Abstract
High-risk human papillomaviruses (HPVs) cause a variety of malignancies of the mucosal epithelium. However, the local immune evasion strategies used by HPV-transformed cells remain unclear. Here, we examined the effect of HPV-positive cancer cells on human peripheral blood monocytes, which are precursors of Langerhans cells, key antigen-presenting cells in the squamous epithelium. HPV-positive cervical cancer cells and HPV-E6 expressing cells inhibited monocyte differentiation to Langerhans cells in a contact-dependent manner. Unlike Langerhans cells, monocytes that differentiated in the presence of HPV16 E6-expressing cells exhibited high levels of endocytic activity. Our results suggest that cells infected by high-risk HPV evade immune surveillance by blocking the differentiation of monocytes into competent antigen presenting cells.
Introduction
Cervical cancer represents a major cause of cancer-related mortality in women worldwide. Human papillomavirus (HPV), particularly the high-risk types (including HPV16 and HPV18), is the primary etiologic agent of cervical cancer (1). Papillomaviruses are double-stranded DNA viruses that replicate exclusively in stratified squamous epithelia, using the differentiation of the epithelium to regulate their replication (2). Infection with high-risk HPV can result in integration of the viral genome into host DNA, which can up-regulate the expression of E6/E7 through multiple mechanisms (3). If integration interrupts the viral E2 gene, overexpression of E6 and E7 proteins occurs due to the loss of E2-mediated transcriptional repression. As a result, HPV-infected cells with integrated HPV DNA acquire extended lifespan, retain the capacity to proliferate, and accumulate mutations attributable to the actions of E6 and E7 proteins (4). The E6 and E7 oncogenes are continuously expressed in human cancer cells and are required for proliferation and survival of the cells (5, 6).
In order for immune elimination of virally infected cells, effector T cells must not only recognize viral antigen presenting target epithelial cells (7), but must also be stimulated by local dendritic cells (DC) (8–11). Restimulation of memory T cells by local antigen-presenting DCs appears to be a pre-requisite for the effector functions of T cells. However, the immunological status and the antigen-presenting function of DCs within cervical cancer lesions are unclear. Previous studies showed that cervical intraepithelial neoplasias are greatly depleted of Langerhans cells (LC) (12–20), which are specialized DCs found within the stratified squamous epithelium. However, the mechanism(s) responsible for LC depletion in these tissues remains unclear. It has been shown that E6 and E7 down-regulate expression of chemoattractants, such as IL-8 (21), MCP-1/CCL2 (22) and MIP-3α/CCL20 (20). Reduced expression of chemoattractants might contribute to the absence of LCs in the squamous intraepithelial lesions. In addition, surface E-cadherin expression is suppressed to some extent by HPV E6, possibly leading to impaired LC retention within the infected epidermis (23). However, because LCs differentiate from a local monocyte precursor within the stratified epithelium (as opposed to fully differentiated LCs migrating from a distal site) (24, 25), a pertinent question is whether HPV might inhibit LC differentiation within the epithelial lesion. In this regard, CD14+ monocytes, which can normally differentiate into LCs, have been reported to accumulate within and around the HPV-positive vulvar intraepithelial neoplasia, and yet, LC numbers remain low within the lesion (26).
In this study, we examine the effects of HPV-transformed cells on human monocyte differentiation and function and demonstrate that HPV-expressing cervical cancer cells inhibit the differentiation of monocytes to LC in vitro, and we probe the viral genes responsible for this inhibition. Further, we examine the functional consequences of co-cultivation with HPV-positive cancer cells on antigen-presenting and T cell differentiating functions.
Materials and Methods
Cell culture
SiHa and C33A cells were purchased from American Type Culture Collection (ATTC, Manassas, VA). HeLa-Sen2 cells (referred to as HeLa throughout the paper), a clonal cell line of HeLa cells selected for sensitivity to SV40 infection, were described previously (27). HeLa, SiHa, and C33A cells were cultured in Dulbecco's MEM (DMEM) with 10% fetal calf serum (FCS) (Sigma, St. Louis, MO), 100 U/ml penicillin, 100 µg/ml streptomycin (Invitrogen). CaSki cells were obtained from Michael Reiss (University of Medicine and Dentistry of New Jersey) and cultured in RPMI-1640 with 10% FCS (Sigma, St. Louis, MO), 100 U/ml penicillin, 100 µg/ml streptomycin.
Transduction of HPV oncogenes into C33A cells
To generate cells that stably express exogenous HPV16 E6 and HPV16 E7, C33A cells were first infected with concentrated LXSN or LXSN 16E6 retroviral stocks prepared as described (28) and selected with 350 µg/ml G418 for nine days. Cells were then infected with concentrated RVY or RVY 16E7 retrovirus and selected in 200 µg/ml hygromycin for 12 days (28). Following selection, cells were maintained in the presence of 175 µg/ml G418 and 100 µg/ml hygromycin.
