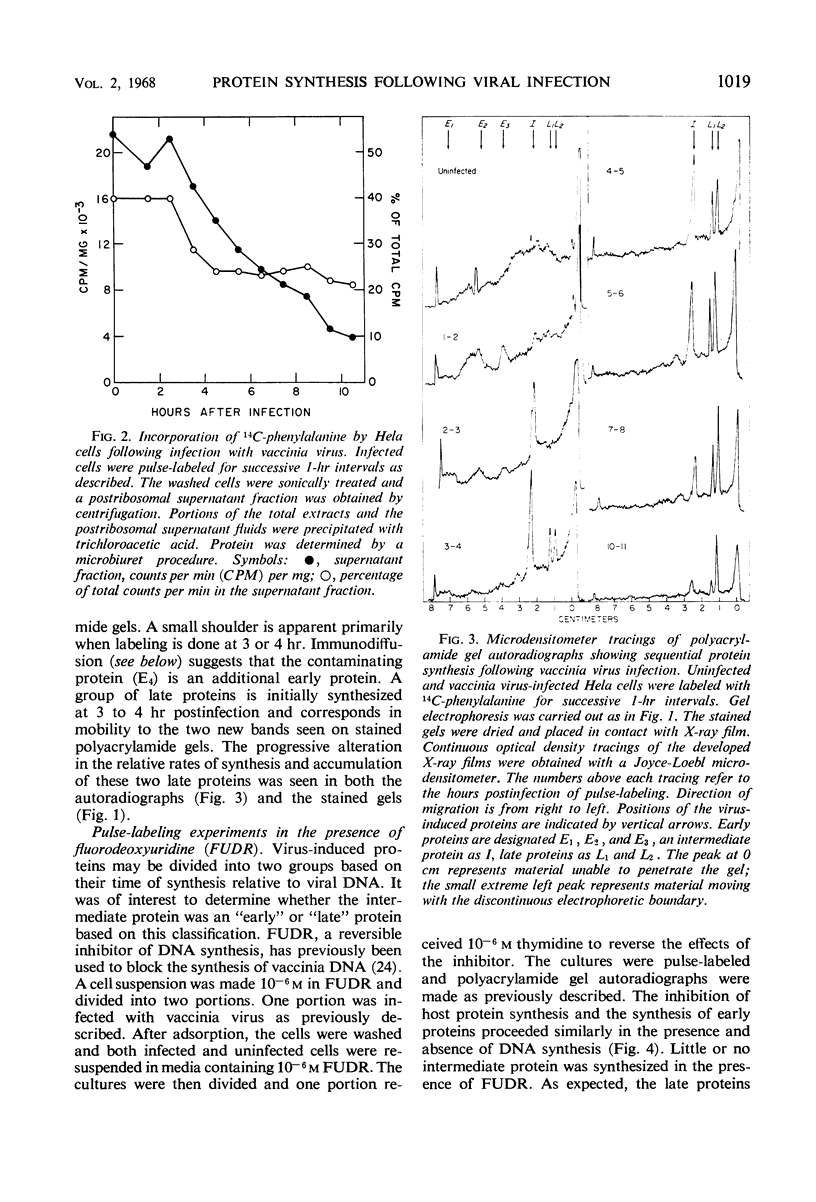
Abstract
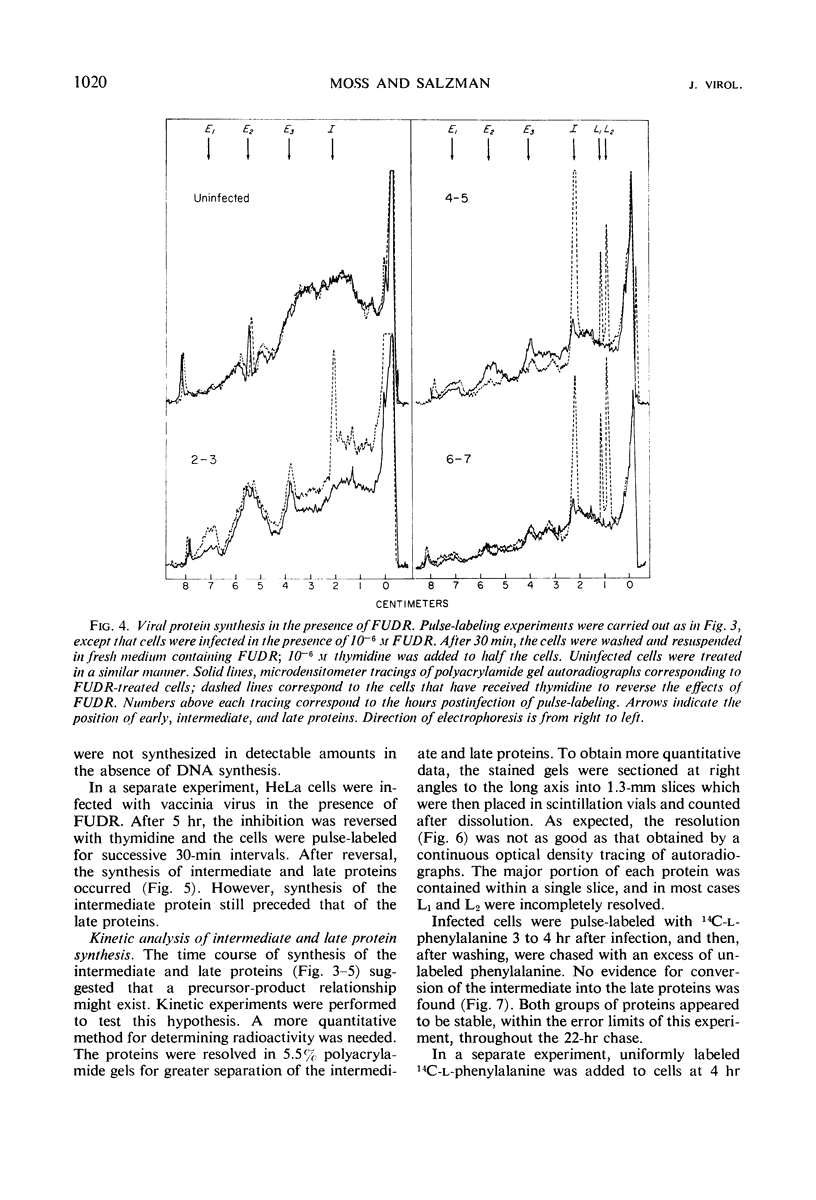
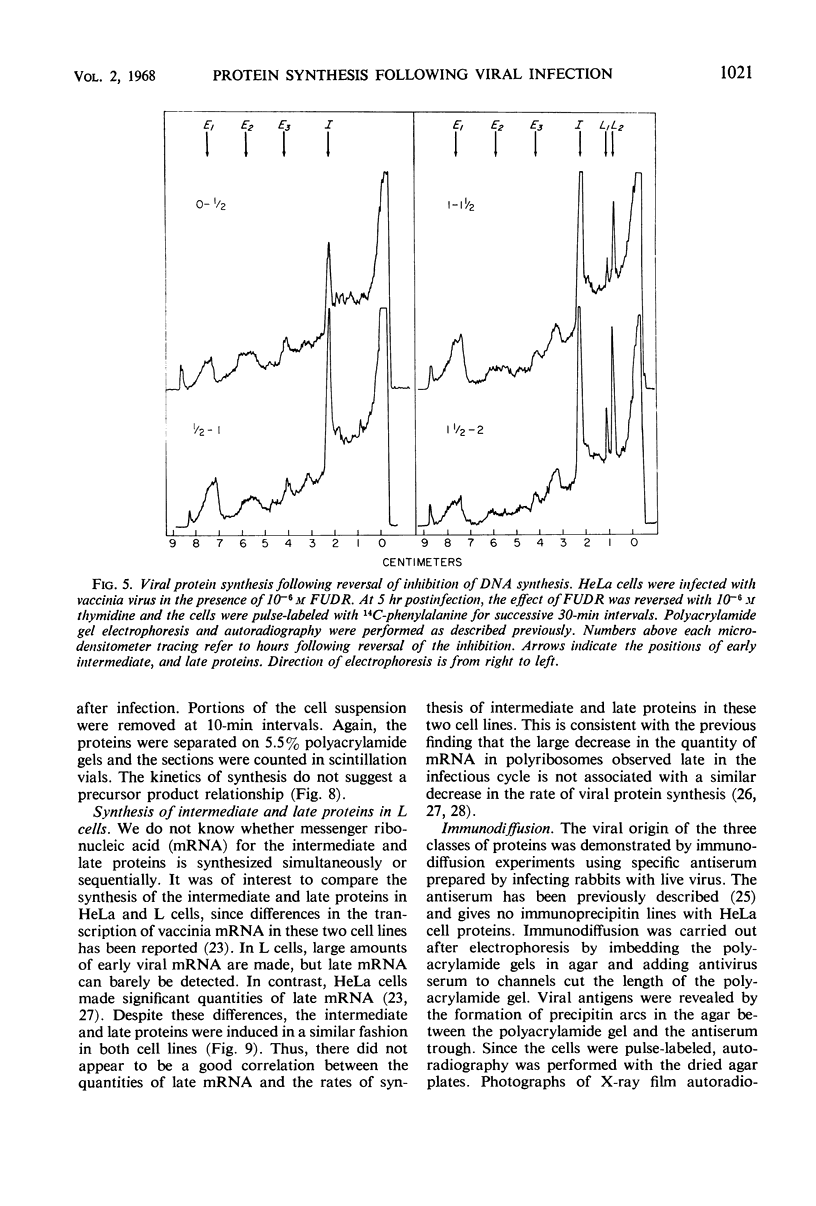
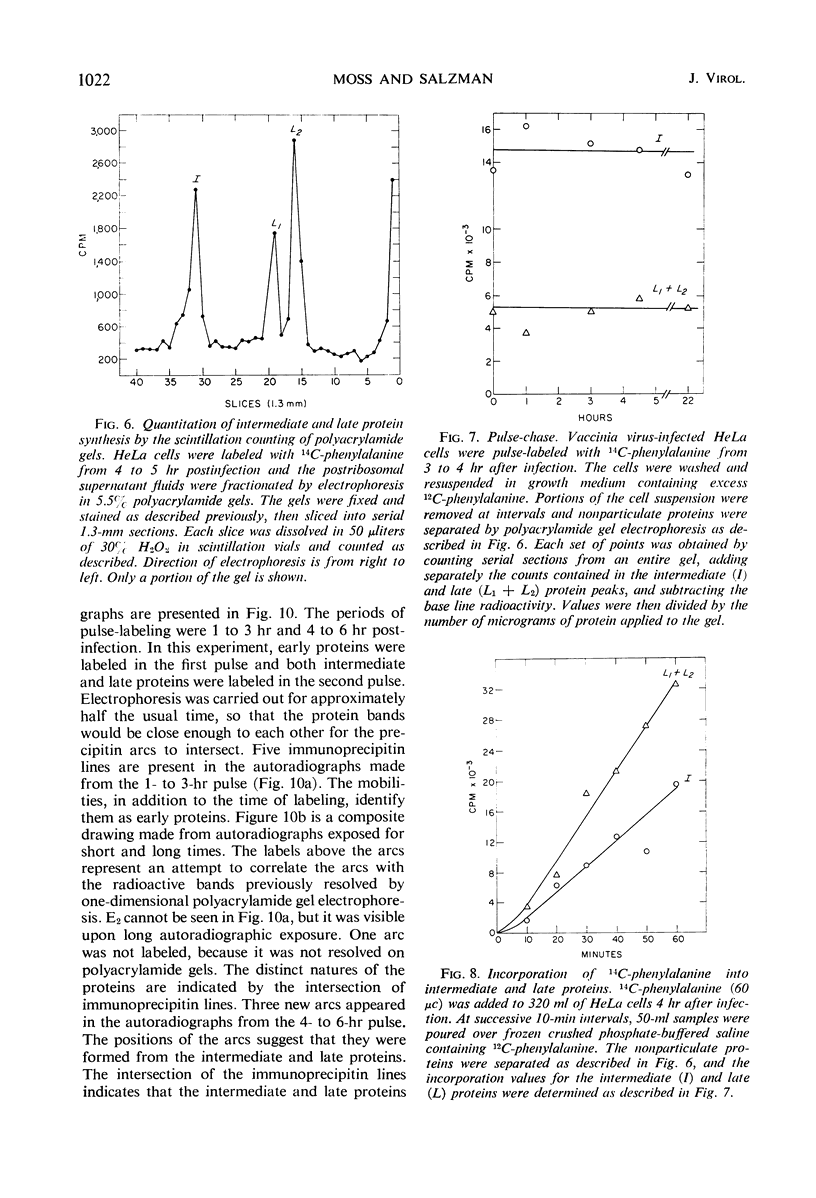
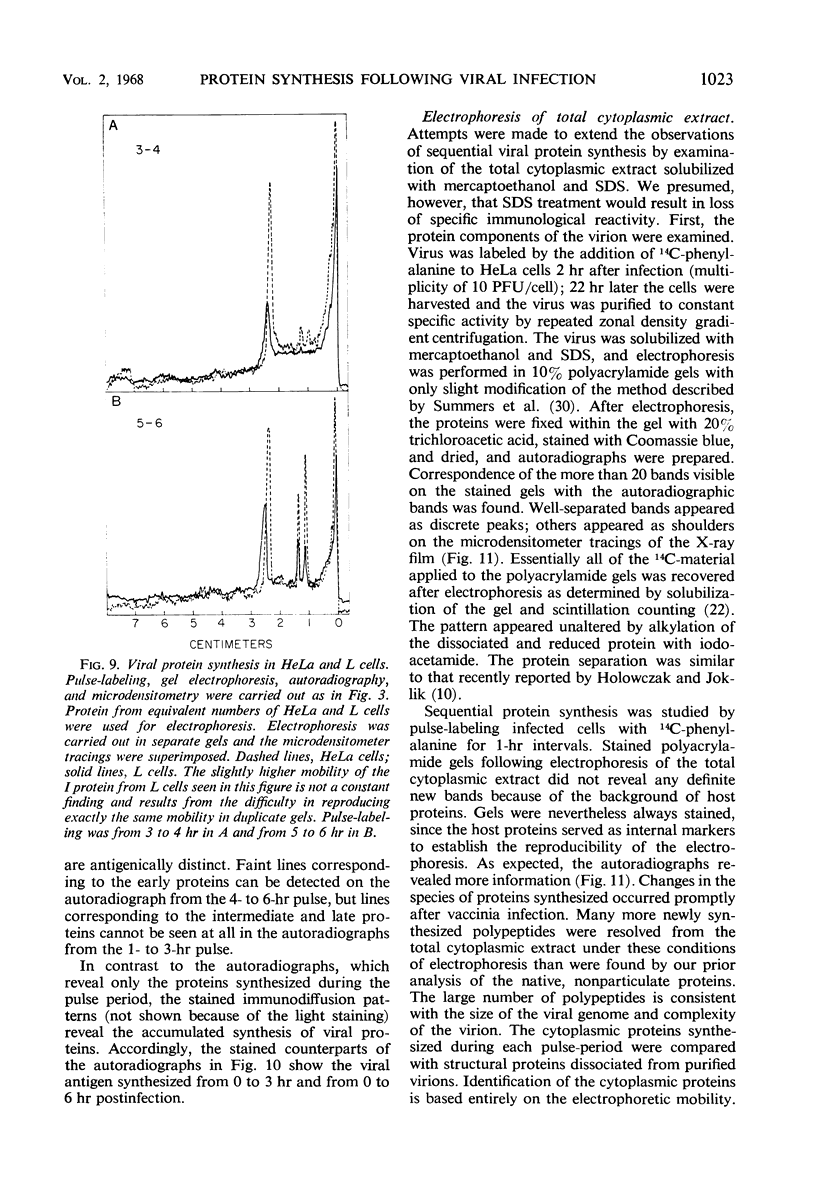
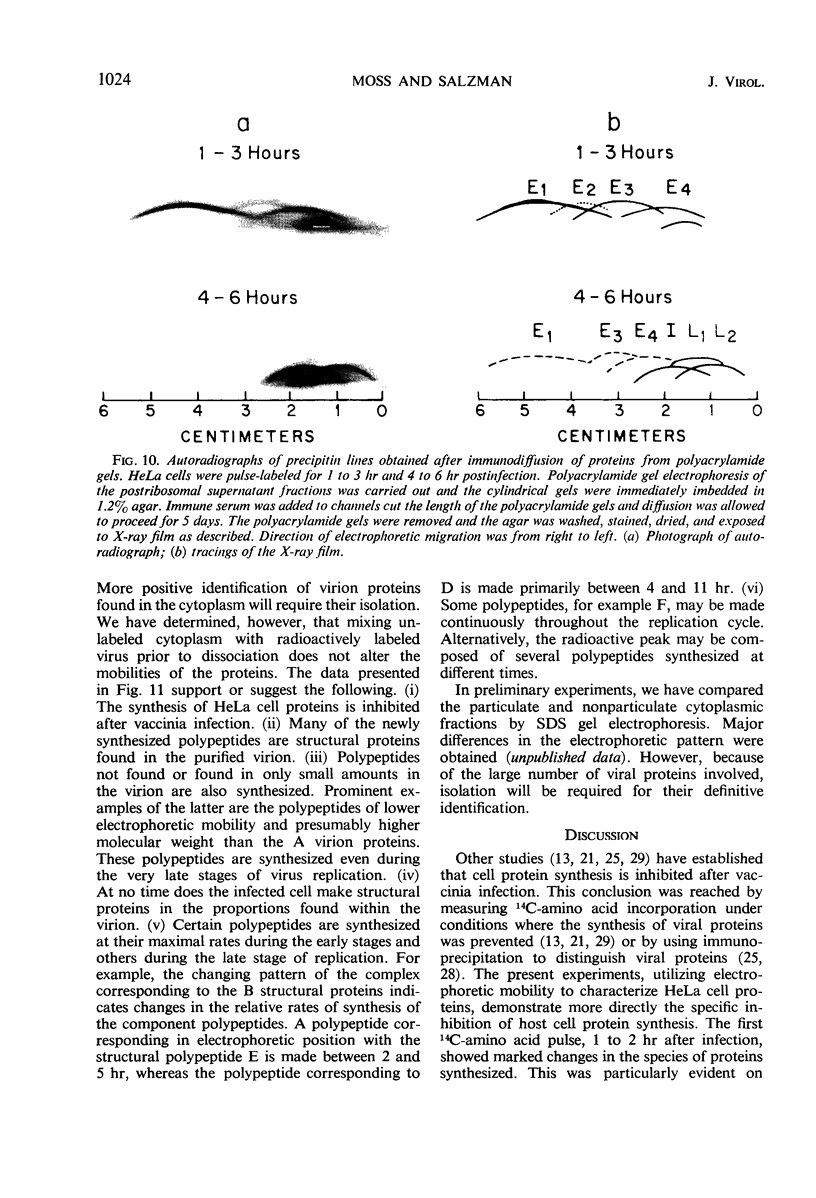
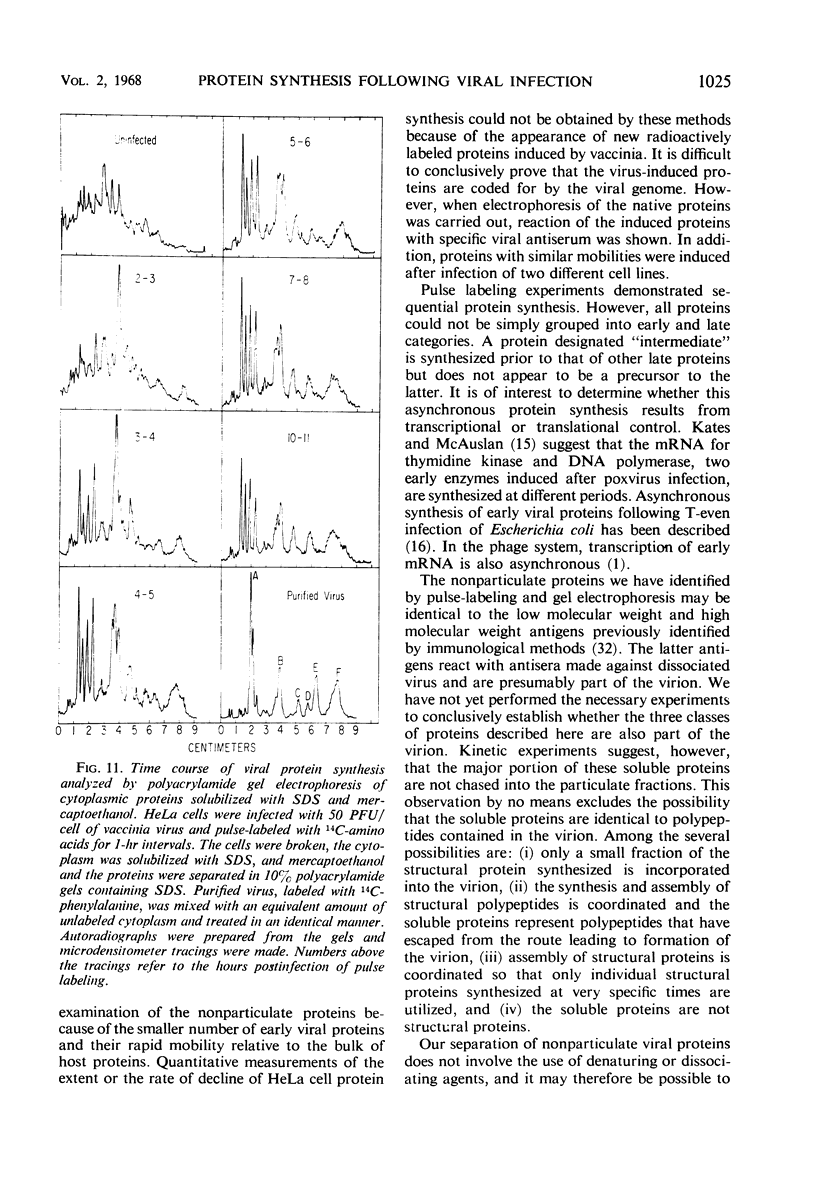
Inhibition of HeLa cell protein synthesis and the sequential synthesis of viral proteins were followed by pulse-labeling infected cells with 14C-phenylalanine. Proteins were resolved by polyacrylamide gel electrophoresis. The viral origin of native proteins was confirmed by immunodiffusion. The inhibition of