Abstract
Several reports have implicated the inoculum effect that some strains of type A beta-lactamase (Bla)-producing, methicillin-susceptible Staphylococcus aureus (MSSA) show against cefazolin as the cause for clinical failures in certain serious deep-seated infections. Here, using a previously reported MSSA strain displaying this phenotype (TX0117), we obtained a Bla-cured derivative (TX0117c) with a combination of novobiocin and high temperature. Both isolates were then used in a rat endocarditis model and treated with cefazolin, nafcillin, and daptomycin, given to simulate human dosing. Animals were treated for 3 days and either sacrificed at 24 h after the last antibiotic dose (standard group) or left untreated for an additional 3 days (relapse group). With TX0117 in the standard treatment group, daptomycin and nafcillin were both significantly better than cefazolin in reducing CFU/g of vegetations, achieving mean log10 reductions compared to levels in untreated rats of 7.1, 5.3, and 1.8, respectively (cefazolin versus daptomycin, P < 0.0001; cefazolin versus nafcillin, P = 0.005; daptomycin versus nafcillin, P = 0.053). In addition, cefazolin was significantly more effective in reducing vegetation titers of TX0117c than of TX0117 (mean log10 reduction of 1.4 versus 5.5, respectively; P = 0.0001). Similar results were observed with animals in the relapse group. Thus, these data show that there can be an in vivo consequence of the in vitro inoculum effect that some MSSA strains display against cefazolin and indicate a specific role for Bla production using a Bla-cured derivative strain against which cefazolin regained both in vitro and in vivo activity.
INTRODUCTION
Staphylococcus aureus continues to be an important pathogen causing invasive infections associated with significant morbidity and mortality. These infections include endocarditis, pneumonia, bacteremia, infections of prosthetic devices and catheters, complicated abscesses, osteomyelitis, and other deep-seeded infections, and methicillin-susceptible S. aureus (MSSA) strains still cause a significant proportion of these infections. Currently, more than 90% of S. aureus isolates produce β-lactamase (Bla), and four different variants of this enzyme (A, B, C, and D) have been identified by different methods (1–4). The type A Bla was shown to have a higher rate of hydrolysis of cefazolin (5), and several cases of cefazolin clinical failures have been reported in patients with endocarditis caused by MSSA strains showing an inoculum effect against this cephalosporin (6–9). These strains typically display an MIC of cefazolin of greater than 50 μg/ml when tested at a high inoculum, associated with cefazolin degradation (7, 8). The clinical impact of S. aureus strains showing an inoculum effect against cefazolin on treatment outcome is of potential concern since (i) cefazolin is an option for the treatment of MSSA endocarditis in patients with non-immediate-type hypersensitivity to penicillin (10, 11), (ii) many physicians discharge patients to complete a course of home intravenous treatment for MSSA infections with cefazolin because of a more convenient dosing schedule, and (iii) in some countries (e.g., Argentina), penicillinase-resistant penicillins are not available. Daptomycin, which is approved for treatment of patients with bacteremia and right-sided endocarditis caused by S. aureus, is a potential alternative for serious MSSA infections. The rapid infusion and once-daily administration make daptomycin an attractive candidate for the outpatient setting. Therefore, the goal of this study was to compare the efficacies of cefazolin, daptomycin, and nafcillin in a rat model of aortic valve endocarditis caused by a well-characterized MSSA strain showing an inoculum effect against cefazolin and its Bla-cured derivative.
MATERIALS AND METHODS
Bacteria used in in vitro and in vivo experiments.
S. aureus strain TX0117, a type A Bla producer with a previously demonstrated inoculum effect against cefazolin, was used in the endocarditis model. From this parental strain, an isogenic Bla-cured strain (TX0117c) was obtained by the combination method with novobiocin and high temperature as described previously (12, 13). In brief, TX0117 was cultured at 43°C for 24 h in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) supplemented with 0.125 μg/ml novobiocin (Sigma, St. Louis, MO) at 200 rpm. This was followed by subculturing daily into fresh BHI broth plus novobiocin at 43°C for 3 consecutive days. On day 4, individual colonies obtained following plating on BHI agar were screened for loss of Bla activity. Screening of Bla positive or negative colonies prior to in vitro and in vivo experiments was done by inoculating colonies into a microtiter tray prefilled with nitrocefin liquid (50 μg/ml) and observing for a rapid color change from yellow to pink. Nitrocefin was acquired from Calbiochem (Billerica, MA).