Generation of monocyte-derived LCs and macrophages
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (Ficoll-Paque™ Plus, GE Healthcare, Waukesha, USA) from buffy coats of healthy anonymous donors (New York Blood Center, New York, NY). CD14+ monocytes were purified from PBMC by using a MACS CD14 isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). After elution from the Midi MACS LS columns, CD14+ monocytes were cultured (2 × 106 cells perT12.5 flask) in media consisting of RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, along with 50 ng/ml GM-CSF, 20 ng/ml human IL-4 and 10 ng/ml TGF-β (for LC generation), or 10 ng/ml GM-CSF (for macrophage generation). All cytokines were purchased from PeproTech, Rocky Hill, NJ. The culture medium was replaced with fresh medium containing cytokines on day 3, and were incubated for additional 3 to 4 days at 37 °C and 5% CO2.
Preparation of HPV-negative and HPV-positive cancer cell lines for the co-culture with CD14+ monocytes
Sub-confluent HPV-negative (C33A and A431) and HPV-positive cell lines (SiHa, HeLa and CaSki) cells were harvested following trypsin treatment. These cells were seeded in 6 well plates at the concentration of 2 ×106 cells/ml and then UV inactivated by exposure to 1 J/cm2 UV light once with a Stratlinker UV cross-linker (Stratagene). These cells (2 × 106 cells) were added to a T12.5 flask containing CD14+ monocytes (1–2 × 106 cells) at day 0 of the co-culture (2 ml of total volume in T12.5 flask). For paraformaldehyde fixation, cancer cell lines were harvested with trypsin, washed in complete media, suspended in 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated for 10 min at ambient temperature (no UV treatment was included prior to fixation). In some experiments, live cancer cells were used. The cells were then washed with culture media three times prior to addition to monocyte co-culture.
Flow cytometry
Phenotypic analysis of monocyte-derived cells by flow cytometry was performed using the following antibodies that were directly conjugated to various fluorescent dyes: CD1a (HI149), HLA-DR (L243), CD45 (H130), CD3 (UCHT1), CD4 (RPAT4) and CD14 (61D3) (Biolegend and eBioscience). Dead cells were excluded by staining with 7-AAD (Biolegend). After flow cytometry on a LSR II flow cytometer(Becton Dickinson), the results were analyzed using the FlowJo software (Tree Star, Ashland, OR).
Endocytosis assay
Differentiated monocyte-derived LC or macrophages generated in the presence of C33A cells or HPV16 E6 and/or E7 transduced C33A cells were incubated with 0.5 mg/ml Fluorescein–conjugated Dextran (70,000 MW, Anionic, Invitrogen) for 1 hour at 37°C or 4°C. After incubation, ice-cold FACS buffer (5% FCS, 0.09% NaN3 in PBS) containing 0.4% trypan blue was added to quench FITC-Dextran on the cell surface, and the cells were incubated for 10 min on ice to stop the endocytic activity. The frequency of FITC+CD1a+HLA-DR+ and FITC+CD1a−HLA-DR+ cells was analyzed by flow cytometry.
Monocyte differentiation using the Transwell system
CD14+ monocytes (2 × 106 cells) were added to upper well or lower well of 0.4 µm pore, polyester membrane, 6-well tissue culture inserts (Costar, Cambridge, MA, USA) in 1 ml of 10% FCS RPMI1640, with cancer cell lines (2 ×106 cell/ml) in the lower or upper well, respectively. CD14+ monocytes and the cancer cells were cultured separately in the presence of 50 ng/ml GM-CSF, 20 ng/ml human IL-4 and 10 ng/ml TGF-β for 7 days. Differentiated LCs from upper or lower well were collected for the analysis of cell surface CD1a and HLA-DR by flow cytometry.
