Abstract
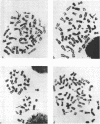
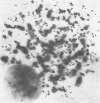
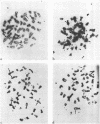
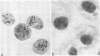
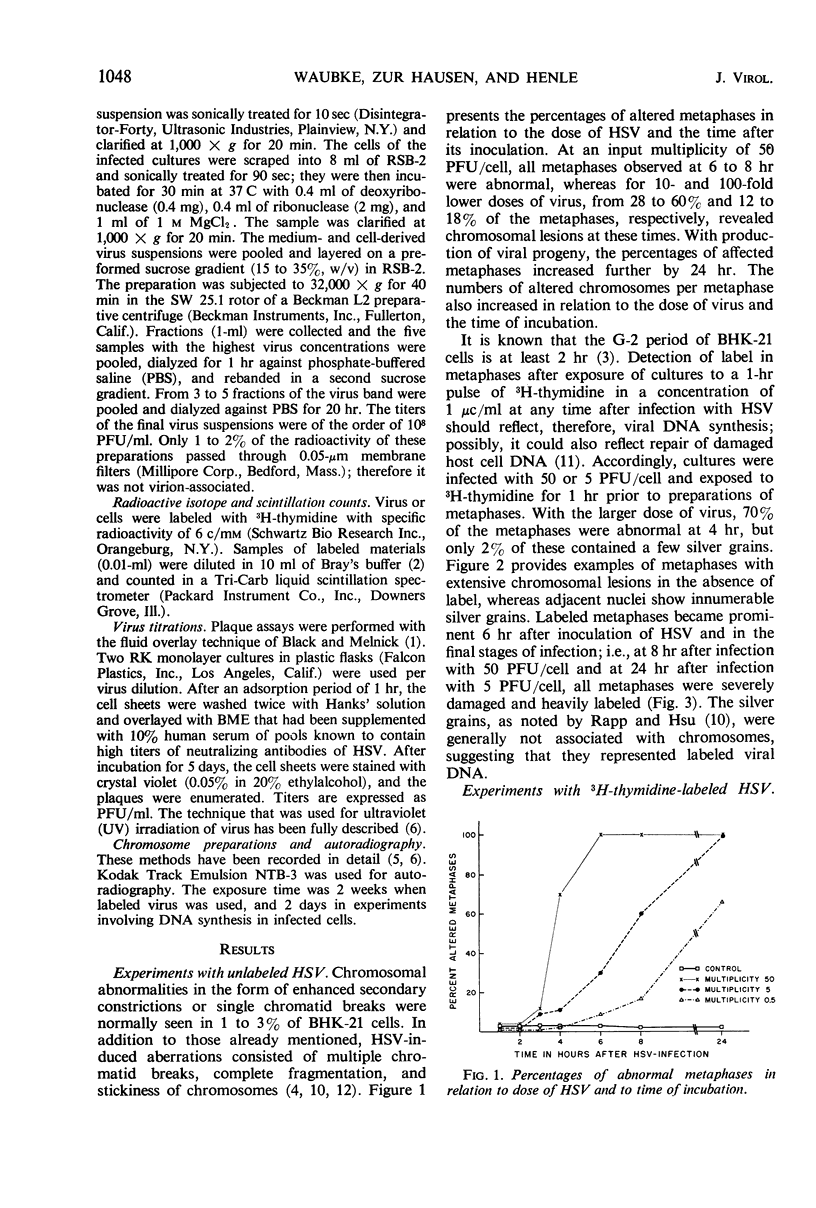
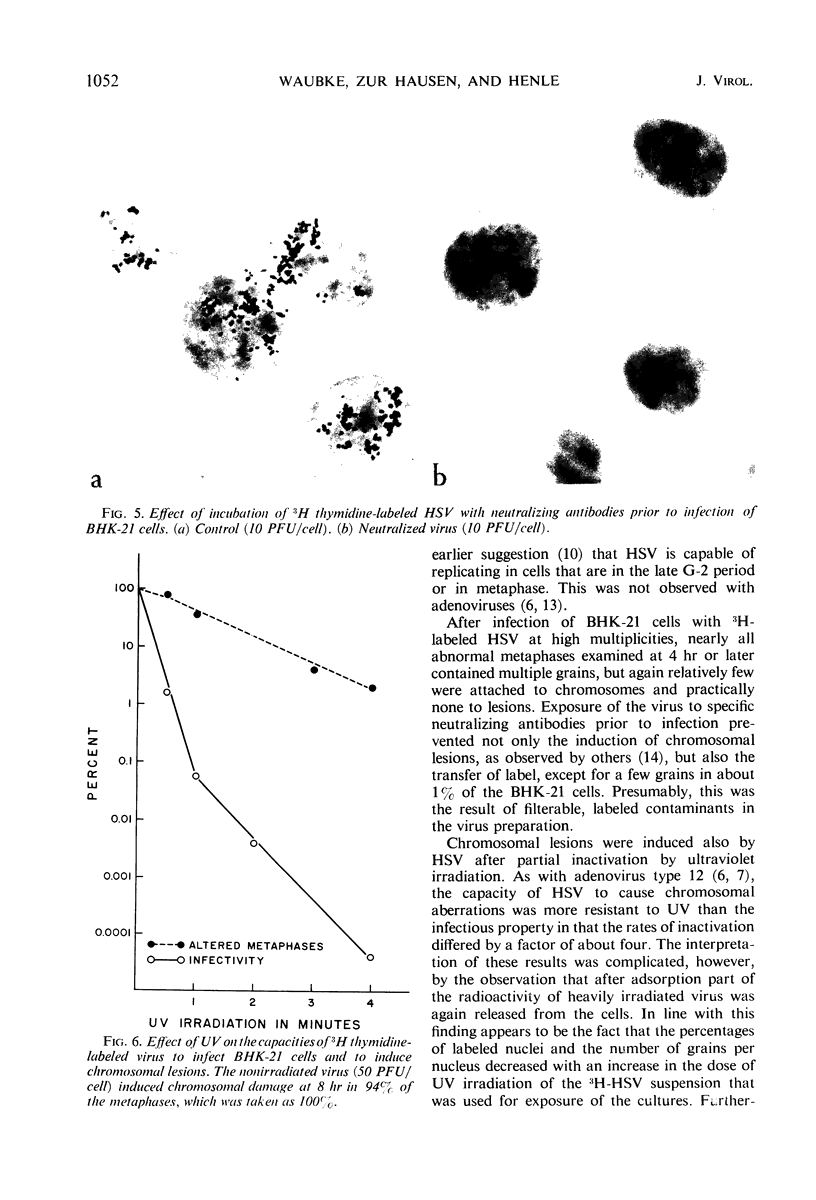
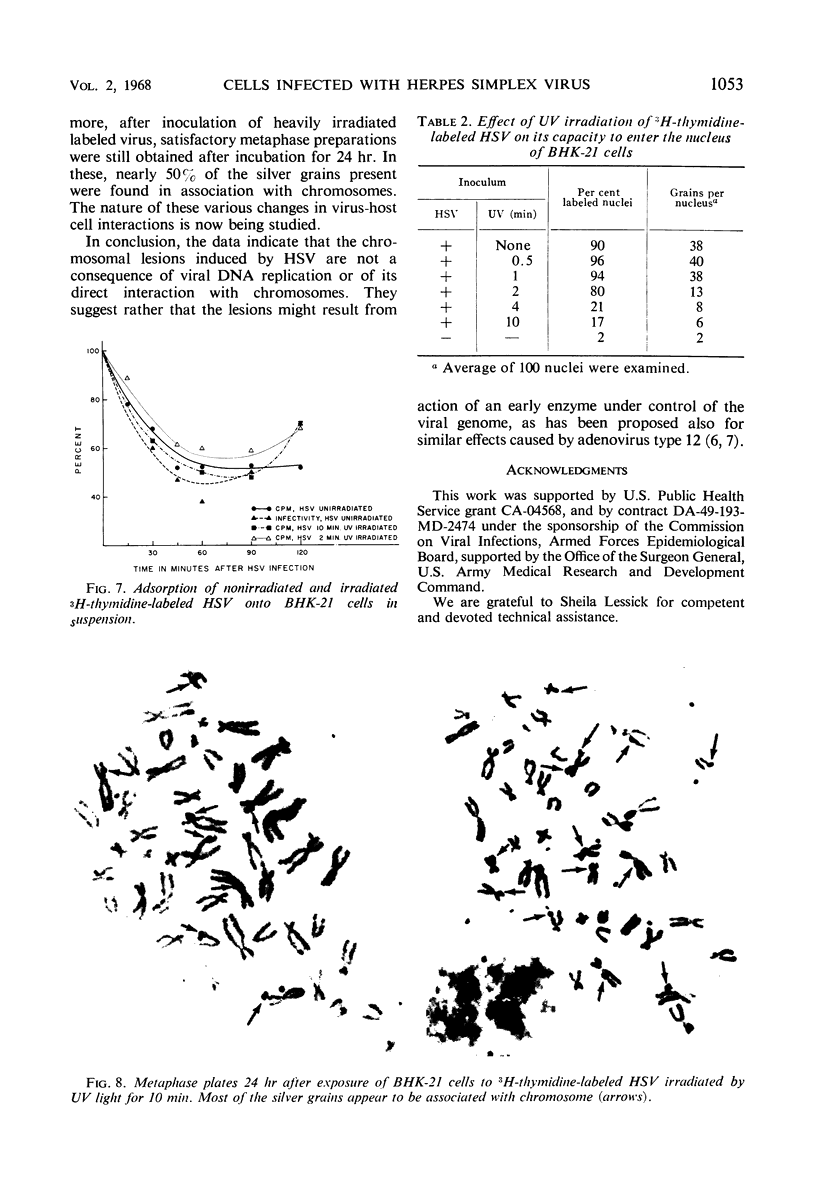
The induction of chromosomal aberrations by herpes simplex virus (HSV) and the interaction between viral deoxyribonucleic acid (DNA) and chromosomes have been studied (i) by infection of the BHK-21 line of hamster kidney cells at multiplicities ranging from 0.5 to 100 followed by 1-hr pulses of 3H-thymidine preceding preparation at varying intervals of metaphases and autoradiography, and (ii) by use of 3H-thymidine-labeled HSV for infection, chromosomal, and autoradiographic analyses at intervals thereafter. The results revealed that (i) chromosomal lesions develop prior to, and thus are independent of, viral DNA synthesis; (ii) HSV is capable of replicating in cells during the late G-2 period or in metaphase; (iii) most of the viral DNA remains unassociated with chromosomes and is not detectable at sites of chromosomal lesions; (iv) the capacity of the virus to cause chromosomal aberrations is four times less sensitive to inactivation by ultraviolet (UV) irradiation than its infectious property; and (v) after large doses of UV, invasion of the nuclei by the irradiated virus is reduced. These observations indicate that the chromosomal lesions induced by HSV result most likely from action of an early enzyme under control of the viral genome. This explanation is proposed also for the effects of adenovirus type 12 on chromosomes.
