Abstract
Inteins are coding sequences that are transcribed and translated with flanking sequences and then are excised by an autocatalytic process. There are two types of inteins in fungi, mini-inteins and full-length inteins, both of which present a splicing domain containing well-conserved amino acid sequences. Full-length inteins also present a homing endonuclease domain that makes the intein a mobile genetic element. These parasitic genetic elements are located in highly conserved genes and may allow for the differentiation of closely related species of the Candida parapsilosis (psilosis) complex. The correct identification of the three psilosis complex species C. parapsilosis, Candida metapsilosis, and Candida orthopsilosis is very important in the clinical setting for improving antifungal therapy and patient care. In this work, we analyzed inteins that are present in the vacuolar ATPase gene VMA and in the threonyl-tRNA synthetase gene ThrRS in 85 strains of the Candida psilosis complex (46 C. parapsilosis, 17 C. metapsilosis, and 22 C. orthopsilosis). Here, we describe an accessible and accurate technique based on a single PCR that is able to differentiate the psilosis complex based on the VMA intein. Although the ThrRS intein does not distinguish the three species of the psilosis complex by PCR product size, it can differentiate them by sequencing and phylogenetic analysis. Furthermore, this intein is unusually present as both mini- and full-length forms in C. orthopsilosis. Additional population studies should be performed to address whether this represents a common intraspecific variability or the presence of subspecies within C. orthopsilosis.
INTRODUCTION
Candida parapsilosis is one of the most common non-albicans Candida species that cause human infections. In some regions in Latin America and Spain, C. parapsilosis occurs at the same or even a higher frequency than does Candida albicans, particularly in bloodstream infections in young children and premature neonates (1–6). C. parapsilosis is considered a normal or transient inhabitant of the skin and is found on the hands of health care workers who install central venous catheters and other medical devices, thus suggesting a nosocomial route of transmission (7–10).
Recently, C. parapsilosis was reclassified into 3 species: C. parapsilosis (sensu stricto), Candida orthopsilosis, and Candida metapsilosis (11). However, species definition within the C. parapsilosis (psilosis) group may be more complex than was initially thought. Prior studies have identified heterogeneity among C. orthopsilosis isolates (12–14), and they have also revealed three polymorphic sites in the internal transcribed spacer (ITS)-5.8S rRNA region of 13 isolates identified as C. orthopsilosis that appeared to separate the species into two genotypes. This conclusion was supported by a mating type locus (MTL) analysis, which placed C. orthopsilosis into one of two groups, type 1 or type 2 (14).
Although the prevalences, distributions, drug susceptibilities, and biofilm productions of these species remain unclear, there are several studies that demonstrate differences among them (12, 15–22). For instance, the recently published genome of C. orthopsilosis shows a reduction in the gene families associated with pathogenesis compared with that of C. parapsilosis (23). This is in agreement with the finding that few infections are caused by C. orthopsilosis and C. metapsilosis, which also suggests that these species are less virulent than C. parapsilosis. Even so, C. orthopsilosis and C. parapsilosis are able to cause similar degrees of tissue damage, whereas C. metapsilosis is less virulent (24), more susceptible to host responses, and less efficiently phagocytosed than other species of the psilosis complex (25). These findings indicate that correct species identification may have a therapeutic impact.
Several molecular techniques have been used to distinguish psilosis species, including PCR amplification of the secondary alcohol dehydrogenase-encoding gene (SADH), followed by digestion with the restriction enzyme BanI (11, 17), analysis of randomly amplified polymorphic DNA (RAPD) (12, 26–28), restriction fragment length polymorphism (RFLP) (28, 29), quantitative PCR (qPCR) (30), qPCR followed by high-resolution melt analysis (HRM) (31), nucleotide sequencing analysis (32), pyrosequencing (33), matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (34, 35), and microsatellite analysis (36). Despite the vast array of techniques described, there is still a need for a simple, rapid, and low-cost method to differentiate these species in molecular diagnostic laboratories in order to monitor the incidence of infection.
Inteins, known as parasitic genetic elements, may prove to be a promising resource for differentiating related species (37, 38) since they are located in highly conserved genes. Inteins are intervening sequences that are transcribed and translated with flanking host protein sequences and then are self-excised by protein splicing; the flanking protein sequences (exteins) are joined by a peptide bond to form the functional protein (39–41).
There are two types of inteins in fungi, the mini-inteins and the full-length inteins, both of which present a splicing domain containing four blocks of relatively well-conserved amino acid sequences (blocks A, B, F, and G) (Fig. 1). In addition, full-length inteins present blocks C, D, E, and H of a homing endonuclease (HE) domain, with the potential to make the intein a mobile genetic element; this results in the occupation of empty alleles and duplication of the parasitic genetic element (42–44).
Fig 1.

Representation of a full-length intein identified with the nomenclature of Perler et al. (40). Motifs A, B, F, and G are important for self-splicing. Motifs C, D, E, and H are associated with the homing process.
Primers designed for the flanking conserved gene regions will PCR amplify and discriminate three genotype types: full-length intein, mini-intein, and absence of intein. In addition, the full-length inteins are expected to have more sequence variation (including size variations due to indels) in the endonuclease domain than in the splicing domain due to a more relaxed selection, mainly when the homing endonuclease is no longer active (37).