Results
High-risk HPV-positive carcinoma cell lines inhibit differentiation of monocytes into Langerhans cells
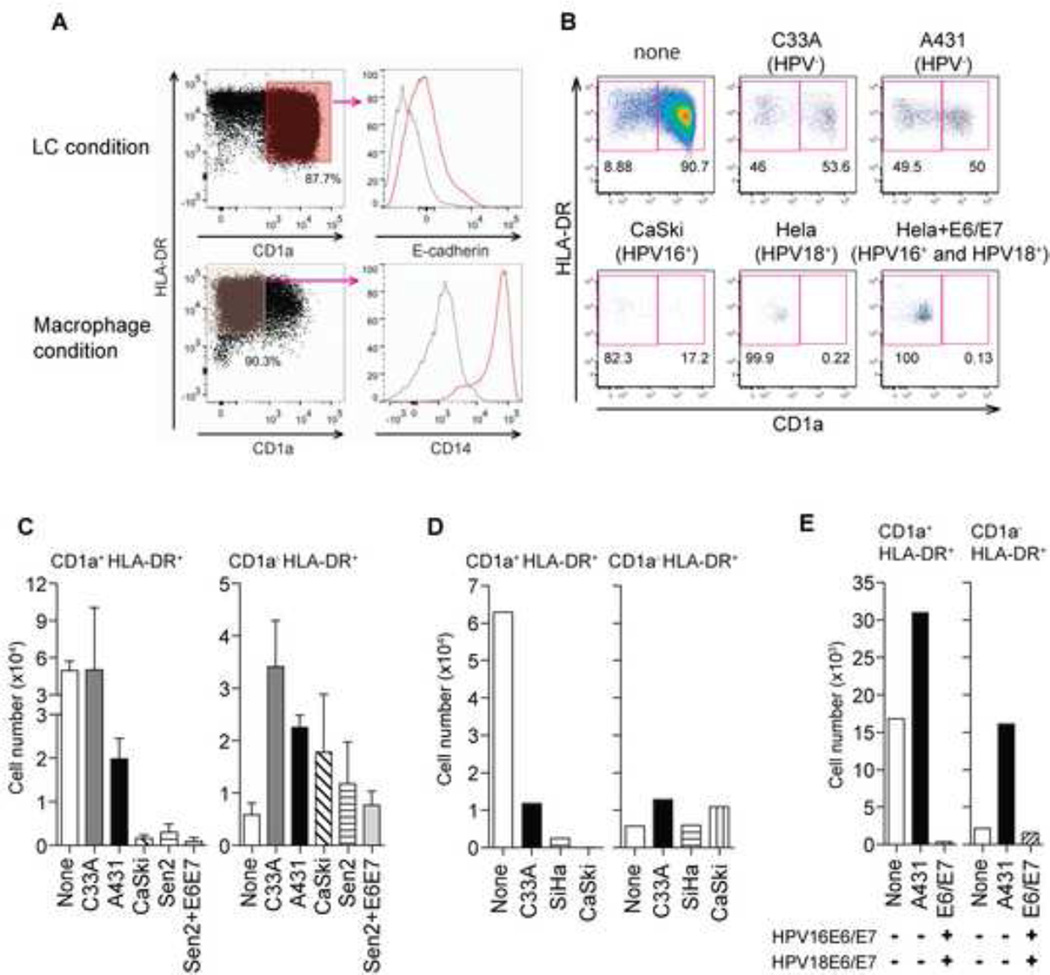
In the presence of GM-CSF, IL-4, and TGF-β primary human peripheral blood monocytes undergo a differentiation program in vitro to become LCs (29). To determine whether HPV-positive cancer cells affect monocyte differentiation, we developed an in vitro co-culture system in which human peripheral blood monocytes are incubated in the presence or absence of various cancer cell lines in the LC differentiation media, and then assessed for LC development. In order to prevent outgrowth of cancer cells in our co-cultures, carcinoma cell lines were UV-irradiated prior to co-incubation with monocytes. After seven days, monocyte-derived cells were analyzed by flow cytometry for the expression of LC markers typically used to define these cells including CD1a, E-cadherin and MHC class II (HLA-DR). In the absence of carcinoma cells, differentiation to LCs was very efficient (~90% CD1a+/HLA-DR+/E-cadherin+/CD14−) (Figure 1A). Co-culture with carcinoma cell lines in general reduced LC differentiation. However, compared to HPV-negative cell lines such as C33A (cervical) and A431 (epidermoid), which reduced LC differentiation to ~50%, HPV-positive cervical cancer cell lines reduced LC differentiation to 17.2% (Caski, HPV16+), or <1% (HeLa, HPV18+) (Figure 1B). In addition to the reduction in frequency of LCs, the average total LC yield, as well as non-LC cell numbers, from four or more donors was dramatically reduced by the presence of HPV-positive cancer cells (Figure 1C).
Figure 1. High-risk HPV-positive cancer cell lines inhibit LC generation from human CD14+ monocytes.
(A) CD14+ monocytes purified from human buffy coats were incubated in the presence of GM-CSF/IL-4/TGF-β (LC condition) or of GM-CSF (macrophage condition) for 7 days, and stained for LC markers (CD1a, E-cadherin, HLA-DR) and a monocyte marker (CD14). Red histograms indicate expression of E-cadherin or CD14 in the indicated condition, while gray histograms indicate expression in the other. (B) Purified monocytes were co-incubated with UV-treated HPV-negative (C33A and A431) or HPV-positive cancer cell lines (CaSki, HeLa, SiHa and HPV16 E6/E7-transduced HeLa), and analyzed for expression of CD1a and HLA-DR. (C) The average total numbers of differentiated LC (CD1a+ HLA-DR+) and non-LC (CD1a−) obtained from at least four donors from co-culture with the indicated cancer cell lines are depicted. Error bars indicate standard error of the mean (SEM). (D, E) CD14+ monocytes were co-incubated with PFA-fixed (D) or live (E) HPV-negative (C33A, A431) or high-risk HPV-positive cell lines (SiHa and CaSki or HPV16 E6/E7-transduced HeLa) in the presence of GM-CSF/IL-4/TGF-β for 7 days. The average total numbers of differentiated LC (CD1a+ HLA-DR+) and non-LC (CD1a−) obtained from at least four donors from co-culture with the indicated cancer cell lines are depicted.