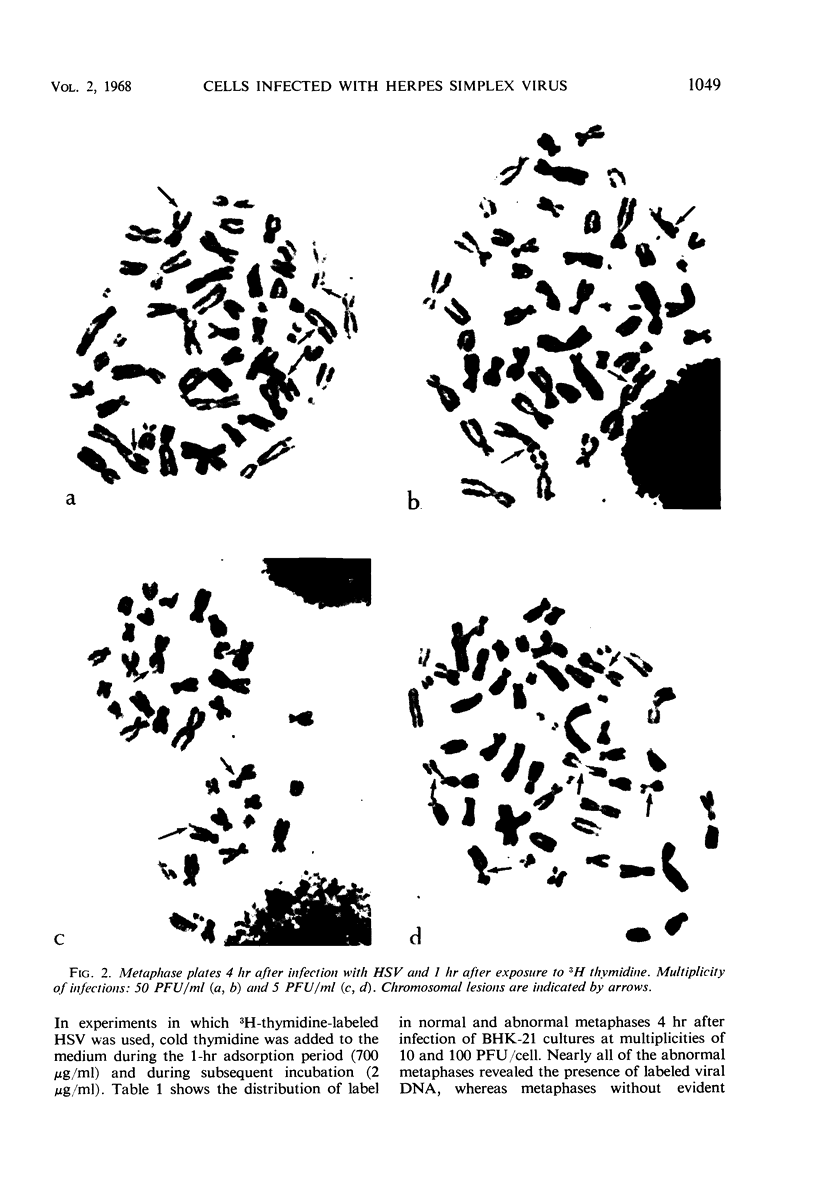
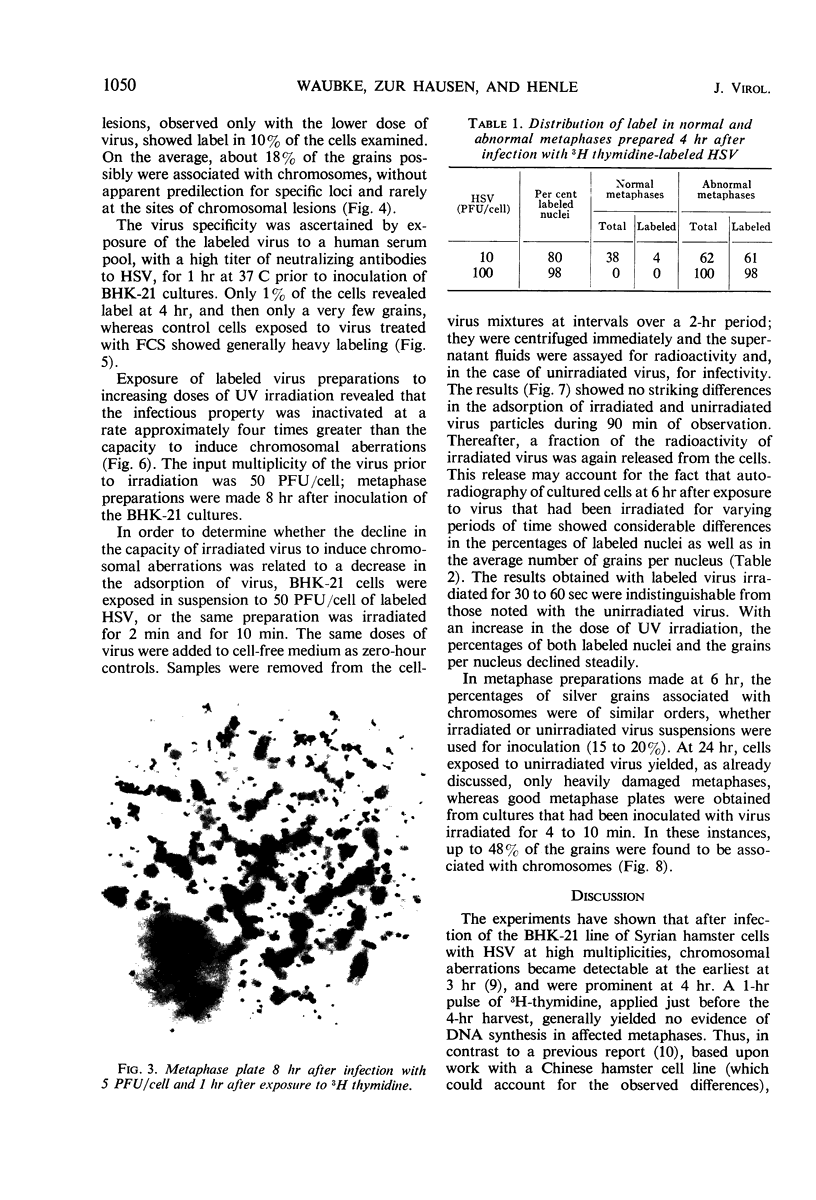
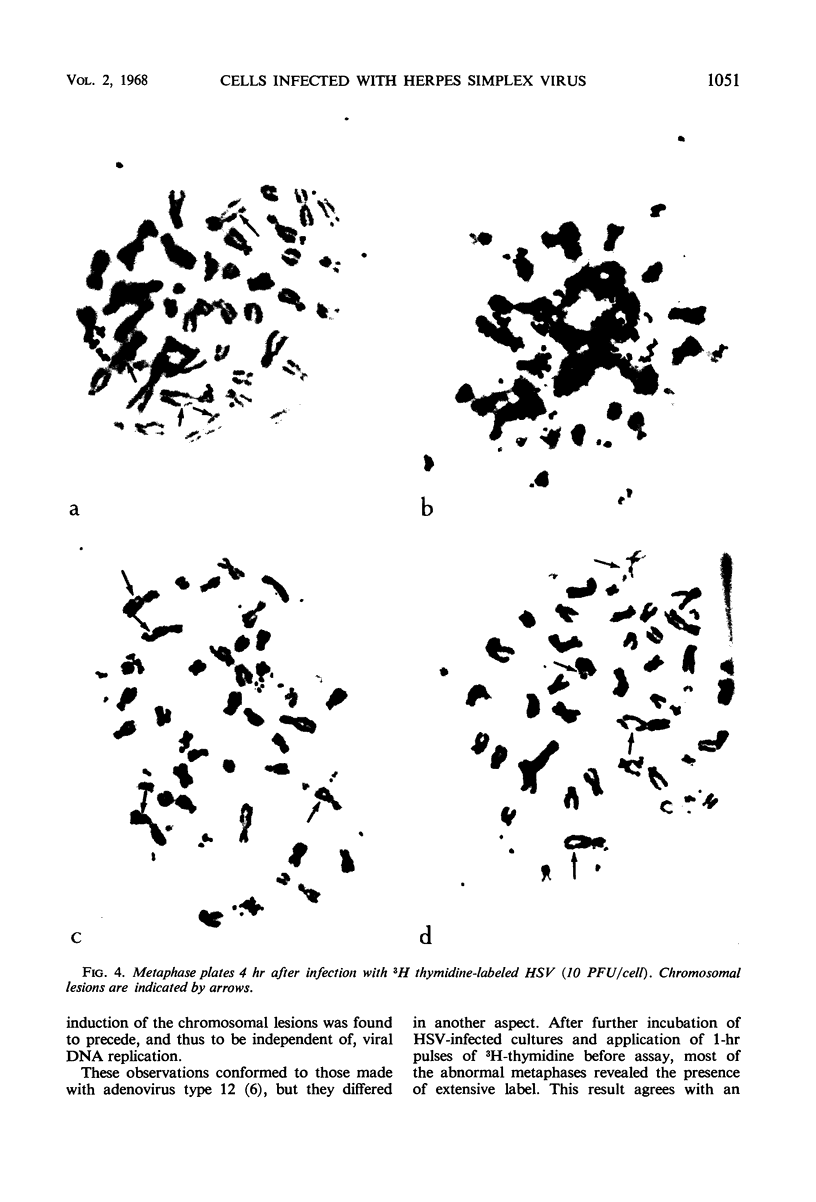
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK F. L., MELNICK J. L. Microepidemiology of poliomyelitis and herpes-B infections: spread of the viruses within tissue cultures. J Immunol. 1955 Mar;74(3):236–242. [PubMed] [Google Scholar]
- HAMPAR B., ELLISON S. A. Cellular alterations in the MCH line of Chinese hamster cells following infection with herpes simplex virus. Proc Natl Acad Sci U S A. 1963 Apr;49:474–480. doi: 10.1073/pnas.49.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J., Becker Y. The effect of cytosine arabinoside on the replication of herpes simplex virus. Virology. 1967 Jan;31(1):129–134. doi: 10.1016/0042-6822(67)90016-5. [DOI] [PubMed] [Google Scholar]
- MAZZONE H. M., YERGANIAN G. Gross and chromosomal cytology of virus infected Chinese hamster cells. Exp Cell Res. 1963 May;30:591–592. doi: 10.1016/0014-4827(63)90336-7. [DOI] [PubMed] [Google Scholar]