In the present work, we evaluate the distribution and phylogeny of two inteins in a representative number of isolates from the psilosis group, the vacuolar ATPase membrane (VMA) extein and the threonyl-tRNA synthetase extein (ThrRS) inteins. The data indicate that distribution and variation among VMA inteins within the psilosis complex may allow for species discrimination using a relatively low-cost PCR approach. In addition, we have also found the existence of two idiomorphic inteins, ThrRS-A (a mini-intein) and ThrRS-B (a full-length intein), at the same insertion site of distinct organisms of C. orthopsilosis species, which may indicate heterogeneity among C. orthopsilosis isolates, different varieties, or even different species.
MATERIALS AND METHODS
Isolates.
A total of 85 strains were used in this study (see Table S1 in the supplemental material). Thirty-four strains (16 from C. metapsilosis and 18 from C. orthopsilosis) were obtained from the Centers for Disease Control and Prevention (CDC) (Atlanta, GA), 48 strains (45 from C. parapsilosis and three from C. orthopsilosis) were taken from patients of the Hospital das Clínicas (HC), Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP), Botucatu, São Paulo state, Brazil, and three reference strains (from C. parapsilosis, C. metapsilosis, and C. orthopsilosis) were obtained from the American Type Culture Collection (ATCC). The identities of all isolates were confirmed by ITS1-5.8S-ITS2 rRNA gene sequencing.
Culture and DNA extraction.
The Candida isolates were cultured on brain heart infusion agar (BHI) at 35°C for 24 to 48 h. DNA was isolated using the DNeasy blood and tissue kit (Qiagen, Valencia, CA), with slight modifications to the manufacturer's instructions. For each isolate, two to three colonies of Candida spp. were picked from the BHI slants and placed in 5-ml polypropylene tubes containing 800 μl Qiagen ATL buffer and 60 U of proteinase K. The mycelia were homogenized using the Omni TH mixer (Omni International, Kennesaw, GA) at low speed for 30 s and then high speed for 30 s, using a clean probe between each isolate. Homogenates were capped and incubated at 55°C for 1 h with frequent vortexing and then were cooled to room temperature. RNase A (Sigma-Aldrich Corp., St. Louis, MO) was then added to obtain a final concentration of 1 mg/ml and the mixture was incubated for 5 min at room temperature (RT), followed by the addition of 900 μl Qiagen buffer AL and vortexing. Homogenates were incubated at 70°C for 10 min and then were transferred to 1.7-ml microcentrifuge tubes and centrifuged at 10,000 × g for 10 min. Clear supernatants (1 ml each) were transferred to clean microcentrifuge tubes, into which a 50% volume of ethanol (Sigma-Aldrich Corp.) was added. The suspensions were vortexed and transferred to Qiagen DNeasy columns, and the manufacturer's instructions were followed throughout the remainder of the procedure. DNA was eluted in 200 μl of 10 mM Tris HCl (pH 8.0) and maintained at −20°C.
Alternatively, some isolates were cultured on Sabouraud dextrose agar (SDA) slants at 35°C and the DNA was extracted by initial cell disruption with glass beads (425 to 600 μm, acid washed) (Sigma, St. Louis, MO) in a solution of 1 M sorbitol and 125 mM EDTA (45).
Amplification and sequencing of the VMA and ThrRS inteins.
The primer design involved a preliminary search in the Candida Genome Database for the sequences of the VMA and ThrRS genes. C. orthopsilosis and C. parapsilosis sequences were aligned in MEGA version 5.0, and two degenerate primer pairs were designed in the flanking exteins, one for amplification of the VMA intein (TP1fwd, 5′-ACTGCTGATTAYCCATTGTTG, and TP2rev, 5′-AGATTGAWGCTTCTCTKGCAG-3′) and the other for amplification of the ThrRS intein (TP3fwd, 5′-GAARGARGCTGCTGAAAGAG, and TP4rev, 5′-TCTTGTTGGAAACGACGAAC-3′). The expected PCR fragment length of the VMA intein and partial extein from C. orthopsilosis was 1,909 bp, while the C. parapsilosis VMA fragment length was an expected 319 bp, since the intein is not present (23, 46). The expected PCR fragment length for the ThrRS intein from C. parapsilosis was 988 bp (46) versus 1,756 in C. orthopsilosis (Candida Genome Database). For the species C. metapsilosis, there was no previous information about the presence or length of either intein.
Each PCR used a reaction volume of 25 μl and contained 23 μl of 1× PCR buffer (200 mM Tris-HCl [pH 8.4], 1.5 mM MgCl2, 50 mM KCl), 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphate (dNTP), 0.4 mM each primer, 1 unit of Taq polymerase (Invitrogen), and 2 μl Candida DNA. Thermal cycling conditions were 95°C for 5 min followed by 40 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min. The PCR products were identified by 1% agarose gel electrophoresis stained with ethidium bromide.
Amplicons (10 μl) were purified enzymatically with 4 μl of ExoSAP-IT (GE Healthcare) for 15 min at 37°C followed by 15 min at 80°C to inactivate the enzyme. The samples were subsequently submitted to a sequencing reaction and capillary electrophoresis in the ABI3500 DNA analyzer (Applied Biosystems) at the Laboratory of Molecular Diagnosis in the Department of Microbiology and Immunology, Instituto de Biociências (IBB)-UNESP. The DNA samples used for the PCR and sequencing reactions are listed in Table S1 in the supplemental material. A total of 85 strains were used for the VMA and 49 for the ThrRS PCRs. The ThrRS intein was sequenced from 49 strains and the VMA intein from three strains.