Paraformaldehyde-fixed HPV-positive carcinoma cell lines, SiHa (HPV16+) and Caski (HPV16+), also inhibited monocyte differentiation to LCs (Figure 1D), suggesting that the inhibition is not likely to be due to residual secretion of soluble factors from UV-treated HPV-positive carcinoma cells. In addition, live HPV-positive HeLa cells transduced with HPV16 E6 and E7 also inhibited LC differentiation as well as recovery of non-LCs (Figure 1E). Collectively, these data demonstrate that HPV-positive cancer cell lines preferentially inhibit LC differentiation and induce death of differentiating cells in general when co-cultured with monocyte precursors.
Monocyte inhibition requires high density of HPV-positive cells
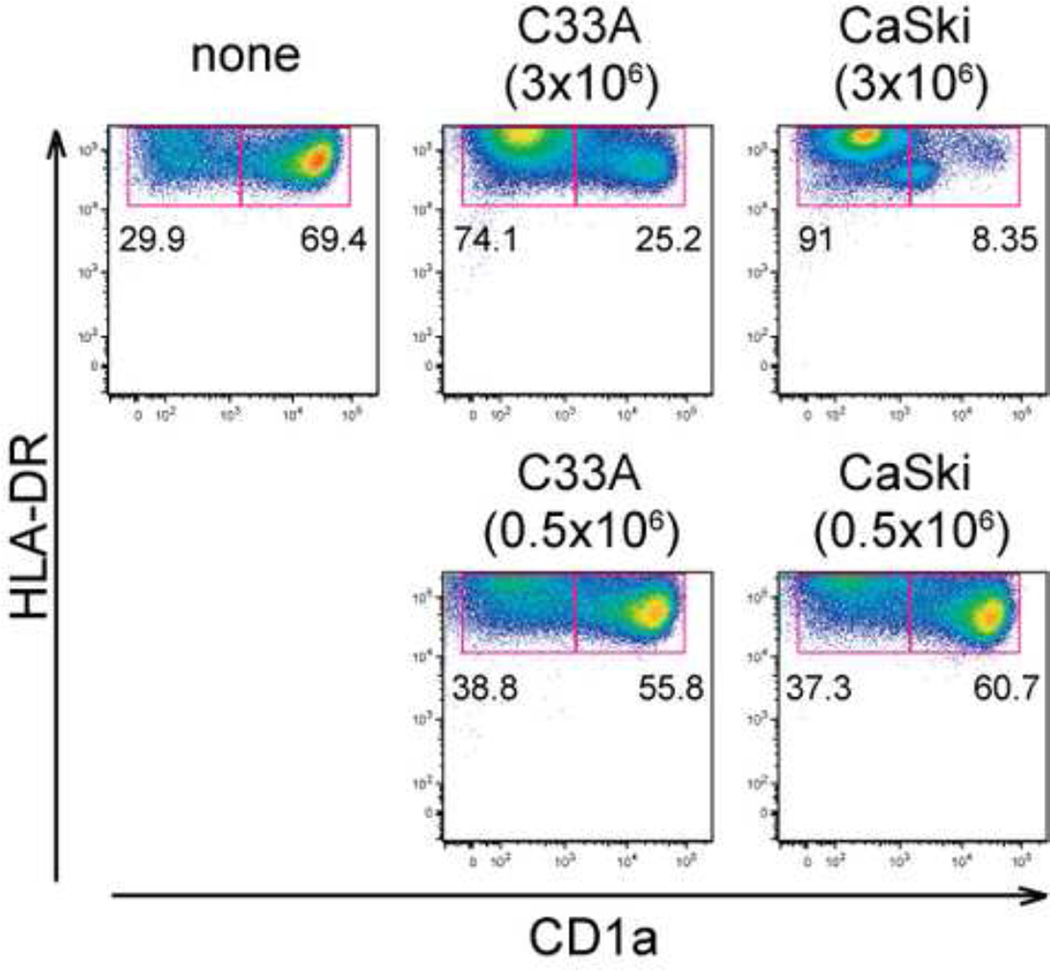
The epithelial layer in a carcinoma in situ would be expected to provide a microenvironment in which the monocytes would encounter a very high density of transformed keratinocytes. Indeed, inhibition of monocyte differentiation to LCs required a high density of cervical carcinoma cells (3 ×106 cells/T12.5 flask), because incubation of monocytes with a low density of cancer cells (0.5 ×106 cells/flask) did not block LC differentiation (Figure 2). These data indicated that HPV-positive carcinoma cells above a certain density threshold appeared to robustly inhibit monocyte differentiation to LCs.