Antibiotic susceptibilities.
Cefazolin and nafcillin were acquired from Sigma-Aldrich Chemicals Co. (St. Louis, MO). Daptomycin (lot number MCB2009) was provided by Cubist Pharmaceuticals, Inc. (Lexington, MA). These antibiotics were reconstituted as recommended by the manufacturers. Cefazolin, nafcillin, and daptomycin MICs were determined by broth microdilution using cation-adjusted Mueller-Hinton II broth, per Clinical and Laboratory Standards Institute (CLSI) guidelines (14). Calcium (50 mg/ml) was added to determine daptomycin MICs. These MICs were determined using standard (5 × 105 CFU/ml) and high (5 × 107 CFU/ml) inocula and were read at 24 h. The cefazolin MICs were also determined for the TX0117c strain at standard and high inocula. S. aureus ATCC 29213 (which is known to produce small amounts of type A Bla) and the Bla-negative S. aureus ATCC 25923 were used as controls.
DNA sequencing.
PCR amplified fragments from TX0117 and TX0117c were used for sequencing to determine the differences in the blaZ coding regions. PCR amplifications, S1 nuclease digestion of genomic DNA, pulsed-field gel electrophoresis, and high-stringency hybridizations (15) were performed to determine the blaZ location in wild-type TX0117 and to confirm the identity of strains TX0117 and TX0117c recovered from in vitro and in vivo experiments.
Rat endocarditis model.
Aortic valve endocarditis was produced in male Sprague-Dawley rats weighing ∼200 g as previously described (16, 17). In brief, animals were anesthetized with isoflurane for placement of intravascular catheters, the right carotid artery was exposed, and a sterile polyethylene catheter (Intramedic PE 10; Clay Adams, Parsippany, NJ) was inserted and advanced into the left ventricle. The catheter was ligated and left in place for the duration of the experiment. Twenty-four hours after placement of the catheter across the aortic valve (16, 17), the bacterial inoculum presuspended in saline and representing ∼10 times the 100% infective dose (ID100), given intravenously via the tail vein, was used for therapeutic experiments.
Antimicrobial therapy.
Antibiotic doses were selected in order to achieve comparable mean serum concentrations attainable in humans treated with standard doses (18–21). Antibiotic treatment was started 48 h after bacterial challenge. Untreated animals were euthanized at initiation of therapy to determine baseline bacterial burdens in aortic valve vegetations. Animals were treated with intramuscular cefazolin at a dose of 50 mg/kg every 8 h or with subcutaneous injections of daptomycin at 45 mg/kg every 24 h or nafcillin at 400 mg/kg every 8 h. These doses were previously reported to simulate, respectively, human dosing of 6 g/day, ∼6 mg/kg/day, and 12 g/day (18, 20, 21) (confirmed with serum samples at baseline and at 3 time points after dosing in a group of animals receiving nafcillin and cefazolin). Microbiological assays (agar well dilution method) were performed to measure the serum antibiotic concentrations using Bacillus subtilis strain ATCC 6633; this bioassay has been shown to be equivalent to methods based on high-pressure liquid chromatography (HPLC) (22, 23). A standard curve with known cefazolin and nafcillin concentrations made in sterile rat serum displayed an excellent correlation between the antibiotic concentration and the inhibition zone. The majority of animals were treated for 3 days and then sacrificed 24 h after the last antibiotic dose (standard group). For each antibiotic, 4 to 6 animals were treated for 3 days and then left untreated for an additional 3 days after the last antibiotic dose in order to evaluate the effect of posttherapy residual infection; this group is referred to as the relapse group. After animals were sacrificed, aortic valve vegetations and surrounding tissues were aseptically removed, weighed, and homogenized in 1 ml of 0.9% saline. Sequential dilutions of homogenized tissues were made, and then entire volume of each dilution was plated on 2 large (150- by 15-mm) BHI agar plates to determine the number of bacteria present. Animals were included in the final analysis only if they survived the first 24 h (16), if catheters were found across the aortic valve in the left ventricle, and if macroscopic vegetations were visualized (18). In the cefazolin-treated, TX0117-inoculated group, one rat died at ∼24 h posttreatment. Bacteria recovered from the vegetations were confirmed to be Bla positive or negative by the nitrocefin liquid test. Since animals were sacrificed 24 h after the last therapeutic dose, we expected drug not to be present in the serial dilutions of the homogenized samples (21).