Sequence analysis of VMA.
The sequences were aligned using the Clustal W algorithm implemented in MEGA v5.0 software (47) to compare the degree of conservation of the splicing and homing endonuclease domains. Full-length VMA inteins were compared to the VMA intein from Saccharomyces cerevisiae (GenBank accession no. Q874G3) in order to observe the presence or absence of the two aspartic acid residues (Asp-218 and Asp-326) that are involved in the activity of the homing endonuclease (48, 49).
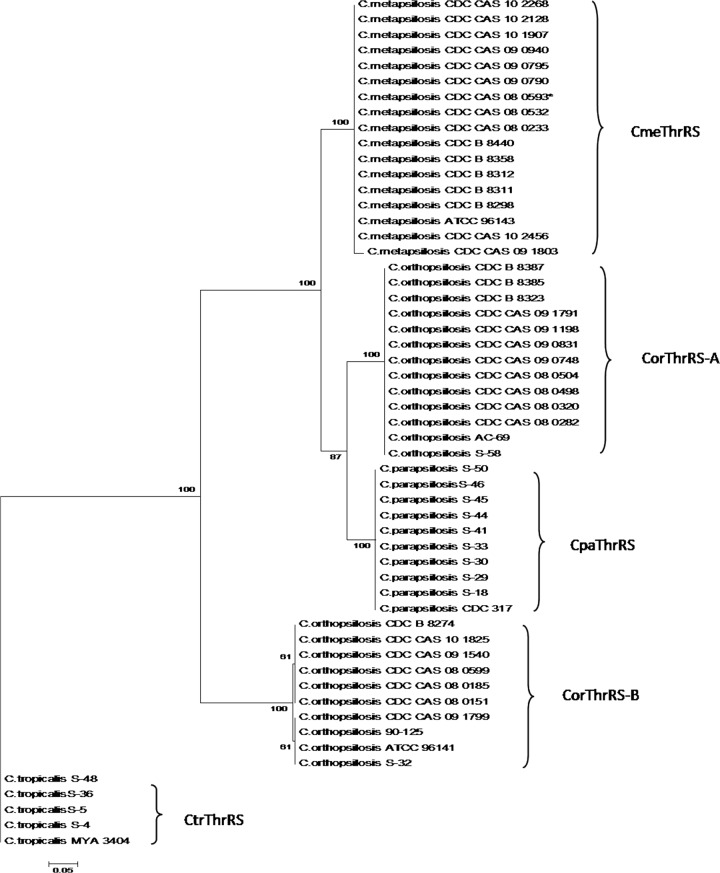
Phylogenetic analysis of ThrRS intein.
Sequences were aligned in MEGA v.5.0 (47), after which phylogenetic analyses based on the splicing domain of C. parapsilosis, C. metapsilosis, and C. orthopsilosis ThrRS inteins (CpaThrRS for C. parapsilosis, CmeThrRS for C. metapsilosis, CorThrRS-A for C. orthopsilosis mini-intein, and CorThrRS-B for C. orthopsilosis full-length intein) were performed by the maximum likelihood (ML) method using the website software version of PhyML (50). The Whelan and Goldman (WAG) model (51) was used as the distance model according to MEGA v5.0. The transition-to-transversion (Ti/Tv) ratio, gamma shape parameter, and proportion of nonvariant sites were estimated by the maximum likelihood method from a neighbor-joining tree (BIONJ algorithm). Bootstrap resampling (52) was applied to assess support for individual nodes, using 1,000 replicates with random additions and tree bisection and reconnection (TBR) branch swapping. The ThrRS intein sequence from Candida tropicalis (CtrThrRS, GenBank accession no. XP002550936) was used as the outgroup.
Polymorphism analysis of ITS (ITS1-5.8S0ITS2) sequence from C. orthopsilosis.
The ITS sequences (ITS1-5.8S-ITS2 rRNA gene) from the 14 isolates of C. orthopsilosis were aligned by Mega 5 in order to detect the two types of C. orthopsilosis (type 1 and type 2) that were already proposed (14) and to evaluate whether the ITS polymorphism of the isolates is correlated with the two groups of CorThrRS inteins, one being a mini-intein and the other one a full-length intein (CorThrRS-A and CorThrRS-B, respectively). The ITS sequencing, with the universal fungal primers ITS4 and ITS5 (53), was performed by the CDC group using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Inc.) in a 3730 DNA analyzer (Applied Biosystems, Inc.).
Nucleotide sequence accession numbers.
The ThrRS intein, VMA intein, and ITS1-5.8S-ITS2 rRNA gene sequence accession numbers used (which are also listed in Table S1 in the supplemental material) are as follows: for the C. parapsilosis ThrRS inteins, KC989746 through KC989748, JQ670685, and JQ670688 through JQ670693; for the C. orthopsilosis ThrRS inteins, KC989749 through KC989770; for the C. metapsilosis ThrRS inteins, KC989771 through KC989786 and JQ670686; for the C. orthopsilosis VMA inteins, KC989788 and KC989789; for the C. metapsilosis VMA intein, KC989787; and for the ITS1-5.8S-ITS2 rRNA gene, KC990354 through KC990357.
RESULTS
Analysis of VMA intein in the psilosis complex.