Figure 2. Block of monocyte-to-LC differentiation by cancer cells occurs at high density.
Differentiation of LC from CD14+ human monocytes (1 × 106 cells/flask) in the presence of low (0.5 × 106 cells/flask) or high (3 × 106 cells) densities of cancer cell lines (C33A cells or CaSki cells) was compared. On day7, expression levels of CD1a and HLA-DR were analyzed by FACS. These data are representative of at least three separate experiments.
Ectopic HPV16 E6 expression inhibits monocyte-to-LC differentiation
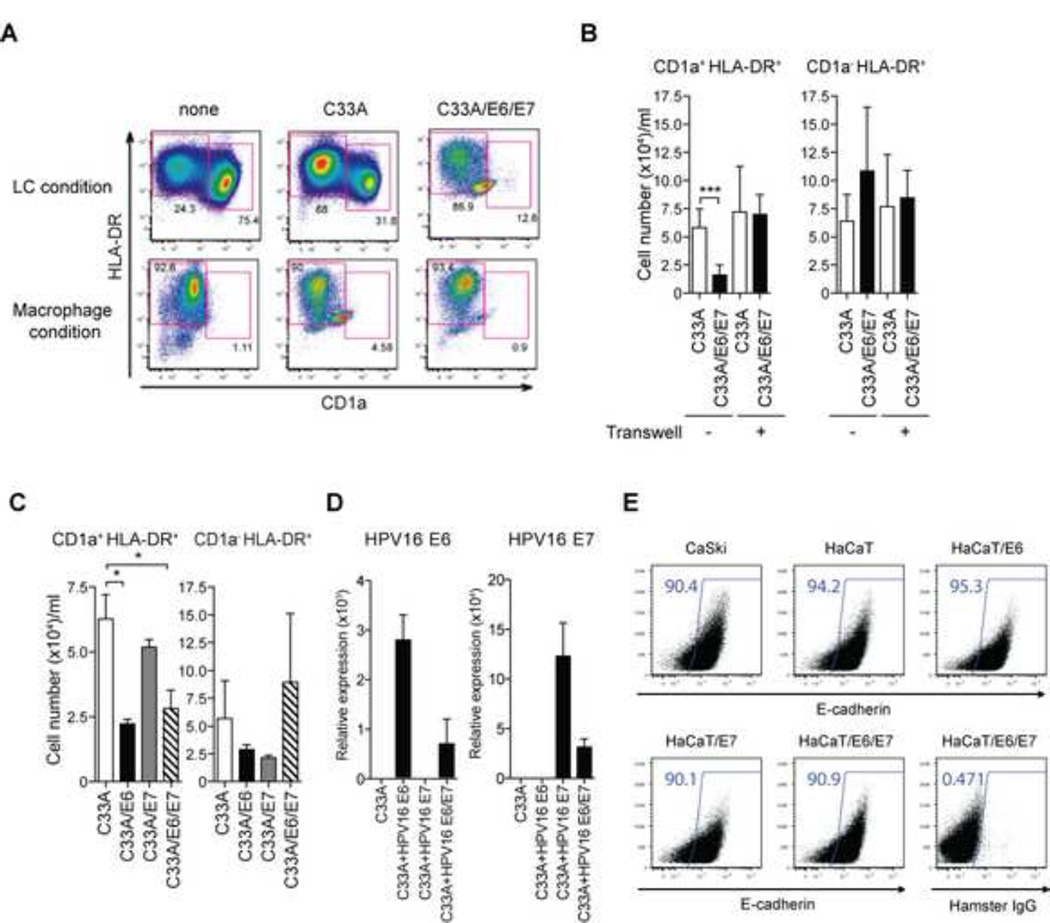
Cervical carcinoma cells express HPV E6 and E7 oncogenes. To determine if E6 and E7 expression is sufficient to reproduce the inhibitory effects of HPV-positive cancer cell lines on monocyte differentiation, the HPV-negative C33A cervical carcinoma cell line was transduced with E6 and E7 genes from high-risk HPV16 to generate C33A/E6/E7 cells. Monocytes incubated with the parental C33A differentiated into LCs (31.8% LC), whereas incubation with C33A/E6/E7 cells reduced LC frequency (to 12.6% LC) (Figure 3A). This inhibitory effect was not due to general toxicity of the E6/E7 cell line, since differentiation of monocytes into macrophages (CD1a− HLADR+) (in the presence of GM-CSF alone) was the same regardless of the presence of C33A cells expressing E6 and E7 (Figure 3A).
Figure 3. High-risk HPV16 E6 but not E7 impairs the differentiation of LCs from blood CD14+ monocytes in a contact-dependent manner.