Data analysis.
Since the entire homogenate was plated, vegetations yielding no growth were scored as sterile and were assigned the value of 1 CFU/g (minimum detection level) for statistical purposes. ID90s for the two infecting strains (TX0117 and TX0117c) were determined by the method of Reed and Muench (24). Comparison between results from bacterial cultures obtained in each treatment group was done by the Mann-Whitney Wilcoxon unpaired test using Prism for Windows (version 4.00; GraphPad Software). Bacterial densities (geometric mean in log10 CFU) in the vegetations were compared. Overall, differences were considered significant at a P level of <0.05 by use of two-tailed significance levels.
This protocol was approved by Animal Welfare Committee of the Center for Laboratory Animal Medicine and Care of the University of Texas Health Science Center at Houston.
RESULTS
Generation of the isogenic Bla-cured strain.
Using the combination of novobiocin and high-temperature exposure, one of 600 colonies of TX0117 screened was negative by the nitrocefin assay screening test. Analysis by S1 nuclease digestion of DNA of this strain showed that there was no plasmid loss. Characterization of these two strains by PCR and DNA sequencing revealed that the type A blaZ gene in the TX0117 strain appears to reside on a transposon, since it amplified with the reverse primer from the inverted repeat region (a common feature described for Tn4002 or Tn552 elements) and the forward primer upstream of the −35 region of blaZ in the Tn4002 transposon. The presence of T at position 651 instead of C suggests that the blaZ gene in this strain is on Tn552 and not on Tn4002, based on what has been reported previously (25). Further DNA sequence analysis of the blaZ gene of TX0117c revealed a single nucleotide deletion (99delA) located 99 nucleotides from the initiation codon (position based on Tn4002 blaZ gene of S. aureus, accession number X16471), leading to a frameshift with subsequent changes in the amino acids following this position, the presumed cause of the lack of Bla activity. Although the presence of other mutations in the strain cannot be excluded, the infectivity of the TX0117c isolate was maintained as demonstrated by finding an ID100 in the endocarditis model similar to that observed with parental strain TX0117.
MICs.
As previously reported, we observed a high cefazolin MIC (64 μg/ml) at the high inoculum when TX0117 was tested. At the same inoculum, but using TX0117c (the Bla-negative, cured isolate), the cefazolin MIC was 1 μg/ml (Table 1). Daptomycin displayed an increase in the MIC up to 4 μg/ml using high inocula in the tested strains, including the ATCC 29213 and ATCC 25923 isolates. The different inocula utilized did not change the MIC of nafcillin.
Table 1.
MICs of cefazolin, daptomycin, and nafcillin for the MSSA isolates tested at standard and high inocula
| Inoculum | MIC (μg/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cefazolin |
Daptomycin |
Nafcillin |
||||||||
| TX0117 | TX0117c | ATCC 29213 | ATCC 25923 | TX0117 | ATCC 29213 | ATCC 25923 | TX0117 | ATCC 29213 | ATCC 25923 | |
| Standard (5 × 105 CFU/ml) | 1 | 0.5 | 0.5 | 0.25 | 0.125 | 0.25 | 0.125 | 0.25 | 0.25 | 0.25 |
| High (5 × 107 CFU/ml) | 64 | 1 | 8 | 1 | 4 | 4 | 4 | 0.5 | 0.5 | 0.5 |
TX0117c, β-lactamase-cured derivative of TX0117; ATCC 29213, low-level producer of type A β-lactamase; ATCC 25923, β-lactamase-negative strain.
Experimental endocarditis model.
