Abstract
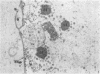
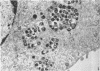
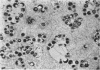
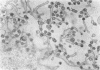
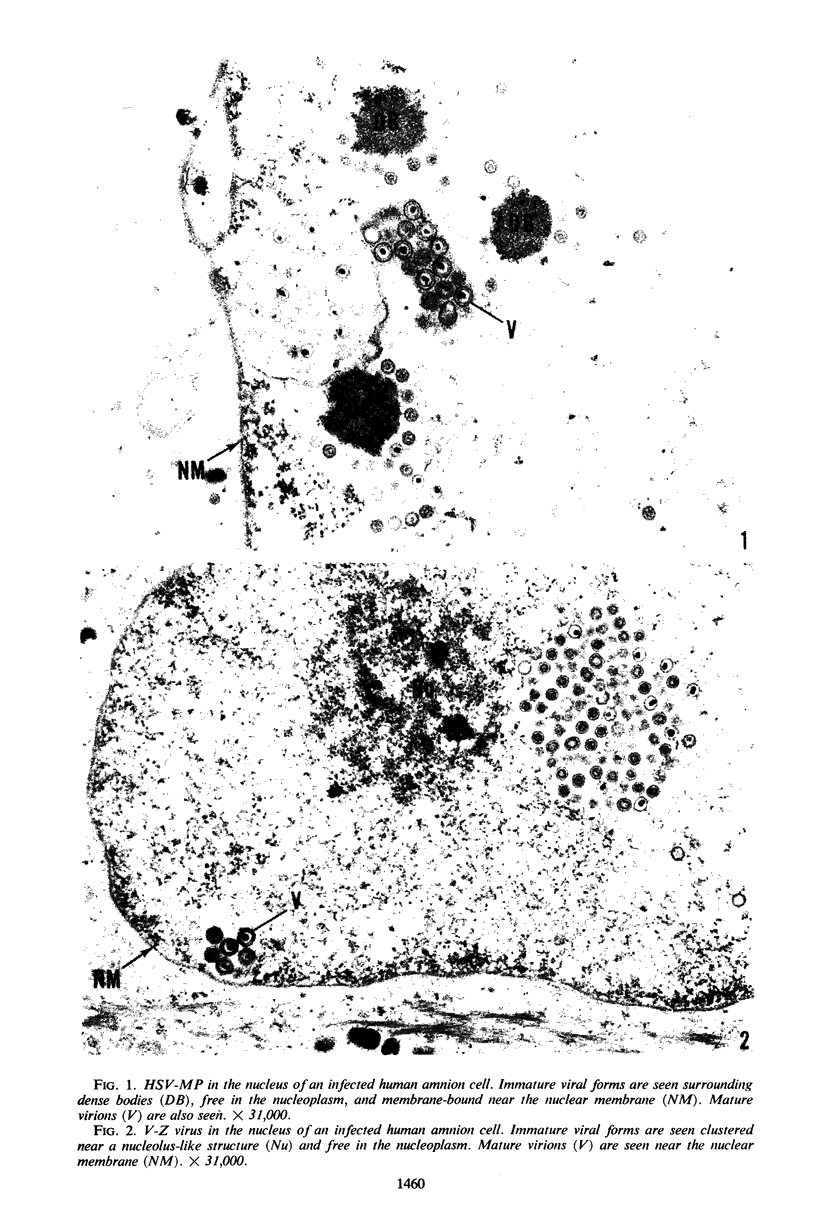
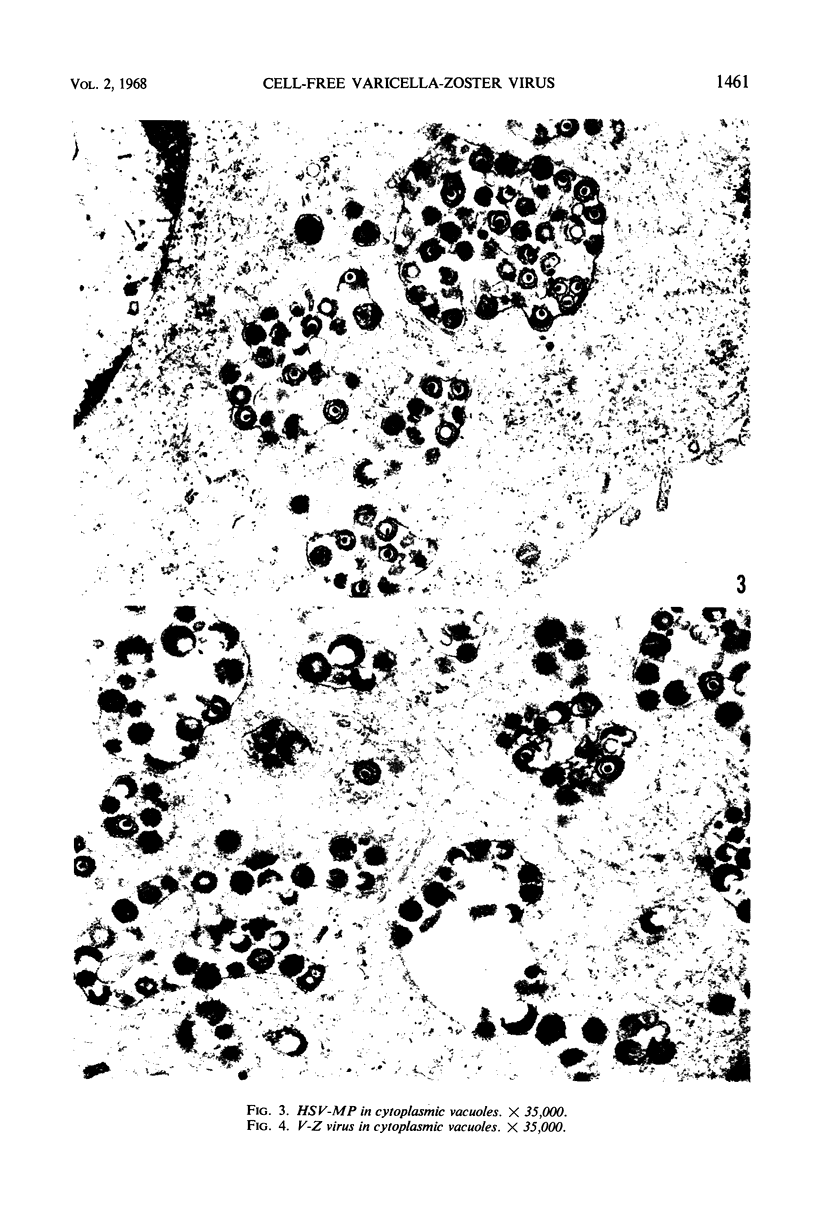
Experiments designed to determine why cell-free varicella-zoster virus replicated in cell culture is noninfectious were performed. Electron micrographs in which varicella-zoster virus (a herpesvirus) was compared to herpes simplex virus in primary human amnion cell cultures showed that the viruses were morphologically indistinguishable inside the nucleus. However, extranuclear varicella-zoster viruses were distinguished from herpes simplex virus by the presence of pleomorphism, incomplete coats, and a resultant loss of central dense cores. This result indicates that varicella-zoster virus possesses a labile coat which is degraded outside the nucleus. It is suggested that the labile coat is a principal reason for the lack of cell-free infectious virus in this system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER P., MELNICK J. L., MAYOR H. D. A MORPHOLOGIC COMPARISON BETWEEN THE DEVELOPMENTAL STAGES OF HERPES ZOSTER AND HUMAN CYTOMEGALOVIRUS. Exp Mol Pathol. 1965 Feb;76:11–23. doi: 10.1016/0014-4800(65)90020-1. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., ALMEIDA J. D. A method for the study of cultured cells by thin sectioning and electron microscopy. J Biophys Biochem Cytol. 1958 Jan 25;4(1):115–118. doi: 10.1083/jcb.4.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADIN S. H., DARBY N. B., Jr Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med. 1958 Jul;98(3):574–576. doi: 10.3181/00379727-98-24111. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkey F. H., Erlandson R. A., Bailey R. B., Babcock V. I., Southam C. M. Virus biographies. II. Growth of herpes simplex virus in tissue culture. Exp Mol Pathol. 1967 Feb;6(1):39–67. doi: 10.1016/0014-4800(67)90005-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D. Chickenpox and herpes zoster. III. Tissue culture studies. Br J Exp Pathol. 1959 Dec;40:521–532. [PMC free article] [PubMed] [Google Scholar]
- TOURNIER P., CATHALA F., BERNHARD W. Ultrastructure et développement intracellulaire du virus de la varicelle observé au microscope électronique. Presse Med. 1957 Jun 29;65(52):1229–1234. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]