CD14+ monocytes were co-incubated with UV-treated HPV− C33A cells containing the empty vector (C33A) or HPV16 E6 and E7-transduced C33A cells in the presence of GM-CSF/IL-4/TGF-β (LC condition) or GM-CSF (Macrophage condition) for 7 days. Cells were analyzed by flow cytometry for the expression of CD1a and HLA-DR. (B) CD14+ monocytes were co-incubated with UV-treated transduced C33A cells either together or separately in Transwell. The average numbers of differentiated CD1a+ HLA-DR+ LCs obtained from at least three donors are depicted. (C) CD14+ monocytes were co-incubated with UV-treated vector-transduced or HPV16 E6 and/or E7-transduced C33A cells in the presence of GM-CSF/IL-4/TGF-β for 7 days. The average numbers of LCs cultured in the presence of C33A transduced with the indicated viral genes obtained from at least three donors are depicted. (D) Expression levels of HPV16 E6 and/or E7 in C33A transduced with the indicated viral genes were analyzed by quantitative RT-PCR. (E) C33A, Caski, HaCaT or HaCaT expression HPV16 E6 and/or E7 were stained with antibody to E-cadherin or isotype control. Error bars indicate SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Next, to determine if cell-to-cell contact was required for inhibitory effect of HPV-positive cancer cells on monocyte differentiation, monocytes were co-cultured with C33A cells or C33A/E6/E7 cells, either together or separated by the permeable membrane in a Transwell insert (Figure 3B). While direct cell contact reduced LC frequency from 34% to 13% in an E6/E7-dependent manner, Transwell separation completely blocked the ability of C33A/E6/E7 to inhibit monocyte differentiation into LCs (Figure 3B). These results indicated that cell-to-cell contact is required for the inhibitory effect of E6/E7-expressing cancer cells.
Next, we tested the individual ability of E6 and E7 to inhibit monocyte differentiation. Human peripheral blood monocytes were cultured in the presence of C33A transduced with HPV 16 E6 (C33A/E6), E7 (C33A/E7) or both (C33A/E6/E7). A significant and comparable reduction in LC number (Figure 3C) was observed in monocytes cultured with C33A expressing E6 or E6/E7, but not E7 alone. Expression levels of the transduced genes were confirmed by quantitative RT-PCR (Figure 3D). These data indicated that, albeit much less efficient than HPV-transformed cervical carcinoma cell lines (Figure 1), high-risk HPV E6 is specifically capable of inhibiting monocyte-to-LC differentiation.
Previous studies indicate that surface E-cadherin expression is suppressed to some extent by HPV E6, possibly leading to impaired LC retention within the infected epidermis (23). However, we did not observe significant changes in the E-cadherin levels between Caski cells (HPV16+) compared to HaCaT cells (HPV-negative keratinocyte cells line), and between control HaCaT cells compared to HaCaT cells expressing E6, E6 + E7 or (Figure 3E). Thus, E-cadherin downregulation is unlikely to explain LC blockade by E6 in our experimental setting.
HPV16 E6 promotes endocytic uptake of antigens by differentiated cells
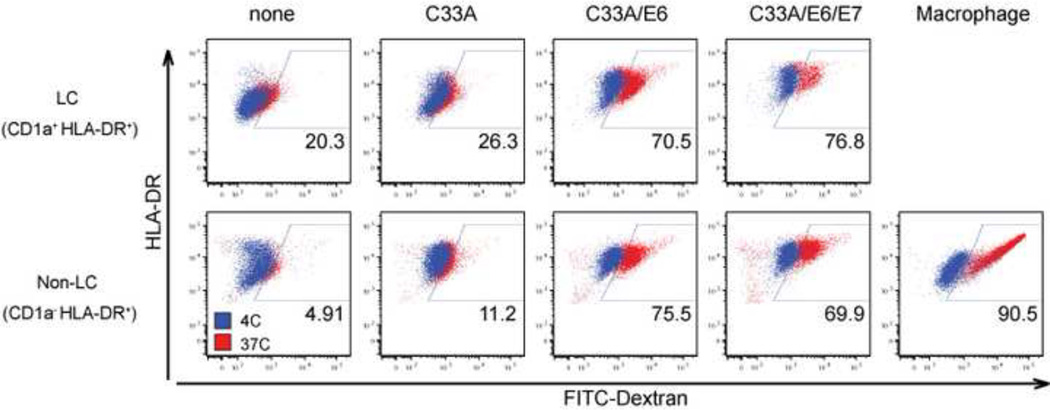
In addition to blocking LC differentiation, co-culture of monocytes with cancer cells expressing E6 may cause them to adopt an altered functional state. To determine if this is the case, we compared the capabilities of E6-conditioned LC-like cells to take up antigens. To this end, the extent of endocytic uptake of FITC-dextran was compared between LC-like cells derived in the presence of C33A, C33A/E6 or C33A/E6/E7. Highly phagocytic macrophages were included as a positive control. Flow cytometric analysis indicated that only a small fraction of monocytes cultured alone or in the presence of C33A cells took up FITC-dextran, and the amount taken up by these cells was low. In contrast, a majority of monocytes cultured in the presence of C33A cells expressing E6 or E6/E7 took up high levels of FITC-dextran (Figure 4). Even the non-LC cells (CD1a− HLADR+) exposed to C33A cells expressing HPV E6 showed similarly high levels of endocytic activity. Incubation of cells with FITC-dextran at 4°C resulted in no positive signals, indicating that an active endocytosis process, and not surface adhesion, is responsible for the observed FITC signal. These data indicated that monocytes that differentiate in the presence of HPV E6 expressing cancer cells exhibit an altered phenotype from wild-type LC in being more endocytic.