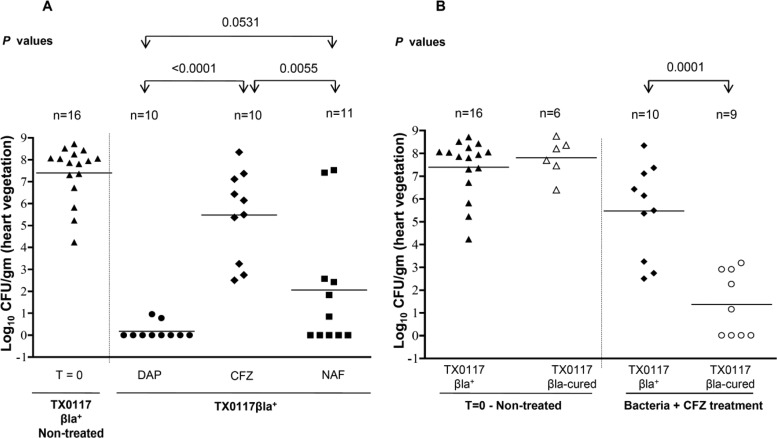
Results for animals infected with TX0117 and TX0117c and treated with cefazolin, daptomycin, and nafcillin for 3 days (standard group) are shown in Fig. 1A and B. Another subset of animals in each treatment arm was included in the relapse group, which was also infected with TX0117 and TX0117c (Fig. 2). Sixteen animals comprising the baseline (T = 0) control group were sacrificed before antibiotic initiation (48 h after inoculation); cultures of the vegetations from these animals yielded a mean log10 ± standard deviation (SD) CFU/g of heart vegetation of 7.3 ± 1.3. Using strain TX0117 in the standard treatment group (sacrificed 24 h after 3 days of treatment), the mean log10 ± SD CFU/g of vegetations were 5.5 ± 2.0, 0.2 ± 0.4, and 2.0 ± 2.9 for cefazolin-, daptomycin-, and nafcillin-treated animals, respectively (cefazolin versus daptomycin, P < 0.0001; cefazolin versus nafcillin, P = 0.005; daptomycin versus nafcillin, P = 0.053) (Fig. 1A). A statistically significant decrease in the CFU/g of vegetation was seen in the cefazolin-treated animals when the infecting strain was the Bla-cured TX0117c (1.4 ± 1.4 CFU/g; P = 0.0001 for comparison with strain TX0117) (Fig. 1B). In those animals infected with the TX0117c strain, there were no significant differences between the three antibiotic groups (data not shown). It should be noted that bacterial growth was observed in only 2 of 10 animals treated with daptomycin (with very low CFU/g), while none of the cefazolin-treated animals had sterile vegetations. Unexpectedly, two animals in the nafcillin arm had high CFU/g of vegetation.
Fig 1.
Efficacy of antibiotic therapy for S. aureus rat endocarditis in the standard treatment study. Rats were treated for 3 days with daptomycin (DAP), nafcillin (NAF), and cefazolin (CFZ) starting 48 h after inoculation. (A) Animals were infected with TX0117; the CFZ-treated group includes one animal that died at ∼24 h posttreatment and from which 2.2 × 108 CFU/g was recovered from the vegetation. (B) Animals infected with TX0117 or TX0117c and treated with CFZ. T = 0, CFU of control animals sacrificed 48 h after inoculation.