- RAPP F., HSU T. C. VIRUSES AND MAMMALIAN CHROMOSOMES. IV. REPLICATION OF HERPES SIMPLEX VIRUS IN DIPLOID CHINESE HAMSTER CELLS. Virology. 1965 Mar;25:401–411. doi: 10.1016/0042-6822(65)90061-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. E., Painter R. B. Radiation-stimulated DNA synthesis in cultured mammalian cells. J Cell Biol. 1966 Apr;29(1):11–19. doi: 10.1083/jcb.29.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICH H. F., HSU T. C., RAPP F. VIRUSES AND MAMMALIAN CHROMOSOMES. I. LOCALIZATION OF CHROMOSOME ABERRATIONS AFTER INFECTION WITH HERPES SIMPLEX VIRUS. Virology. 1964 Apr;22:439–445. doi: 10.1016/0042-6822(64)90064-9. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Yohn D. S. Mutagenic capacity of adenoviruses for mammalian cells. Nature. 1967 Dec 30;216(5122):1292–1294. doi: 10.1038/2161292a0. [DOI] [PubMed] [Google Scholar]
- TANZER J., THOMAS M., STOITCHKOV Y., BOIRON M., BERNARD J. ALT'ERATIONS CHROMOSOMIQUES OBSERV'EES DANS DES CELLULES DE REIN DE SINGE INFECT'EES IN VITRO PAR LE VIRUS DE L'HERP'ES. Ann Inst Pasteur (Paris) 1964 Sep;107:366–373. [PubMed] [Google Scholar]
- TOKUMARU T. STUDIES OF HERPES SIMPLEX VIRUS BY THE GEL DIFFUSION TECHNIQUE. I. DISTRIBUTION OF PRECIPITATING ANTIBODIES AMONG HUMAN SERA. J Immunol. 1965 Jul;95:181–188. [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H. Association of adenovirus type 12 deoxyribonucleic acid with host cell chromosomes. J Virol. 1968 Mar;2(3):218–223. doi: 10.1128/jvi.2.3.218-223.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H. Induction of specific chromosomal aberrations by adenovirus type 12 in human embryonic kidney cells. J Virol. 1967 Dec;1(6):1174–1185. doi: 10.1128/jvi.1.6.1174-1185.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Chromosomal changes of similar nature in seven established cell lines derived from the peripheral blood of patients with leukemia. J Natl Cancer Inst. 1967 May;38(5):683–696. [PubMed] [Google Scholar]