The VMA intein is absent in C. parapsilosis isolates, while it is present as a full-length intein in all C. orthopsilosis and C. metapsilosis isolates. The expected PCR fragment length of 319 bp that contains only the extein regions of VMA was observed in all C. parapsilosis isolates evaluated, whereas the fragment lengths for C. orthopsilosis and C. metapsilosis were 1,909 bp and 1,681 bp, respectively, due to the presence of the inteins (Table 1 and Fig. 2). In Fig. 2, the representative gel electrophoresis demonstrates the possibility of distinguishing the three species of the psilosis complex by comparing the PCR fragment length generated by the same pairs of degenerated primers that anneal in the extein regions of VMA.
Table 1.
PCR fragment length, type, and size of the inteins VMA and ThrRS in Candida species from the psilosis complex
| Candida species | Size of intein (bp) |
Type of intein |
PCR fragment length (bp)a |
|||
|---|---|---|---|---|---|---|
| VMA | ThrRS | VMA | ThrRS | VMA | ThrRS | |
| C. parapsilosis | Absent | 549 | Absent | Mini | 319 | 988 |
| C. metapsilosis | 1,362 | 516 | Full-length | Mini | 1,681 | 955 |
| C. orthopsilosis A (mini-intein) | 1,590 | 540 | Full-length | Full-length | 1,909 | 979 |
| C. orthopsilosis B (full-intein) | 1,590 | 1,317 | Full-length | Mini | 1,909 | 1,756 |
The PCR products include the intein plus the 5′and 3′ portions of the host gene (extein), except for the CpaVMA, whose amplicon consists only of the extein portion.
Fig 2.
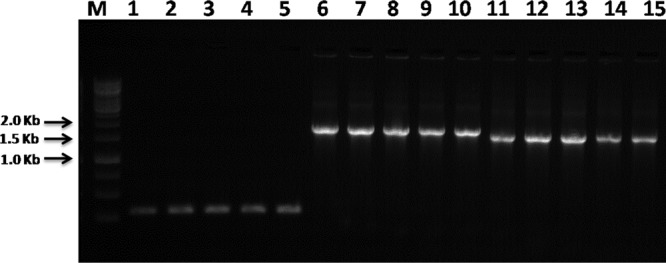
Agarose gel electrophoresis of PCR carried out with TP1 and TP2 primers for amplification of VMA inteins from C. parapsilosis (lanes 1 to 5), C. orthopsilosis (lanes 6 to 10), and C. metapsilosis (lanes 11 to 15). Lane M, 1-kb DNA ladder (Promega).
All amino acid residues involved in protein splicing were found in CorVMA and CmeVMA inteins, with the exception of the Thr-x-x-His motif in block B (46), which is absent (see Fig. S1 in the supplemental material). In addition, the highly conserved blocks A, B, F, and G indicate that the splicing function operates in both CorVMA and CmeVMA. Compared to the VMA intein from Saccharomyces cerevisiae, SceVMA (GenBank accession no. Q874G3), we found that the two aspartic acid residues (Asp-218 and Asp-326) that are critical to the activity of the homing endonuclease (48, 49) are conserved in CorVMA. In CmeVMA, the first aspartate was changed to asparagine, while the second one is conserved (see Fig. S1 in the supplemental material).
Analysis of ThrRS intein in the psilosis complex.
The degenerate primers used in PCR were designed in the N and C extein terminals of C. parapsilosis and C. orthopsilosis ThrRS and detected the presence of mini-inteins, herein named CpaThrRS (549 bp), CmeThrRS (516 bp), and CorThrRS-A (540 bp). These mini-inteins were located at the same insertion site in all isolates of C. parapsilosis and C. metapsilosis and in 13 of the 22 isolates of C. orthopsilosis. In the other 9 C. orthopsilosis isolates, a full-length intein (CorThrRS-B; 1,317 bp) was observed (Table 1).
The splicing domains were highly conserved in all CpaThrRS, CmeThrRS, CorThrRS-A, and CorThrRS-B inteins (see Fig. S2 in the supplemental material). All of them presented with cysteine (C) as the first amino acid and asparagine (N) the last (essential for the splicing mechanism), and contained the Thr-x-x-His motif in block B. The full-length intein CorThrRS-B also presented an addition of 3 amino acids (G-R-G and G-K-G, depending on the strain) before block B in the splicing domain, and its HE domain contained both of the critical aspartates, suggesting that it may be active (see Fig. S3 in the supplemental material). The mini-inteins of the three species (CpaThrRS, CmeThrRS, and CorThrRS-A) presented similar amplified fragment sizes (Table 1) that were indistinguishable in agarose (Fig. 3), yet their deduced amino acid sequences differed greatly (see Fig. S2 in the supplemental material).
Fig 3.
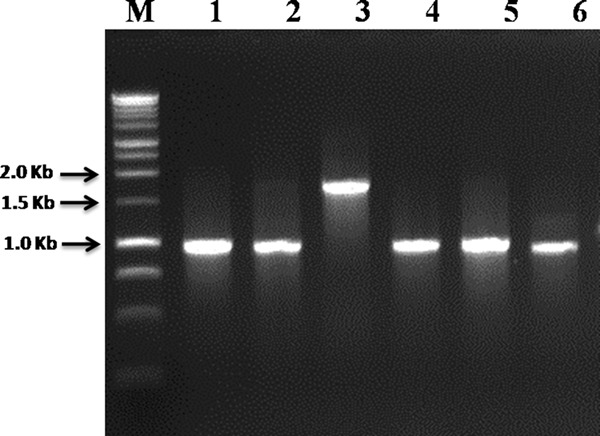
Agarose gel electrophoresis of the PCR carried out with TP3 and TP4 primers for amplification of ThrRS inteins from C. parapsilosis (lane 1), C. orthopsilosis (lanes 2, 4, and 5, strains with ThrRS-A, and lane 3, strain with ThrRS-B intein), and C. metapsilosis (lane 6). Lane M, 1-kb DNA ladder (Promega).
