Abstract
The production of aerial conidia of Lecanicillium lecanii 41185, a highly virulent fungus, by solid-state fermentation was studied for use as a biocontrol agent against aphids. Among several agro-industrial solid media, steamed polished rice was found to produce the highest amount of aerial conidia. The optimal conditions for aerial conidia production were determined to be a 28.5% moisture content in the rice, 25℃ culture temperature, rice pH of 6.0, 75% ambient relative humidity, 4-dold seeding culture, 0.6% KNO3, and 12 d of culture time. The conidia yield increased from 5.7 × 109 conidia/g polished rice to 18.2 × 109 conidia/g polished rice following application of these optimized conditions.
Keywords: Aerial conidia, Lecanicillium lecanii, Mycoinsecticide, Optimum condition, Solid-state fermentation
Aphids are among the most serious pests throughout the world. Aphids transmit various plants pathogenic viruses and are very difficult to control using organic pesticides alone, because of the development of insecticide resistance and rapid increase in population size. Consumers demand pesticide-free foods and an unpolluted environment. Entomopathogenic fungi that parasitize insects are a valuable weapon for biocontrol and have an important role in integrated pest management (Cooke, 1977; Deshpande, 1999). To date, Lecanicillium spp. has been used to control aphids and insects in the field as well as in the greenhouse (Cuthbertson and Walters, 2005; Jackson et al., 1985; Steenberg and Humber, 1999; Vu et al., 2007). L. lecanii is a common insect pathogen infecting the aphids Trialeurodes vaporariorum, and Macrosiphoniella sanborni (Jackson et al., 1985; Tanada and Kaya, 1993; Meyer and Meyer, 1996; Charnley, 1997; Lee et al., 2002; Khalil et al., 2003; Kim, 2004; Vu et al., 2007). Among the various entomopathogenic fungi, a Korean isolate of L. lecanii 41185 has previously been selected to control the aphids Myzus persicae and Aphis gossypii (Vu et al., 2007) at fluctuating temperatures and relative humidity conditions present in green houses.
Both solid and liquid fermentation systems have been used for mass production of biocontrol agents. Solid-state fermentation (SSF) allows some fungi to produce hard and healthy conidia (Bartlett and Jaronski, 1988). As oil-based formulation for application at ultra-low volume rates requires the production of lipophilic conidia that are easily suspend, the hydrophilic submerged conidia produced from liquid fermentation are not the most favorable (Jenkins et al., 1988). Blastospores produced via submerged liquid fermentation are hydrophilic, but they also have been found to lose their viability quickly during storage (Rombach, 1989; Kleespies and Zimmermann, 1992). Therefore, SSF has been considered the appropriate system for mass production of conidia for use in oil formulation (Jenkins et al., 1988). SSF also has additional advantages when compared to submerged fermentation: it is simpler, requires lower capital, has superior productivity, has a reduced energy requirement, requires simpler fermentation media, does not require rigorous control of fermentation parameters, uses less water and produces lower wastewater, allows easy control of bacterial contamination, and has a lower cost requirement for downstream processing (Pandey, 1994; Babu and Satyanarayana, 1995).
To obtain desirable and fast control of pests by Lecanicillium sp., more than 1 × 108 conidia/ml must be sprayed to control aphids and whitefly (Lee et al., 2002; Kim, 2004; Kim et al., 2007). Other entomopathogenic fungi such as Beauveria bassiana and Metarhizium anisopliae also require high concentrations, on the order of 1 × 108 to 1 × 109 conidia/ml (Feng et al., 1994, 2004; Kirkland et al., 2004) for successful control of pests (Hidalgo et al., 1998; Kaaya and Hassan, 2000; Benjamin et al., 2002). In the case of L. lecanii 41185, however, the control values obtained when applied at 1 × 107 to 1 × 108 conidia/ml were successful, and 1 × 107 conidia/ml was recommended for effective control of aphids (Vu et al., 2007). Therefore, L. lecanicillium 41185 can be used as an economical mycoinsecticide because lower concentrations of conidia are required.
Since the conidial number for spray application is important for proper pest control, it is very desirable to produce as high concentration of aerial conidia as possible while using the same amount of substrate so as to reduce production costs. Therefore, the present study was carried out to determine optimum solid culture medium and culture conditions to produce a high density of aerial conidia of L. lecanii 41185 via SSF.