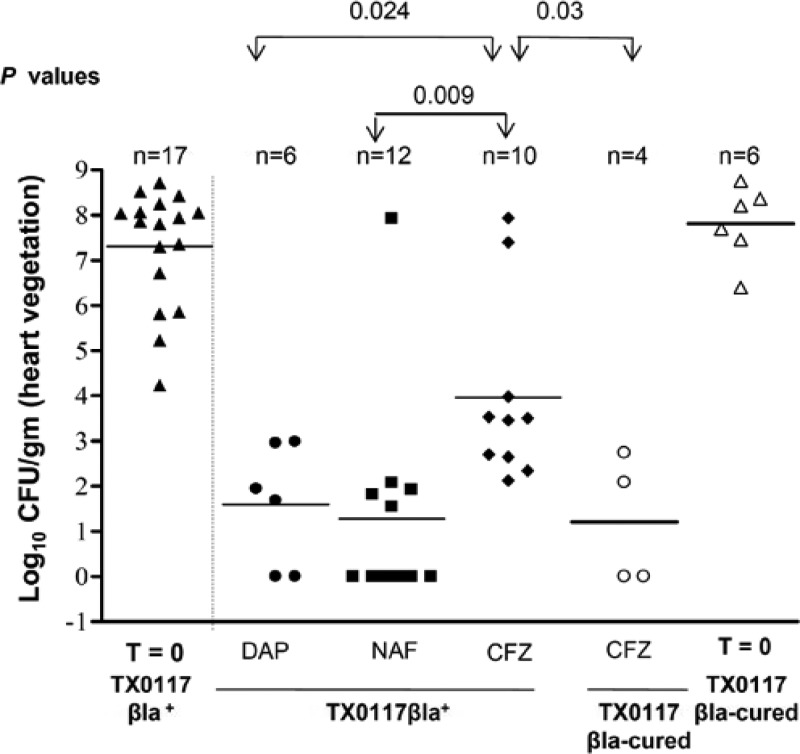
Fig 2.
Efficacy of antibiotic therapy for S. aureus rat endocarditis in the relapse treatment study. Animals were infected with strains TX0117 and TX0117c; antibiotics were given for 3 days, and animals were sacrificed 3 days after stopping them. Two rats, both in the CFZ-treated, TX0117-infected group, died 24 h after stopping antibiotics and had 2.7 × 107 and 8.6 × 107 CFU/g recovered from the vegetations. T = 0, CFU of control animals sacrificed 48 h after inoculation.
Analysis of the relapse groups infected with the TX0117 strain (Fig. 2) revealed that those animals receiving cefazolin, daptomycin, and nafcillin had a mean log10 ± SD CFU/g of vegetation of 4.0 ± 2.0, 1.6 ± 1.3, and 1.3 ± 2.3 3 days after stopping therapy, respectively (cefazolin versus daptomycin, P = 0.024; cefazolin versus nafcillin, P = 0.009; daptomycin versus nafcillin, P = not significant [NS]). Two rats died before the end of the 72-h observation period; both were in the cefazolin group (TX0117-infected animals). As in the standard treatment group, there were a significantly lower mean log10 ± SD CFU/g in those animals in the relapse group treated with cefazolin when the infecting organism was TX0117c (1.2 ± 1.4) versus TX0117 (4.0 ± 2.0) (P = 0.03).
DISCUSSION
Several case reports have associated failure of cefazolin for the treatment of severe MSSA infections with the presence of strains that efficiently hydrolyze cefazolin at high inocula (6–9). However, animal studies have shown conflicting results. In the 1980s, Goldman and Petersdorf found higher mortality in cefazolin-treated rabbits in an experimental endocarditis model using an MSSA strain with a significant inoculum effect against cefazolin than with a nonisogenic Bla-negative MSSA strain (26). Carrizosa et al., however, could not confirm these findings using the same strain and model (26, 27). In a murine model of intraperitoneal infection, Chapman and Steigbigel showed greater mortality in cefazolin-treated animals when the infecting strain displayed an inoculum effect against cefazolin than when strains did not have the inoculum effect, a result that was ameliorated when higher doses were administered (28).
In the current study, with a well-established model in rats of aortic valve endocarditis caused by a clinical type A Bla producer MSSA strain showing an inoculum effect against cefazolin, we found that daptomycin and nafcillin were significantly more effective in decreasing the CFU/g of vegetation than cefazolin. We also demonstrated, using a derivative strain cured of Bla activity (TX0117c), that cefazolin regained its activity against this strain, confirming the important role of Bla production. This derivative strain showed a negative nitrocefin test and no increase in the cefazolin MICs when tested at the high inoculum, and it was confirmed to be equally virulent as the parent strain. These results imply that the production of sufficient Bla type A to generate an in vitro inoculum effect leads to decreased efficacy of cefazolin, at least in this model. The chosen dose of cefazolin was intended to simulate dosing in humans, serum levels of cefazolin were measured in several animals, and concentrations at 1 h after dosing were similar as those previously reported (18, 29). Since the half-life of cefazolin in rats is shorter than that in humans (30), it is possible that higher or more frequent doses of cefazolin would have resulted in increased effectiveness of this antibiotic; however, the administered dose of cefazolin used here has been effectively used in prior animal studies of endocarditis (18) and osteomyelitis (31). Moreover, this cefazolin dosing schedule was effective in this model against the TX0117c strain. Higher or more frequent cefazolin doses, prolonged infusion, and/or the coadministration of a Bla inhibitor might be alternative ways to overcome the in vivo cefazolin inoculum effect.