Phylogenetic analysis of the splicing domain regions of all ThrRS inteins produced a tree topology that distinguished the three species of the psilosis complex, mainly when considering the three mini-inteins (CpaThrRS, CmeThrRS, and CorThrRS-A). The mini- and the full-length ThrRS inteins of C. orthopsilosis (CorThrRS-A and CorThrRS-B) do not group together, while the intein CorThrRS-A is closely related to CmeThrRS and CpaThrRS, and the intein CorThrRS-B is genetically more distant from the clade that encompasses CmeThRS, CpaThrRS, and CorThrRS-A (Fig. 4). The ITS sequences of 14 C. orthopsilosis isolates, 8 presenting the mini-intein CorThrRS-A and 6 presenting the full-length intein CorThrRS-B, indicate that there is no correlation with the polymorphism pattern types 1 and 2 that were already proposed (14) (Table 2).
Fig 4.
Molecular phylogenetic analysis by maximum likelihood method of amino acid sequences from the splicing domain of the ThrRS inteins. Maximum likelihood (ML) phylogenetic analyses were performed using the website software version of PhyML (50).
Table 2.
Polymorphism analysis of the C. orthopsilosis intein ThrRS in relation to the ITS (ITS1-5.8S-ITS2) sequence
| Intein type in Candida orthopsilosis | Strain | Presence of indicated polymorphism in ITS sequence |
|||||
|---|---|---|---|---|---|---|---|
| Polymorphism 1 |
Polymorphism 2 |
Polymorphism 3 |
|||||
| Type 1 | Type 2 | Type 1 | Type 2 | Type 1 | Type 2 | ||
| A (mini-intein) | CAS08-0282 | + | + | + | |||
| CAS08-0320 | + | + | + | ||||
| CAS08-0498 | + | + | + | ||||
| CAS08-0504 | + | + | + | ||||
| CAS09-0748 | + | + | + | ||||
| CAS09-0831 | + | + | + | ||||
| CAS09-1198 | + | + | + | ||||
| CAS09-1791 | + | + | + | ||||
| B (full-length intein) | CAS08-0151 | + | + | + | |||
| CAS08-0185 | + | + | + | ||||
| CAS08-0599 | + | + | + | ||||
| CAS08-1540 | + | + | + | ||||
| CAS09-1799 | + | + | + | ||||
| CAS10-1825 | + | + | + | ||||
DISCUSSION
The differentiation of the psilosis complex into C. parapsilosis, C. orthopsilosis, and C. metapsilosis species is important for epidemiological purposes and also for changes in resistance to antifungal therapy, since C. orthopsilosis and C. metapsilosis are more susceptible to some currently used antifungals (16, 17, 19) and may have a lower capacity for biofilm formation (19, 21).
Ever since C. parapsilosis sensu lato was divided into a complex of three species, several authors have proposed different molecular identification techniques that are lengthy or costly, since they require further processing after DNA amplification. In contrast, we were able to differentiate species of the psilosis complex by means of a single PCR assay using degenerate primers that are complementary to the highly conserved VMA gene sites that flank the intein. We found that C. metapsilosis presents a VMA intein smaller than the one observed in C. orthopsilosis, while C. parapsilosis lacks an intein in VMA. This pattern of VMA intein distribution was systematically observed in all of the study isolates, indicating that this approach might be useful to unequivocally distinguish the three species of the psilosis complex. The identification of species using VMA intein sizing (Fig. 1) is an attractive tool for routine use in a clinical laboratory because it is accurate, rapid, and less expensive than other methods that have been previously proposed.
The amino acid residues involved in protein splicing were present in all of the VMA and ThrRS intein sequences analyzed, indicating that the splicing function is operating in both inteins, which was expected since both genes are considered essential for cell function. The Thr-x-x-His motif in block B that is known to assist in the N-terminal transesterification reaction leading to intein splicing (54, 55) is absent in CorVMA and CmeVMA (see Fig. S1 in the supplemental material). However, any residue that can form similar hydrogen bonds can replace these conserved residues without compromising splicing activity (46).
The presence of the essential aspartates Asp218 and Asp 326 in the inteins CorVMA and CorThrRS-B suggests that their HE domains may be active, while the absence of the first aspartate, as well as the degeneration observed in the HE domain of the CmeVMA intein, leads us to speculate that it is not functional, as is the case in most of the yeast VMA inteins (48). This is in agreement with the homing cycle proposed by Burt and Koufopanou (56) for these parasitic genetic elements that present a typical “rise and fall” cycle in the population structure of the affected species: once most of the alleles in a population are occupied by intein invasion, due to the homing process, there is no further constrained selection of a functional endonuclease, which might degenerate and become nonfunctional, so that empty sites may reemerge.