host protein synthesis and the synthesis of early viral proteins occur 1 to 3 hr after infection. This early sequence of events also occurs in the presence of 5-fluorodeoxyuridine, an inhibitor of deoxyribonucleic acid synthesis. Other viral proteins are synthesized at a later time. Those proteins which are not made in the absence of viral deoxyribonucleic acid synthesis can be further subdivided into intermediate and late classes. The intermediate protein is synthesized before the late proteins but does not appear to be a precursor of them. Many more viral polypeptides were resolved by polyacrylamide gel electrophoresis after solubilization of the entire cytoplasmic fraction with sodium dodecyl sulfate. Virion and nonvirion proteins were identified. Kinetic experiments suggested that certain structural proteins as well as certain nonstructural proteins are made early, whereas others of both classes are made primarily at later times.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Chrambach A. Device for sectioning of polyacrylamide gel cylinders and its use in determining biological activity in the sections. Anal Biochem. 1966 Jun;15(3):544–548. doi: 10.1016/0003-2697(66)90121-7. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOH P. C., RIGGS J. L. Demonstration of the sequential development of vaccinial antigens and virus in infected cells: observations with cytochemical and differential fluorescent procedures. J Exp Med. 1961 Jul 1;114:149–160. doi: 10.1084/jem.114.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Moss B., Ingram V. M. The repression and induction by thyroxin of hemoglobin synthesis during amphibian metamorphosis. Proc Natl Acad Sci U S A. 1965 Sep;54(3):967–974. doi: 10.1073/pnas.54.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN D AND VACCINIA VIRUS INFECTION OF HELA CELLS. Nature. 1963 Jul 27;199:357–358. doi: 10.1038/199357a0. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. The formation of vaccinia virus protein in the presence of 5-fluorodeoxyuridine. Virology. 1963 Jun;20:292–301. doi: 10.1016/0042-6822(63)90118-1. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Sebring E. D. Sequential formation of vaccinia virus proteins and viral deoxyribonucleic acid replication. J Virol. 1967 Feb;1(1):16–23. doi: 10.1128/jvi.1.1.16-23.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Salzman N. P. Metabolic properties of early and late vaccinia virus messenger ribonucleic acid. J Virol. 1967 Jun;1(3):550–558. doi: 10.1128/jvi.1.3.550-558.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Wilcox W. C., Cohen G. H. Soluble antigens of vaccinia-infected mammalian cells. II. Time course of synthesis of soluble antigens and virus structural proteins. J Virol. 1967 Jun;1(3):500–508. doi: 10.1128/jvi.1.3.500-508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Recovery of S35 radioactivity from protein-bearing polyacrylamide gel. Anal Biochem. 1965 May;11(2):389–391. doi: 10.1016/0003-2697(65)90030-8. [DOI] [PubMed] [Google Scholar]