Abstract
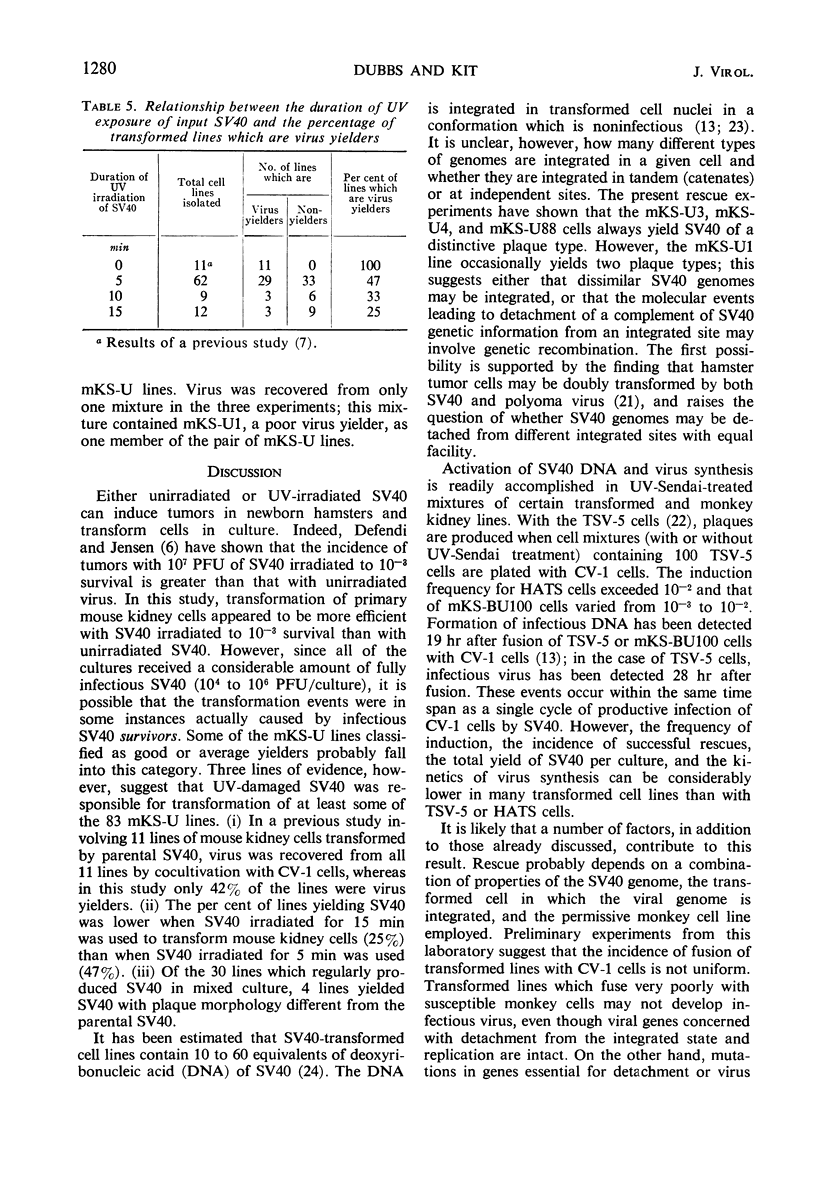
Rescue of simian virus 40 (SV40) from hamster and murine cell lines transformed by nonirradiated or by ultraviolet (UV)-irradiated SV40 (10−3 to 10−5 survival) was studied. A combination of tests was employed to detect induction of SV40 synthesis: (i) co-cultivation with susceptible monkey kidney (CV-1) cells; (ii) treating mixtures of transformed and CV-1 cells with UV-irradiated Sendai virus (UV-Sendai) prior to co-cultivation; and (iii) plating untreated or UV-Sendai-treated mixtures of transformed and CV-1 cells with freshly trypsinized CV-1 cells. The first and second tests provided a measure of the total infectious SV40 yield per culture, and the third test provided a measure of the frequency of induction (fraction of transformed cells giving rise to infectious centers). With the combination of tests, SV40 was rescued in all trials from TSV-5 hamster cells, mKS-BU100 mouse cells, and from several lines of mouse kidney cells transformed by UV-irradiated SV40 (mKS-U lines). The frequency of induction was about 7 × 10−2 for TSV-5 cells, about 3 × 10−3 for mKS-BU100 cells, greater than 10−4 for the mKS-U lines which were “good” yielders, and about 10−5 to 10−4 for the mKS-U lines which were “average” yielders. SV40 of a plaque type different from parental virus was rescued from four of the mKS-U cell lines. Virus was also easily rescued from: (i) tumor cells produced from the mKS-A line of transformed mouse kidney cells; (ii) mouse kidney cells transformed by SV40 which had been rescued from mKS-BU100 cells; and (iii) tumor cells (HATS) which had been produced by inoculating newborn hamsters with SV40 rescued from mKS-BU100 cells. The frequency of induction of HATS cells was of the same order of magnitude as the frequency of induction of TSV-5 cells. In a study of the kinetics of virus induction, it was shown that SV40 could be detected 28, 40, and 48.5 hr after UV-Sendai treatment of mixtures of CV-1 and TSV-5, HATS, or mKS-BU100 cells, respectively. Although all of the mKS-U lines contained the SV40-specific tumor antigen, some were poor virus yielders (SV40 was recovered in less than 50% of the trials) and five lines were rare virus yielders (SV40 recovered only once in four or more trials). Forty-eight mKS-U lines were nonyielders; SV40 was never recovered by any test used thus far. UV-Sendai-treated mixtures of pairs of nonyielder mKS-U lines with CV-1 cells also did not yield infectious virus. Various factors affecting rescue have been discussed. The mKS-U lines which were poor virus yielders, rare yielders, or which never yielded virus have been classified tentatively as “defective lysogens” which contain mutational lesions at loci essential for detachment of SV40 from integration sites or for SV40 replication, or for both.