Even though we observed an increase in the daptomycin MICs (up to 4 μg/ml) using a high inoculum among the three MSSA strains tested, daptomycin displayed excellent activity in this model of endocarditis. This modest in vitro inoculum effect was previously reported (32, 33), is of unclear importance, is seen with other antibiotics, and is unrelated to enzymatic destruction of the drug. The dose of daptomycin used in this study was shown to be equivalent to the human dose of ∼6 mg/kg/day (21). This is the approved dose of daptomycin in adults with bacteremia or right-sided endocarditis, although many experts currently recommend 8 to 10 mg/kg/day for the treatment of S. aureus bacteremia/endocarditis (34, 35). Prior studies have shown the efficacy of this compound against methicillin-resistant S. aureus (MRSA) strains in similar animal models (21, 36, 37).
We also found that the blaZ gene in the TX0117 strain likely resides on Tn552. This gene has long been associated with large plasmids (38, 39), although the Bla structural gene, blaZ, and its two regulators, blaI and blaR, have been identified in several transposons other than Tn552, such as Tn4002, Tn4201, and Tn3852 (25, 40). In addition, both plasmid-located and chromosomally located blaZ genes have been described in transposon-like elements of Enterococcus faecalis (41, 42). These transposons may arrive at a chromosomal site by transposing from a plasmid.
The exact mechanism by which some type A Bla producer MSSA strains are associated with higher rates of cefazolin hydrolysis is unknown. Mutations in the blaI regulator might lead to derepression of blaZ expression, resulting in production of a larger amount of enzyme without an inducer (43). Alternatively, strains without an inoculum effect might not secrete the enzyme into the extracellular space, which could result from mutations at the normal proteolytic cleavage site where the enzyme separates from the leader peptide (44).
The clinical impact of infections caused by MSSA strains with this phenotype has not been established. Several case reports have shown an association between cefazolin failure and the presence of a cefazolin inoculum effect (6–9). In 2009, we found that among patients on hemodialysis with MSSA bacteremia, the frequency of a cefazolin inoculum effect among infecting strains was numerically higher in those who experienced cefazolin failure (3 of 6) than in those who were successfully treated (none of 6) (P = 0.09) (7). A group from South Korea failed to show any differences in clinical outcomes between two matched groups of patients with MSSA bacteremia treated with cefazolin or with nafcillin (45). Another recent study found similar results (46). However, the retrospective nature of these studies, the few patients with endocarditis who were included, and the lack of determination of high-inoculum MICs make it difficult to draw definitive conclusions. Whether the treatment of an episode of MSSA bacteremia would be affected by the inoculum effect most likely depends on the source/cause of the bacteremia and the density nidus of organisms. The presence of this trait in a clinical isolate is unlikely, by itself, to cause cefazolin clinical failure. The impact of the inoculum effect is a dynamic process with other factors playing important roles, such as the degree of this inoculum effect, the dose of cefazolin used, the bacterial load and antibiotic penetration at the infection site, the host immune system, and whether a “debulking” procedure was performed (e.g., abscess drainage, debridement, foreign material removal or replacement, valve surgery, etc.).
The incidence of strains with an inoculum effect against cefazolin is likely to vary from region to region. Among a repository of worldwide clinical isolates of MSSA causing invasive infections collected between 2005 and 2007, we found that 19% of them had a significant inoculum effect to cefazolin (MICs ≥ 16 μg/ml) (7). Another group reported a rate of only 4% of this type of strains among bloodstream MSSA isolates recovered in the United States during 2010 (47). More recently, these strains were found in 36% of 357 MSSA strains isolated from patients with osteomyelitis and bloodstream infections in Colombia, Ecuador, Colombia, Peru, and Venezuela (48). Again, however, it should be emphasized that the number of situations in which the inoculum effect might have a clinical impact is surely much lower.
