Abstract
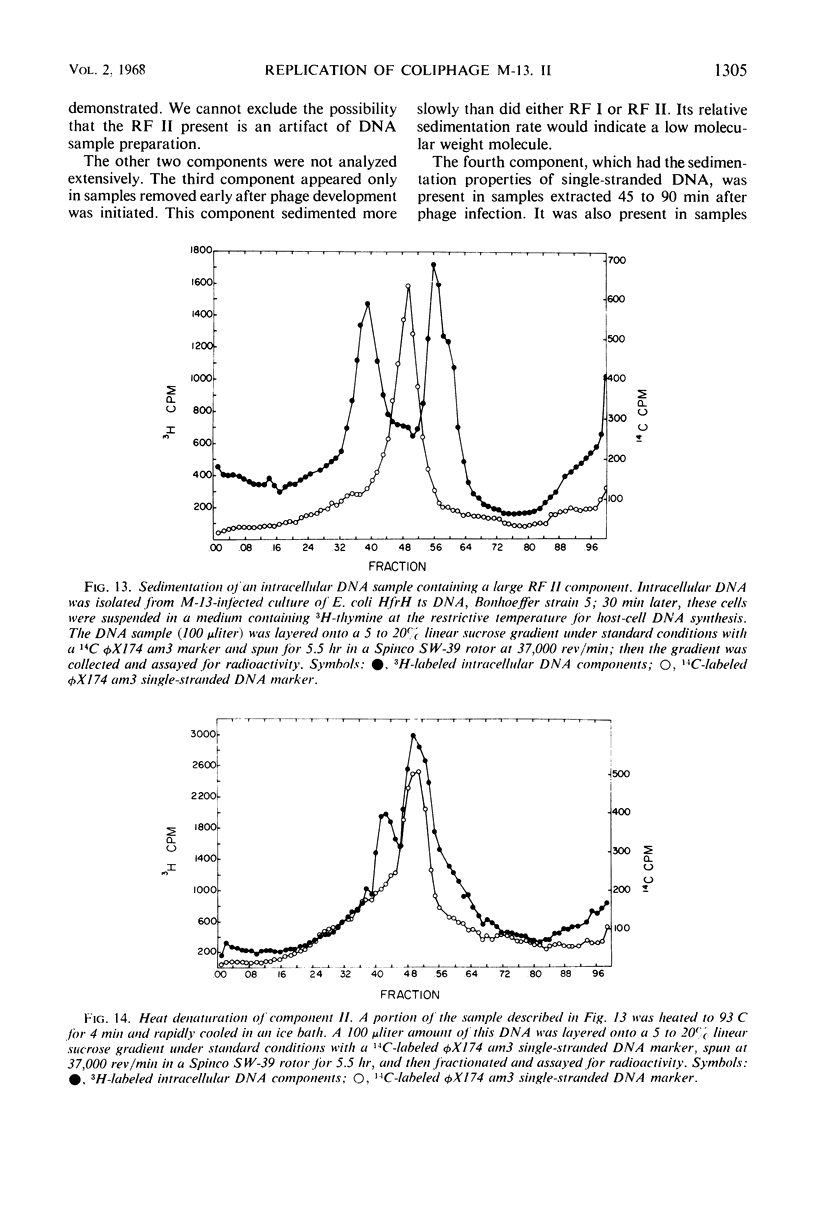
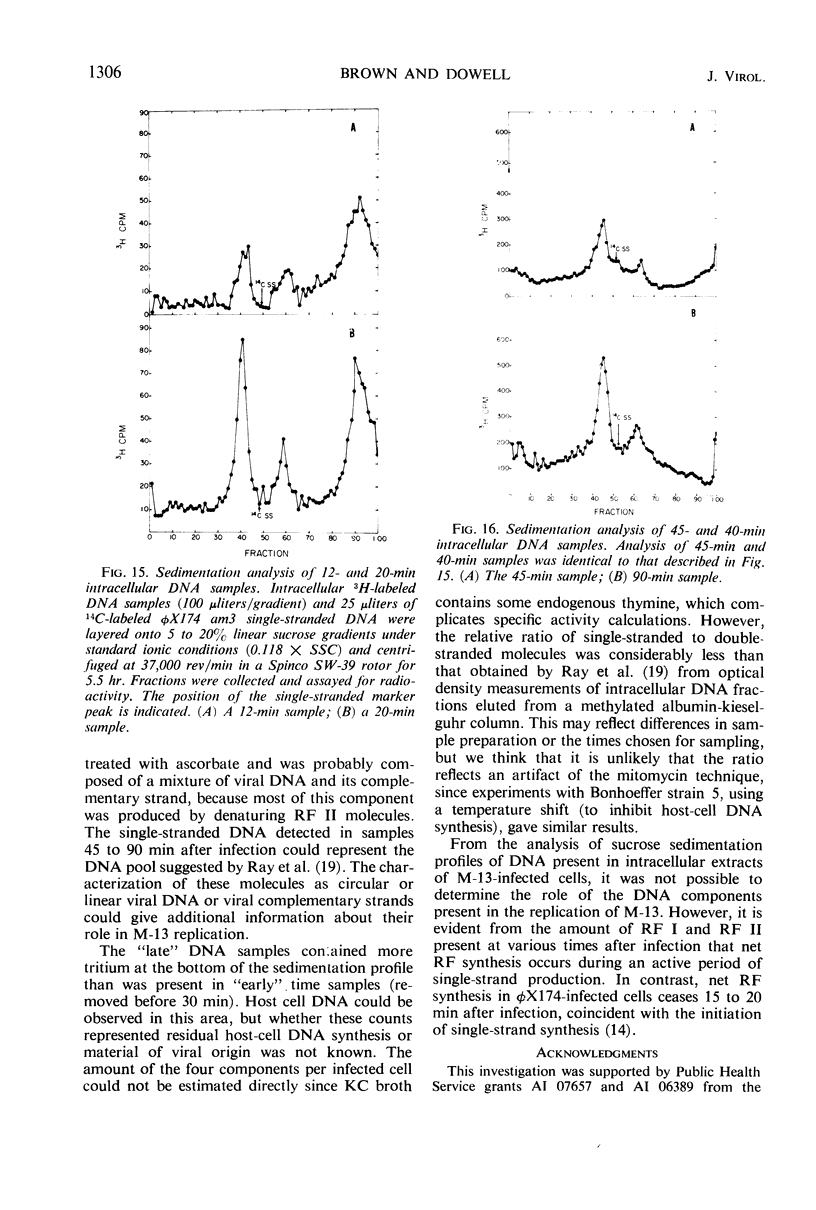
Intracellular deoxyribonucleic acid (DNA) forms associated with bacteriophage M-13 infection have been isolated and characterized. Escherichia coli HF4704 (F+, hcr−, thy−) cells were treated with mitomycin C to inhibit host-cell DNA synthesis and were then infected with phage M-13. This treatment permitted radioactive labeling of phage-specific DNA forms with 3H-thymine. These labeled DNA components were characterized by sucrose density sedimentation and equilibrium density gradient centrifugation in neutral and ethidium bromide CsCl gradient. Two double-stranded circular forms were found with properties analogous to the replicative form I and replicative form II of φX174. A third component, identified as single-stranded DNA, was isolated in some samples removed 45 min after phage synthesis was initiated.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. I. Effects on host cells after synchronized infection. J Virol. 1968 Nov;2(11):1290–1295. doi: 10.1128/jvi.2.11.1290-1295.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A., Sinsheimer R. L. The process of infection with bacteriophage phi-X174 VII. Ultracentrifugal analysis of the replicative form. J Mol Biol. 1965 Dec;14(2):327–347. doi: 10.1016/s0022-2836(65)80185-1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 3. Phage maturation and lysis after synchronized infection. J Mol Biol. 1965 Jul;12(3):641–646. doi: 10.1016/s0022-2836(65)80318-7. [DOI] [PubMed] [Google Scholar]
- Dowell C. E., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. IX. Studies on the physiology of three phi-X174 temperature-sensitive mutants. J Mol Biol. 1966 Apr;16(2):374–386. doi: 10.1016/s0022-2836(66)80180-8. [DOI] [PubMed] [Google Scholar]