Full text
PDF

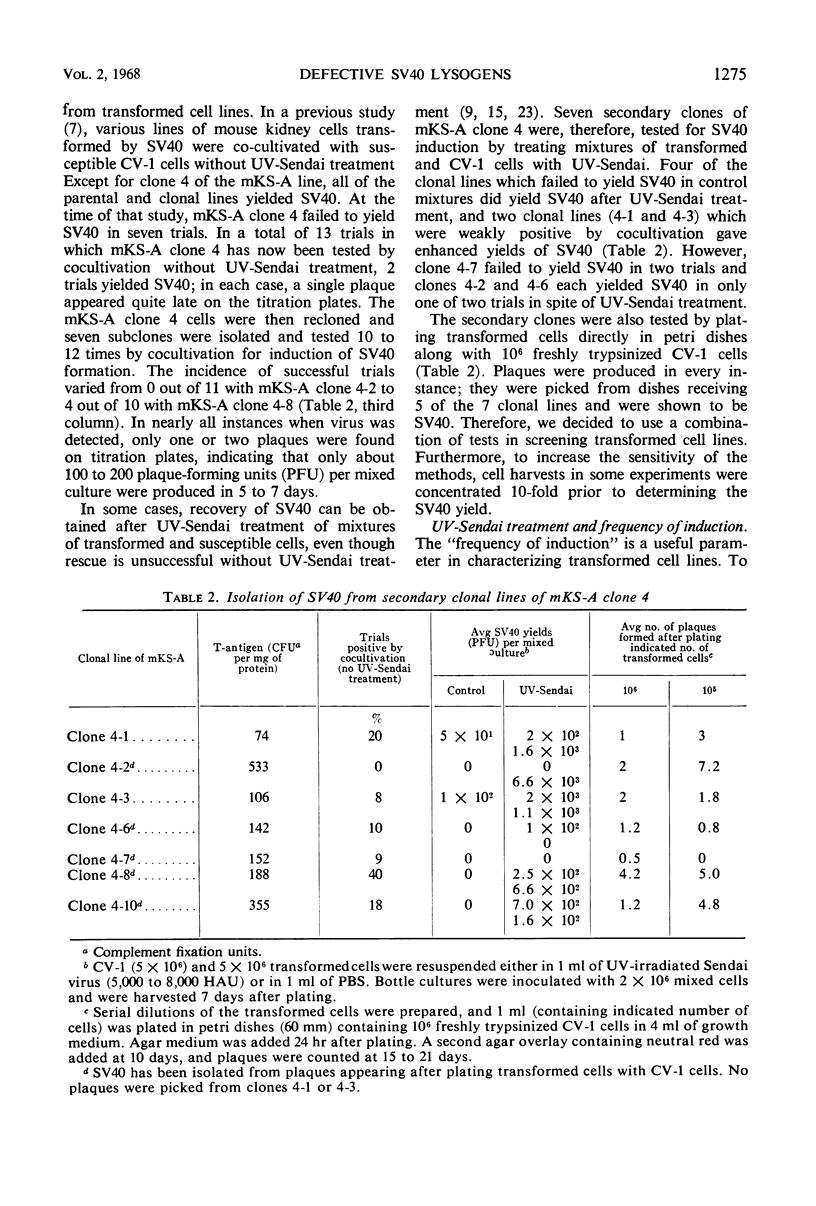

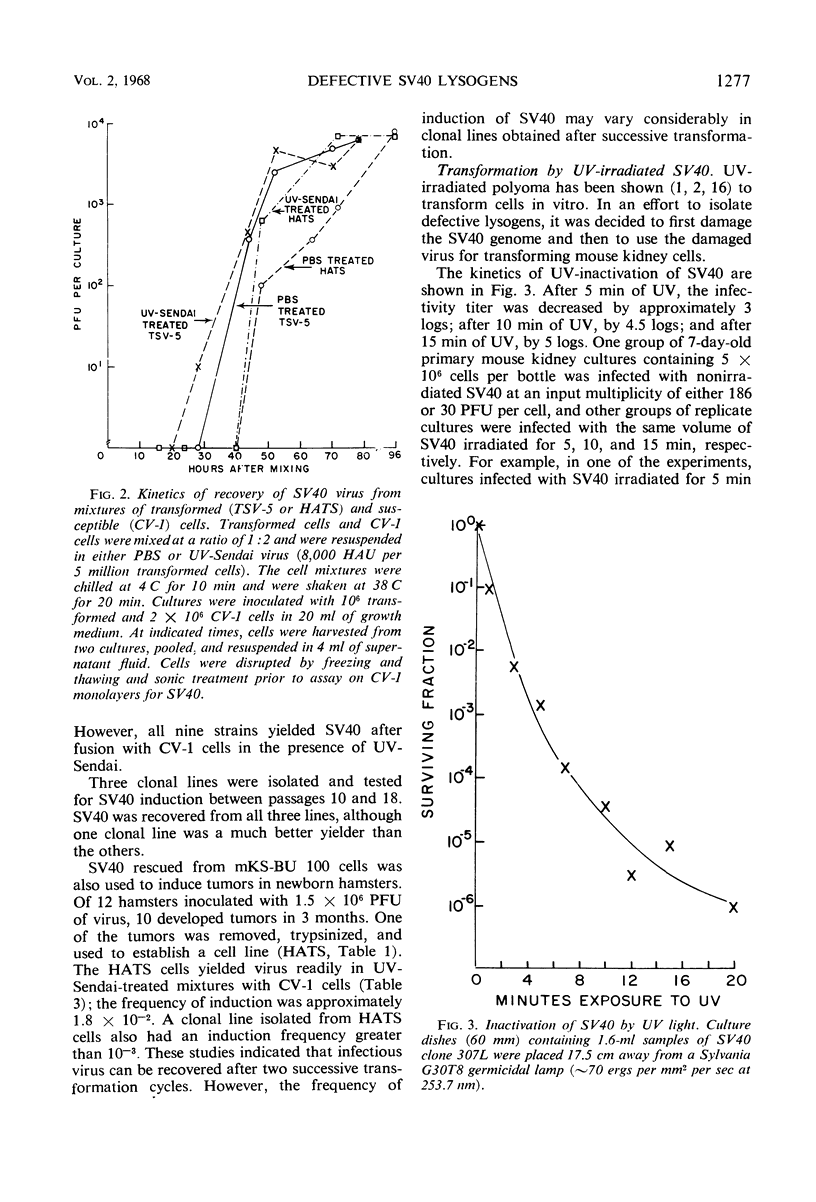

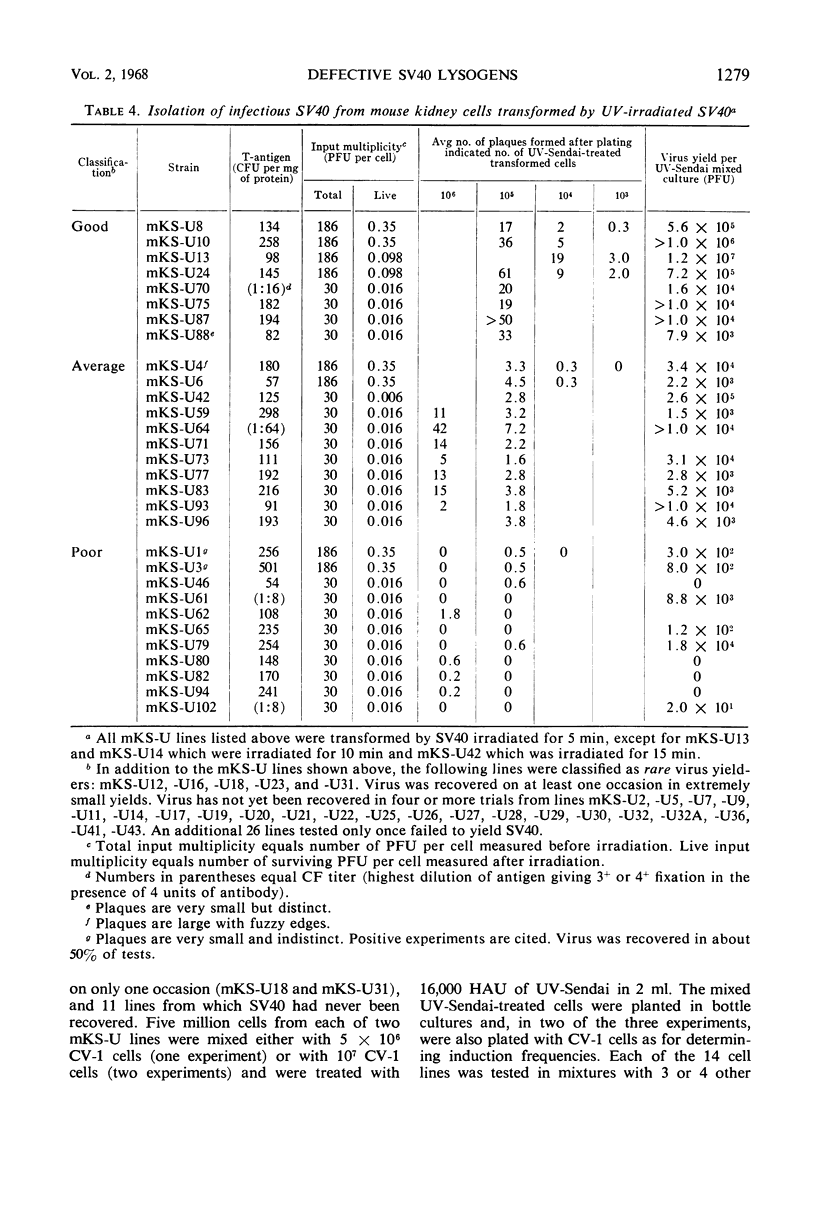


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P., COOPER H. L. AN ANALYSIS OF SV 40-INDUCED TRANSFORMATION OF HAMSTER KIDNEY TISSUE IN VITRO. II. STUDIES OF THREE CLONES DERIVED FROM A CONTINUOUS LINE OF TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Nov;50:847–854. doi: 10.1073/pnas.50.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. H. An analysis of SV40-induced transformation of hamster kidney tissue in vitro. 3. Persistence of SV40 viral genome in clones of transformed hamster cells. J Natl Cancer Inst. 1966 Oct;37(4):487–493. [PubMed] [Google Scholar]
- Defendi V., Jensen F. Oncogenicity by DNA tumor viruses: enhancement after ultraviolet and cobalt-60 radiations. Science. 1967 Aug 11;157(3789):703–705. doi: 10.1126/science.157.3789.703. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S., De Torres R. A., Anken M. Virogenic properties of bromodeoxyuridine-sensitive and bromodeoxyuridine-resistant simian virus 40-transformed mouse kidney cells. J Virol. 1967 Oct;1(5):968–979. doi: 10.1128/jvi.1.5.968-979.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER P., KIRSCHSTEIN R. L. SV40-induced ependymomas in newborn hamsters. I. Virus-tumor relationships. Virology. 1962 Dec;18:582–588. doi: 10.1016/0042-6822(62)90061-2. [DOI] [PubMed] [Google Scholar]
- GERBER P. VIROGENIC HAMSTER TUMOR CELLS: INDUCTION OF VIRUS SYNTHESIS. Science. 1964 Aug 21;145(3634):833–833. doi: 10.1126/science.145.3634.833. [DOI] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Salvi M. L., Dubbs D. R. Activation of infectious SV40 synthesis in transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1239–1246. doi: 10.1073/pnas.60.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latarjet R., Cramer R., Montagnier L. Inactivation, by UV-, x-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967 Sep;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., KHERA K. S., RAPP F. PAPOVAVIRUS SV40: FAILURE TO ISOLATE INFECTIOUS VIRUS FROM TRANSFORMED HAMSTER CELLS SYNTHESIZING SV40-INDUCED ANTIGENS. Virology. 1964 Jul;23:430–432. doi: 10.1016/0042-6822(64)90267-3. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Knowles B. B., Koprowski H. The mechanism of internuclear transmission of SV40-induced complement fixation antigen in heterokaryocytes. Proc Natl Acad Sci U S A. 1968 Mar;59(3):769–776. doi: 10.1073/pnas.59.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Habel K. Hamster tumor cells doubly transformed by SV40 and polyoma viruses. Virology. 1966 Sep;30(1):20–28. doi: 10.1016/s0042-6822(66)81005-x. [DOI] [PubMed] [Google Scholar]