The idiomorphic occurrence of a mini- (CorThrRS-A) and a full-length intein (CorThrRS-B) at the same insertion site of C. orthopsilosis had not been observed previously. This interesting observation may indicate intraspecific genetic variation among C. orthopsilosis isolates, different varieties, or even different species. The polymorphism analysis of ITS (ITS1-5.8S-ITS2) sequences of the isolates harboring either CorThrRS-A or CorThrRS-B does not corroborate the division into 2 types (types 1 and 2) of C. orthopsilosis (14) (Table 2). However, the presence of polymorphisms in the ITS region does not always agree with other molecular markers. For instance, in another study using RAPD and ITS sequencing, the authors (27) divided C. orthopsilosis isolates into two groups (P2 and P3). Although the P3 group presents an identical ITS sequence to those of type 2 isolates, there are differences between the P2 group and type 1 isolates. Indeed, despite having been indicated as fungal barcoding (57), analysis of the ITS region does not appear to be suitable for discrimination between very closely related groups. This was observed, for example, among the cryptic species denominated S1, PS2, and PS3 from the Paracoccidioides brasiliensis species complex (58).
Although the phylogeny of the ThrRS inteins clearly distinguished other Candida species from the psilosis complex, it did not corroborate the species phylogeny proposed using the ITS region of rRNA (11) or by the analysis of 1,334 partial gene sequences (23); this indicates that C. metapsilosis and C. orthopsilosis share a more recent common ancestor and comprise the sister clade of C. parapsilosis. Furthermore, in our analysis, the intein CorThrRS-B does not group with the CorThrRS-A intein of C. orthopsilosis, which might reflect the occurrence of independent intein invasions through two possible scenarios. In the first scenario, the ancestor of C. orthopsilosis might have had its ThrRS gene invaded by an intein (ThrRS-A) that followed the homing cycle rules proposed by Burt and Koufopanou (56). The intein may have eventually become fixed in most of the population, leading to the degeneration of its HE domain (which might explain the current mini-intein structure). With the HE degenerated and therefore not functional, empty sites may have increased in the C. orthopsilosis population and been reoccupied by another intein, ThrRS-B. In the second scenario, the same site of ThrRS gene might have been occupied at the same moment by two different inteins, ThrRS-A in some populations and ThrRS-B in others. However, the two inteins are not present in the same phase of the homing cycle, because while ThrRS-A is a mini-intein without an HE, ThrRS-B is a full-length intein, probably with a functional HE, which may be an indication that this intein is in the “invasion phase” of the homing cycle and that empty sites might still exist in C. orthopsilosis species.
The finding of two types of allelic inteins, mini- and full-length, in a single population is unusual and might provide useful data for future epidemiological and population studies. The discovery of the biological meaning of this observation, whether it represents only an intraspecific polymorphism or two reproductively isolated groups (cryptic species), is still pending and requires additional population studies, especially those that employ sequences from many loci, such as the multilocus sequence typing (MLST), which has been largely used for phylogenetic recognition in many fungal species (59–61). In addition, future studies will also be important to determine whether the two-intein pattern has clinical relevance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mary Brandt and Nina Grossman from the CDC for their assistance, and Augusto C. Montelli and Terue Sadatsune for providing the Brazilian isolates.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants no. 2012/04003-1 and 2012/07741-3.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official findings and conclusions of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 19 June 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00981-13.
REFERENCES
- 1.Bruder-Nascimento A, Camargo CH, Sugizaki MF, Sadatsune T, Montelli AC, Mondelli AL, Bagagli E. 2010. Species distribution and susceptibility profile of Candida species in a Brasilian tertiary hospital. BMC Res. Notes 3:1. 10.1186/1756-0500-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J, Brazilian Network Candidemia Study 2006. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 44:2816–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, Moncada-Barrón D, Alvarez-Verona E, Hernández-Delgado L, Torres-Narváez P, Lavalle-Villalobos A. 2010. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur. J. Pediatr. 169:783–787 [DOI] [PubMed] [Google Scholar]
- 4.das Neves Miranda L, Rodrigues ECA, Costa SF, van der Heijden IM, Dantas KC, Lobo RD, Basso M, Varkulja GF, Krebs VLJ, Gibelli MABC, Criado PR, Levin AS. 2012. Candida parapsilosis candidaemia in a neonatal unit over 7 years: a case series study. BMJ Open 2:e000992. 10.1136/bmjopen-2012-000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikaku AS, Melo ASA, Colombo AL. 2010. Geographic trends in invasive candidiasis. Curr. Fungal Infect. Rep. 4:210–218 [Google Scholar]
- 6.Reissa E, Lasker BA, Iqbal NJ, James M, Arthington-Skaggs BA. 2008. Molecular epidemiology of Candida parapsilosis sepsis from outbreak investigations in neonatal intensive care units. Infect. Genet. Evol. 8:103–109 [DOI] [PubMed] [Google Scholar]