Figure 4. Increased phagocytic capacity of monocytes differentiated in the presence of HPV16 E6-transduced cell line.
CD14+ monocytes were co-incubated with UV-irradiated HPV-negative (C33A), HPV16 E6 C33A cells, or HPV16 E6 + E7 C33A cells in the presence of GM-CSF/ IL-4/TGF-β (LC condition) or GM-CSF (Macrophage condition) for 7 days. (A) Differentiated monocytes were incubated with FITC-Dextran for 1 hour at 37°C or 4°C. Phagocytic activity of CD1a+HLA-DR+ (upper row) and CD1a− HLADR+ (bottom row) populations at 37°C (red dots) and 4°C (blue dots) was analyzed by flow cytometry. These data were representative of three separate experiments.
Discussion
Immune elimination of virally-infected cells requires robust CD8 T cell responses and a population of local DCs to elicit recall responses from effector T cells (8–10). A number of studies have established the paucity of Langerhans’ cells within cervical cancer and precancerous lesions, but the molecular basis for this defect is not well understood. Our data demonstrate that HPV-transformed cancer cell lines directly inhibited the ability of monocytes, the precursor for LCs in the squamous epithelial layer, to differentiate into antigen-presenting LCs. This inhibition required cell contact between HPV-positive cancer cells and monocytes. This effect was likely mediated via cell surface interactions as even UV or formaldehyde-killed HPV containing cells retained full repressive activity. This activity also depended on the HPV E6 but not E7 oncogenes. Further, monocyte-derived LCs that were exposed to cells expressing HPV-16 E6 acquired higher levels of endocytic capacity compared to those differentiated in the presence of parental cell line.
Our data suggest that monocytes within the HPV-positive lesions fail to differentiate into antigen presenting cells and fail to support effector T cell recall response in situ. Currently, the mechanism by which high-risk HPV E6 protein exerts inhibitory effects on monocyte differentiation is unknown. E6 likely mediates the inhibition of monocyte differentiation via a surface expressed factor, since cell-to-cell contact was required for E6-expressing cells to inhibit monocyte differentiation to LCs. In addition to blocking differentiation, HPV-positive cancer cell lines also appeared to induce death of precursor monocytes as well as differentiating cells, resulting in general decrease in cell yield (Figure 1). Future studies are needed to identify the E6 target(s) responsible for inhibition of monocyte-to-LC transition.
Our findings reveal an important axis of immunosuppression by HPV-infected cells, at the level of differentiation of antigen-presenting cells. Inhibition of immunosurveillance enforced by blockade of LC differentiation provides the virus with the chance to replicate in a more protected environment than would exist in the normal stratified squamous epithelium. Thus, in addition to the previously-described mechanisms of LC exclusion including impairment in recruitment and retention of LCs, blockade of LC differentiation within the cervical intraepithelial neoplasia could contribute to immune evasion of HPV-infected cells. As a consequence of these viral evasion strategies, HPV-infected keratinocytes might accumulate mutations with little immunological pressures that eventually lead to carcinogenic progression. Future strategies to restore LC differentiation in the HPV-infected cervical intraepithelial neoplasia may be key to successful implementation of immunotherapeutic intervention against HPV infections and the cancers they cause.
HPV-transformed cells inhibit monocyte differentiation into Largerhans cell in culture.
Inhibition of monocyte differentiation requires cell-to-cell contact between monocytes and HPV positive cervical cancer cells.
Ectopic expression of HPV E6, but not E7, confers this inhibitory activity.