In summary, we have shown, in an experimental model of endocarditis, an in vivo correlation between the previously reported cefazolin inoculum effect using a clinical isolate producing type A Bla and its Bla-negative derivative. A definitive answer on whether these strains can be as reliably treated with cefazolin as with other agents when they cause deep-seated and high-inoculum infections can come only from prospective studies.
Footnotes
Published ahead of print 24 June 2013
REFERENCES
- 1.Richmond MH. 1975. Immunological techniques for studying beta-lactamases. Methods Enzymol. 43:86–100 [DOI] [PubMed] [Google Scholar]
- 2.Kernodle DS, McGraw PA, Stratton CW, Kaiser AB. 1990. Use of extracts versus whole-cell bacterial suspensions in the identification of Staphylococcus aureus beta-lactamase variants. Antimicrob. Agents Chemother. 34:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kernodle DS, Stratton CW, McMurray LW, Chipley JR, McGraw PA. 1989. Differentiation of beta-lactamase variants of Staphylococcus aureus by substrate hydrolysis profiles. J. Infect. Dis. 159:103–108 [DOI] [PubMed] [Google Scholar]
- 4.Voladri RK, Kernodle DS. 1998. Characterization of a chromosomal gene encoding type B beta-lactamase in phage group II isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 42:3163–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zygmunt DJ, Stratton CW, Kernodle DS. 1992. Characterization of four beta-lactamases produced by Staphylococcus aureus. Antimicrob. Agents Chemother. 36:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant RE, Alford RH. 1977. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 237:569–570 [PubMed] [Google Scholar]
- 7.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG, Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob. Agents Chemother. 53:3437–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J. Infect. Dis. 128(Suppl):S386–S389 [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Guerrero ML, de Gorgolas M. 2005. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin. Infect. Dis. 41:127. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WR, Karchmer AW, Dajani AS, Taubert KA, Bayer A, Kaye D, Bisno AL, Ferrieri P, Shulman ST, Durack DT. 1995. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. American Heart Association. JAMA 274:1706–1713 [PubMed] [Google Scholar]
- 11.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434 [DOI] [PubMed] [Google Scholar]
- 12.McHugh GL, Swartz MN. 1977. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob. Agents Chemother. 12:423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shore AC, Brennan OM, Ehricht R, Monecke S, Schwarz S, Slickers P, Coleman DC. 2010. Identification and characterization of the multidrug resistance gene cfr in a Panton-Valentine leukocidin-positive sequence type 8 methicillin-resistant Staphylococcus aureus IVa (USA300) isolate. Antimicrob. Agents Chemother. 54:4978–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15.Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Entenza JM, Hohl P, Heinze-Krauss I, Glauser MP, Moreillon P. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. 2005. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob. Agents Chemother. 49:380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murillo O, Garrigos C, Pachon ME, Euba G, Verdaguer R, Cabellos C, Cabo J, Gudiol F, Ariza J. 2009. Efficacy of high doses of daptomycin versus alternative therapies against experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catherall EJ, Gillon V, Hurn S, Irwin R, Mizen L. 1992. Efficacy of ticarcillin-clavulanic acid for treatment of experimental Staphylococcus aureus endocarditis in rats. Antimicrob. Agents Chemother. 36:458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakoulas G, Eliopoulos GM, Alder J, Eliopoulos CT. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wold JS. 1977. Rapid analysis of cefazolin in serum by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 11:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuluaga AF, Agudelo M, Rodriguez CA, Vesga O. 2009. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clin. Pharmacol. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493–497 [Google Scholar]
- 25.Rowland SJ, Dyke KG. 1989. Characterization of the staphylococcal beta-lactamase transposon Tn552. EMBO J. 8:2761–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman PL, Petersdorf RG. 1980. Importance of beta-lactamase inactivation in treatment of experimental endocarditis caused by Staphylococcus aureus. J. Infect. Dis. 141:331–337 [DOI] [PubMed] [Google Scholar]