- 7.Almirante B, Rodríguez D, Cuenca-Estrella M, Almela M, Sanchez F, Ayats J, Alonso-Tarres C, Rodriguez-Tudela JL, Pahissa A. 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44:1681–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, Brandt ME, Webb RM, Currier M, Flowers RH, Fridkin SK, Hajjeh RA. 2004. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 10:4468–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 2:255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin AS, Costa SF, Mussi NS, Basso M, Sinto SI, Machado C, Geiger DC, Villares MC, Schreiber AZ, Barone AA, Branchini ML. 1998. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn. Microbiol. Infect. Dis. 4:243–249 [DOI] [PubMed] [Google Scholar]
- 11.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavanti A, Hensgens LAM, Ghelardi E, Campa M, Senesi S. 2007. Genotyping of Candida orthopsilosis clinical isolates by amplification fragment length polymorphism reveals genetic diversity among independent isolates and strain maintenance within patients. J. Clin. Microbiol. 45:1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Asbeck EC, Clemons KV, Markham AN, Stevens DA. 2009. Correlation of restriction fragment length polymorphism genotyping with internal transcribed spacer sequence, randomly amplified polymorphic DNA and multilocus sequence groupings for Candida parapsilosis. Mycoses 52:493–498 [DOI] [PubMed] [Google Scholar]
- 14.Sai S, Holland L, McGee CF, Lynch DB, Butler G. 2011. Evolution of mating within the Candida parapsilosis species group. Eukaryot. Cell 10:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantón E, Espinel-Ingroff A, Pemán J, del Castillo L. 2010. In vitro fungicidal activities of echinocandins against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis evaluated by time-kill studies. Antimicrob. Agents Chemother. 54:2194–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-López A, Alastruey-Izquierdo A, Rodriguez D, Almirante B, Pahissa A, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 52:1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. 2008. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 46:2659–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Asbeck E, Clemons KV, Martinez M, Tong AJ, Stevens DA. 2008. Significant differences in drug susceptibility among species in the Candida parapsilosis group. Diagn. Microbiol. Infect. Dis. 62:106–109 [DOI] [PubMed] [Google Scholar]
- 19.de Toro M, Torres MJ, Maite R, Aznar J. 2011. Characterization of Candida parapsilosis complex isolates. Clin. Microbiol. Infect. 17:418–424 [DOI] [PubMed] [Google Scholar]
- 20.Lattif AA, Mukherjee PK, Chandra J, Swindell K, Lockhart SR, Diekema DJ, Pfaller MA, Ghannoum MA. 2010. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int. J. Med. Microbiol. 300:265–270 [DOI] [PubMed] [Google Scholar]
- 21.Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. 2011. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 49:253–262 [DOI] [PubMed] [Google Scholar]
- 22.Song JW, Shin JH, Shint DH, Jung SI, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW. 2005. Differences in biofilm production by three genotypes of Candida parapsilosis from clinical sources. Med. Mycol. 43:657–661 [DOI] [PubMed] [Google Scholar]
- 23.Riccombeni A, Vidanes G, Proux-Wéra E, Wolfe KH, Butler G. 2012. Sequence and analysis of the genome of the pathogenic yeast Candida orthopsilosis. PLoS One 7:e35750. 10.1371/journal.pone.0035750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gácser A, Schäfer W, Nosanchuk JS, Salomon S, Nosanchuk JD. 2007. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet. Biol. 44:1336–1341 [DOI] [PubMed] [Google Scholar]
- 25.Orsi CF, Colombari B, Blasi E. 2010. Candida metapsilosis as the least virulent member of the ‘C. parapsilosis ' complex. Med. Mycol. 48:1024–1033 [DOI] [PubMed] [Google Scholar]
- 26.Kocsubé S, Tóth M, Vágvölgyi C, Dóczi I, Pesti M, Pócsi I, Szabó J, Varga J. 2007. Occurrence and genetic variability of Candida parapsilosis sensu lato in Hungary. J. Med. Microbiol. 56:190–195 [DOI] [PubMed] [Google Scholar]
- 27.Tay ST, Na SL, Chong J. 2009. Molecular differentiation and antifungal susceptibilities of Candida parapsilosis isolated from patients with bloodstream infections. J. Med. Microbiol. 58:185–191 [DOI] [PubMed] [Google Scholar]
- 28.van Asbeck EC, Clemons KV, Markham AN, Stevens DA, Candida parapsilosis Global Epidemiology Group 2008. Molecular epidemiology of the global and temporal diversity of Candida parapsilosis. Scand. J. Infect. Dis. 40:827–834 [DOI] [PubMed] [Google Scholar]
- 29.Mirhendi H, Bruun B, Schønheyder HC, Christensen JJ, Fuursted K, Gahrn-Hansen B, Johansen HK, Nielsen L, Knudsen JD, Arendrup MC. 2010. Molecular screening for Candida orthopsilosis and Candida metapsilosis among Danish Candida parapsilosis group blood culture isolates: proposal of a new RFLP profile for differentiation. J. Med. Microbiol. 59:414–420 [DOI] [PubMed] [Google Scholar]
- 30.Souza AC, Ferreira RC, Gonçalves SS, Quindós G, Eraso E, Bizerra FC, Briones MR, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) by use of mitochondrial DNA and real-time PCR. J. Clin. Microbiol. 50:2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays C, Duhamel C, Cattoir V, Bonhomme J. 2011. Rapid and accurate identification of species belonging to the Candida parapsilosis complex by real-time PCR and melting curve analysis. J. Med. Microbiol. 60:477–480 [DOI] [PubMed] [Google Scholar]