- GAREN A., SIDDIQI O. Suppression of mutations in the alkaline phosphatase structural cistron of E. coli. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1121–1127. doi: 10.1073/pnas.48.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., BURTON A., SINSHEIMER R. L. ELECTRON MICROSCOPY OF THE REPLICATIVE FORM OF THE DNA OF THE BACTERIOPHAGE PHI-X174. Science. 1963 Nov 15;142(3594):961–961. doi: 10.1126/science.142.3594.961. [DOI] [PubMed] [Google Scholar]
- Knippers R., Komano T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XXI. Replication and fate of the replicative form. Proc Natl Acad Sci U S A. 1968 Feb;59(2):577–581. doi: 10.1073/pnas.59.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Knippers R., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XXII. Synthesis of progeny single-stranded DNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):911–916. doi: 10.1073/pnas.59.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi X174. XVI. Synthesis of the replicative form and its relationship to viral single-stranded DNA synthesis. J Mol Biol. 1968 Mar 14;32(2):285–302. doi: 10.1016/0022-2836(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Schaller H. The topology of DNA from the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):1–7. doi: 10.1016/s0022-2836(66)80204-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- SEDAT J., SINSHEIMER R. L. STRUCTURE OF THE DNA OF BACTERIOPHAGE PHI-X174. V. PURINE SEQUENCES. J Mol Biol. 1964 Aug;9:489–497. doi: 10.1016/s0022-2836(64)80221-7. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kilgore W. W. Decomposition of ribosomal particles in Escherichia coli treated with mitomycin C. J Bacteriol. 1967 Sep;94(3):666–676. doi: 10.1128/jb.94.3.666-676.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- Takeya K., Amako K. A rod-shaped Pseudomonas phage. Virology. 1966 Jan;28(1):163–165. doi: 10.1016/0042-6822(66)90317-5. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]