- 27.Carrizosa J, Kobasa WD, Snepar R, Kaye KM, Kaye D. 1982. Cefazolin versus cephalothin in beta-lactamase-producing Staphylococcus aureus endocarditis in a rabbit experimental model. J. Antimicrob. Chemother. 9:387–393 [DOI] [PubMed] [Google Scholar]
- 28.Chapman SW, Steigbigel RT. 1983. Staphylococcal beta-lactamase and efficacy of beta-lactam antibiotics: in vitro and in vivo evaluation. J. Infect. Dis. 147:1078–1089 [DOI] [PubMed] [Google Scholar]
- 29.Kozatani J, Okui M, Matsubara T, Nishida M. 1972. Cefazolin, a new semisynthetic cephalosporin antibiotic. VI. Excretion and metabolism of cefazolin-14 C in rats after intramuscular administration. J. Antibiot. 25:86–93 [PubMed] [Google Scholar]
- 30.Lee FH, Pfeffer M, Van Harken DR, Smyth RD, Hottendorf GH. 1980. Comparative pharmacokinetics of ceforanide (BL-S786R) and cefazolin in laboratory animals and humans. Antimicrob. Agents Chemother. 17:188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel R, Piper KE, Rouse MS, Steckelberg JM. 2000. Linezolid therapy of Staphylococcus aureus experimental osteomyelitis. Antimicrob. Agents Chemother. 44:3438–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamp KC, Rybak MJ, Bailey EM, Kaatz GW. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueroa DA, Mangini E, Amodio-Groton M, Vardianos B, Melchert A, Fana C, Wehbeh W, Urban CM, Segal-Maurer S. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177–180 [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 36.Tattevin P, Basuino L, Bauer D, Diep BA, Chambers HF. 2010. Ceftobiprole is superior to vancomycin, daptomycin, and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:610–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marco F, de la Maria CG, Armero Y, Amat E, Soy D, Moreno A, del Rio A, Almela M, Mestres CA, Gatell JM, Jimenez de Anta MT, Miro JM. 2008. Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2538–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang PZ, Novick RP. 1987. Nucleotide sequence and expression of the beta-lactamase gene from Staphylococcus aureus plasmid pI258 in Escherichia coli, Bacillus subtilis, and Staphylococcus aureus. J. Bacteriol. 169:1763–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan PT. 1986. Nucleotide sequence of the Staphylococcus aureus PC1 beta-lactamase gene. Nucleic Acids Res. 14:5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy E, Novick RP. 1979. Physical mapping of Staphylococcus aureus penicillinase plasmid pI524: characterization of an invertible region. Mol. Gen. Genet. 175:19–30 [DOI] [PubMed] [Google Scholar]
- 41.Rice LB, Marshall SH. 1992. Evidence of incorporation of the chromosomal beta-lactamase gene of Enterococcus faecalis CH19 into a transposon derived from staphylococci. Antimicrob. Agents Chemother. 36:1843–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray BE, Mederski-Samaroj B. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Invest. 72:1168–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen S, Sweeney HM. 1968. Constitutive penicillinase formation in Staphylococcus aureus owing to a mutation unlinked to the penicillinase plasmid. J. Bacteriol. 95:1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.East AK, Curnock SP, Dyke KG. 1990. Change of a single amino acid in the leader peptide of a staphylococcal beta-lactamase prevents the appearance of the enzyme in the medium. FEMS Microbiol. Lett. 57:249–254 [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob. Agents Chemother. 55:5122–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z, Leibovici L, Bishara J. 2011. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin. Microbiol. Infect. 17:1581–1586 [DOI] [PubMed] [Google Scholar]
- 47.Livorsi DJ, Crispell E, Satola SW, Burd EM, Jerris R, Wang YF, Farley MM. 2012. Prevalence of blaZ gene types and the inoculum effect with cefazolin among bloodstream isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 56:4474–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rincon S, Reyes J, Carvajal LP, Rojas N, Cortes F, Panesso D, Guzman M, Zurita J, Adachi JA, Murray BE, Nannini EC, Arias CA. 21 June 2013, posting date Cefazolin high-inoculum effect in methicillin-susceptible Staphylococcus aureus from South American hospitals. J. Antimicrob. Chemother. [Epub ahead of print.] 10.1093/jac/dkt254 [DOI] [PMC free article] [PubMed] [Google Scholar]