- 32.Yong PV, Chong PP, Lau LY, Yeoh RS, Jamal F. 2008. Molecular identification of Candida orthopsilosis isolated from blood culture. Mycopathologia 165:81–87 [DOI] [PubMed] [Google Scholar]
- 33.Borman AM, Linton CJ, Oliver D, Palmer MD, Szekely A, Odds FC, Johnson EM. 2009. Pyrosequencing analysis of 20 nucleotides of internal transcribed spacer 2 discriminates Candida parapsilosis, Candida metapsilosis, and Candida orthopsilosis. J. Clin. Microbiol. 47:2307–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubesová A, Šalplachta J, Horká M, Rùžička F, Šlais K. 2012. Candida “psilosis”–electromigration techniques and MALDI-TOF mass spectrometry for phenotypical discrimination. Analyst 137:1937–1943 [DOI] [PubMed] [Google Scholar]
- 35.Quiles-Melero I, García-Rodríguez J, Gómez-López A, Mingorance J. 2012. Evaluation of matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry for identification of Candida parapsilosis, C. orthopsilosis and C. metapsilosis. Eur. J. Clin. Microbiol. Infect. Dis. 31:67–71 [DOI] [PubMed] [Google Scholar]
- 36.Lasker BA, Butler G, Lott TJ. 2006. Molecular genotyping of Candida parapsilosis group I clinical isolates by analysis of polymorphic microsatellite markers. J. Clin. Microbiol. 44:750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler MI, Poulter RT. 2005. The PRP8 inteins in Cryptococcus are a source of phylogenetic and epidemiological information. Fungal Genet. Biol. 42:452–463 [DOI] [PubMed] [Google Scholar]
- 38.Theodoro RC, Bagagli E, Oliveira C. 2008. Phylogenetic analysis of PRP8 intein in Paracoccidioides brasiliensis species complex. Fungal Genet. Biol. 45:1284–1291 [DOI] [PubMed] [Google Scholar]
- 39.Chong S, Shao Y, Paulus H, Benner J, Perler FB, Xu MQ. 1996. Protein splicing involving the Saccharomyces cerevisiae VMA intein. The steps in the splicing pathway, side reactions leading to protein cleavage, and establishment of an in vitro splicing system. J. Biol. Chem. 271:22159–22168 [DOI] [PubMed] [Google Scholar]
- 40.Perler FB, Xu MQ, Paulus H. 1997. Protein splicing and autoproteolysis mechanisms. Curr. Opin. Chem. Biol. 1:292–299 [DOI] [PubMed] [Google Scholar]
- 41.Xu M, Southworth MW, Mersha FB, Hornstra LJ, Perler FB. 1993. In vitro protein splicing of purified precursor and the identification of a branched intermediate. Cell 75:1371–1377 [DOI] [PubMed] [Google Scholar]
- 42.Liu XQ. 2000. Protein-splicing intein: genetic mobility, origin and evolution. Annu. Rev. Genet. 34:61–76 [DOI] [PubMed] [Google Scholar]
- 43.Wu H, Hu Z, Liu XQ. 1998. Protein trans-splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. U. S. A. 95:9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gimble FS, Stephens BW. 1995. Substitutions in conserved dodecapeptide motifs that uncouple the DNA binding and DNA cleavage activities of PI-SceI endonuclease. J. Biol. Chem. 270:5849–5856 [DOI] [PubMed] [Google Scholar]
- 45.McCullough MJ, DiSalvo AF, Clemons KV, Park P, Stevens DA. 2000. Molecular epidemiology of Blastomyces dermatitidis. Clin. Infect. Dis. 30:328–335 [DOI] [PubMed] [Google Scholar]
- 46.Perler FB. 2002. InBase, the intein database. Nucleic Acids Res. 28:344–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 10:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Posey KL, Koufopanou V, Burt A, Gimble FS. 2004. Evolution of divergent DNA recognition specificities in VDE homing endonucleases from two yeast species. Nucleic Acids Res. 32:3947–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koufopanou V, Burt A. 2005. Degeneration and domestication of a selfish gene in yeast: molecular evolution versus site-directed mutagenesis. Mol. Biol. Evol. 22:1535–1538 [DOI] [PubMed] [Google Scholar]
- 50.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321 [DOI] [PubMed] [Google Scholar]
- 51.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18:691–699 [DOI] [PubMed] [Google Scholar]
- 52.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–787 [DOI] [PubMed] [Google Scholar]
- 53.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed). PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA [Google Scholar]
- 54.Klabunde T, Sharma S, Telenti A, Jacobs WR, Jr, Sacchettini JC. 1998. Crystal structure of GyrA intein from Mycobacterium xenopi reveals structural basis of protein splicing. Nat. Struct. Biol. 5:31–36 [DOI] [PubMed] [Google Scholar]
- 55.Poland BW, Xu MQ, Quiocho FA. 2000. Structural insights into the protein splicing mechanism of PI-SceI. J. Biol. Chem. 275:16408–16413 [DOI] [PubMed] [Google Scholar]
- 56.Burt A, Koufopanou V. 2004. Homing endonuclease genes: the rise and fall and rise again of a selfish element. Curr. Opin. Genet. Dev. 14:609–615 [DOI] [PubMed] [Google Scholar]
- 57.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. U. S. A. 109:6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. 2009. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 52:273–283 [DOI] [PubMed] [Google Scholar]
- 59.Kasuga T, White TJ, Koenig G, McEwen J, Restrepo A, Castañeda E, Da Silva Lacaz C, Heins-Vaccari EM, De Freitas RS, Zancopé-Oliveira RM, Qin Z, Negroni R, Carter DA, Mikami Y, Tamura M, Taylor ML, Miller GF, Poonwan N, Taylor JW. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12:3383–3401 [DOI] [PubMed] [Google Scholar]
- 60.Matute DR, McEwen JG, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Niño-Veja G, Taylor JW. 2006. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 23:65–73 [DOI] [PubMed] [Google Scholar]
- 61.Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31:21–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.