Acknowledgement
We thank Peter Howley, Elizabeth White and Justin Garyu for helpful discussions and M. Sasai for technical help. This work is supported by Yale Cancer Center multi-investigator pilot award and Yale Cancer Center Pilot Research Grant to A. I., a generous donation from Ms. Laurel Schwartz, and grants from the NIH to AI (AI054359, AI062428), and D.D. (CA16038). N. I. is a recipient of the study-abroad grant of Kanae Foundation for the promotion of medical science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009 Feb 20;384(2):260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J. The papillomavirus life cycle. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005 Mar;32(Suppl 1):S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010 Aug;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magaldi TG, Almstead LL, Bellone S, Prevatt EG, Santin AD, DiMaio D. Primary human cervical carcinoma cells require human papillomavirus E6 and E7 expression for ongoing proliferation. Virology. 2012 Jan 5;422(1):114–124. doi: 10.1016/j.virol.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gainey MD, Rivenbark JG, Cho H, Yang L, Yokoyama WM. Viral MHC class I inhibition evades CD8+ T-cell effector responses in vivo but not CD8+ T-cell priming. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):E3260–E3267. doi: 10.1073/pnas.1217111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005 May;22(5):561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zammit DJ, Lefrancois L. Dendritic cell-T cell interactions in the generation and maintenance of CD8 T cell memory. Microbes Infect. 2006 Apr;8(4):1108–1115. doi: 10.1016/j.micinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008 Jan 11;319(5860):198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 11.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of experimental medicine [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] 2008 Dec 22;205(13):3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayati AR, Zulkarnaen M. An immunohistochemical study of CD1a and CD83-positive infiltrating dendritic cell density in cervical neoplasia. Int J Gynecol Pathol. 2007 Jan;26(1):83–88. doi: 10.1097/01.pgp.0000225850.90115.bc. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Munoz-Molina R, Balderas-Carrillo O, de la Luz Diaz-Soberanes M, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology. 2006 Feb;117(2):220–228. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubert P, Caberg JH, Gilles C, Bousarghin L, Franzen-Detrooz E, Boniver J, et al. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre)neoplastic lesions. J Pathol. 2005 Jul;206(3):346–355. doi: 10.1002/path.1771. [DOI] [PubMed] [Google Scholar]
- 15.Uchimura NS, Ribalta JC, Focchi J, Simoes MJ, Uchimura TT, Silva ES. Evaluation of Langerhans' cells in human papillomavirus-associated squamous intraepithelial lesions of the uterine cervix. Clin Exp Obstet Gynecol. 2004;31(4):260–262. [PubMed] [Google Scholar]
- 16.Connor JP, Ferrer K, Kane JP, Goldberg JM. Evaluation of Langerhans' cells in the cervical epithelium of women with cervical intraepithelial neoplasia. Gynecol Oncol. 1999 Oct;75(1):130–135. doi: 10.1006/gyno.1999.5559. [DOI] [PubMed] [Google Scholar]
- 17.Viac J, Guerin-Reverchon I, Chardonnet Y, Bremond A. Langerhans cells and epithelial cell modifications in cervical intraepithelial neoplasia: correlation with human papillomavirus infection. Immunobiology. 1990 Jun;180(4–5):328–338. doi: 10.1016/s0171-2985(11)80296-2. [DOI] [PubMed] [Google Scholar]
- 18.Hughes RG, Norval M, Howie SE. Expression of major histocompatibility class II antigens by Langerhans' cells in cervical intraepithelial neoplasia. J Clin Pathol. 1988 Mar;41(3):253–259. doi: 10.1136/jcp.41.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Poole IC, Elmasri WM, Denman CJ, Kroll TM, Bommiasamy H, Lyons Eiben G, et al. Langerhans cells and dendritic cells are cytotoxic towards HPV16 E6 and E7 expressing target cells. Cancer Immunol Immunother. 2008 Jun;57(6):789–797. doi: 10.1007/s00262-007-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. Journal of virology. 2005 Dec;79(23):14852–14862. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S-M, McCance DJ. Down Regulation of the Interleukin-8 Promoter by Human Papillomavirus Type 16 E6 and E7 through Effects on CREB Binding Protein/p300 and P/CAF. Journal of virology. 2002 Sep 1;76(17):8710–8721. doi: 10.1128/JVI.76.17.8710-8721.2002. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleine-Lowinski K, Rheinwald JG, Fichorova RN, Anderson DJ, Basile J, Munger K, et al. Selective suppression of monocyte chemoattractant protein-1 expression by human papillomavirus E6 and E7 oncoproteins in human cervical epithelial and epidermal cells. Int J Cancer. 2003 Nov 10;107(3):407–415. doi: 10.1002/ijc.11411. [DOI] [PubMed] [Google Scholar]
- 23.Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. Journal of virology. 2003 Aug;77(15):8378–8385. doi: 10.1128/JVI.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature immunology. 2002 Dec;3(12):1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, et al. Langerhans cells arise from monocytes in vivo. Nature immunology. 2006 Mar;7(3):265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terlou A, van Seters M, Kleinjan A, Heijmans-Antonissen C, Santegoets LA, Beckmann I, et al. Imiquimod-induced clearance of HPV is associated with normalization of immune cell counts in usual type vulvar intraepithelial neoplasia. Int J Cancer. 2010 Dec 15;127(12):2831–2840. doi: 10.1002/ijc.25302. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin EC, Yang E, Lee CJ, Lee HW, DiMaio D, Hwang ES. Rapid induction of senescence in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):10978–10983. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. Journal of virology. 2003 Jan;77(2):1551–1563. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. The Journal of experimental medicine. 1998 Mar 16;187(6):961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006 Feb;16(1):53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]