Materials and Methods
Fungal strain
The entomopathogenic fungus L. lecanii 41185 with a high virulent pathogenicity against aphids M. persicae and Aphis gossypii (Vu et al., 2007), originally isolated from M. persicae and T. vaporariorum in Korea, was used in this study. The fungus was cultured on potato dextrose agar (PDA) at 25℃ and stored at 4℃ until use.
Preparation of liquid seeding culture
For preparation of the inoculum, one plug of 7-d-old (10 × 10 mm2) mycelium grown on a PDA plate was cultivated in a 250 ml Erlenmeyer flask containing 100 ml potato dextrose broth with an initial pH of 5.8 at 25℃ using a rotary shaking incubator operated at 200 rpm.
Solid state fermentation for aerial conidia production
One hundred grams of solid medium was distributed in a 900 ml plastic bottle (62 × 92 × 160 mm3) and autoclaved at 121℃ for 25 min. Once the bottle of moistened solid media was cooled, 10 ml of 3-d-old liquid culture was added and mixed thoroughly using a sterilized spoon. The bottles were plugged with ventilated cap in order to minimize contamination and to allow passive aeration during growth and conidiogenesis. The bottles were then incubated under various conditions of temperature, initial pH, and relative humidity over the desired time period, depending on the experimental protocol. The contents of the bottle was gently shaken every 12 h. Unless otherwise mentioned, these conditions were maintained throughout the experiments.
Selection of substrate
Ten different agro-industrial solid media were examined: wheat bran powder, polished rice, rice bran, rice husks, peat moss, polished rice mixed with rice bran (mixing ratio of 2 : 1, w/w), polished rice mixed with peat moss (2 : 1, w/w), rice mixed with flour (1 : 2, w/w), wheat bran, and polished rice mixed with broken rice (1 : 2, w/w). The solid media were supplemented with distilled water to the desired moisture content of about 30% and sterilized at 121℃ for 25 min. Inoculated solid media were incubated at 25℃ and 50~60% ambient relative humidity (RH). Conidia number/g solid media was determined after 7 to 12 d of incubation.
Effect of moisture content in the polished rice on aerial conidia production
The polished rice was submerged in tap water for 3 h and allowed to drain for 1.0, 2.0, 2.5, 3.0, and 3.5 h to achieve the desired moisture contents of 23.5, 25.5, 27.0, 28.5, and 31.0%, respectively. One hundred grams of moistened rice was dried at 105℃ for 24 h and weighed to determine the water content of the moistened rice. The bottles containing the inoculated rice were then incubated at 25℃ for 7 to 12 d and conidia number/g rice was determined.
Effect of cultivation time on aerial conidia production
Conidia production was conducted at 25℃ and 50~60% RH using steamed polished rice (pH 6.5) containing 28.5% moisture. After 5 d of cultivation and subsequent 1 d intervals, conidial number/g polished rice was determined.
Effect of temperature on aerial conidia production
The inoculated steamed polished rice (pH 6.5) containing 28.5% moisture was incubated at 15℃, 20℃, 25℃, 30℃, and 35℃, with an RH of 50~60%. The conidia number/g polished rice was determined after 12 d of cultivation.
Effect of rice pH on aerial conidia production
The rice submerged in tap water was adjusted to the desired pH (3.0, 3.5, 4.0, 4.5 5.0, 5.5, 6.0, or 6.5) using 10% HCl, left for 3 h, and then drained for 2 h. The inoculated steamed rice samples with various initial pHs were incubated at 25℃ with a RH of 50~60%. After 12 of cultivation, conidia number/g polished rice was determined.
Effect of different ambient RH on aerial conidia production
Plastic bottles containing inoculated steamed polished rice (pH 6.0) were placed in chambers at 25℃ with different ambient RHs of 45%, 60%, or 75% for aerial conidia production. The different constant RHs inside the chamber were maintained using the method of Goettel and Inglis (1997).
Effect of seeding-culture age on the aerial conidia production
Different seeding-culture ages of 2-, 3-, 4-, and 5-d-old were inoculated into sterilized steamed rice (pH 6.0) containing 28.5% moisture at an inoculum size of 10% (v/w). The conidial production process was conducted at 25℃ and 75% RH, and the conidia number/g polished rice was determined after 7 d and 12 d of cultivation.
Effect of inorganic salts on aerial conidia production
Either 0.6 g of KNO3; a mixture of 0.02 g MgSO4 and 0.1 g KHPO4; or a mixture of 0.02 g MgSO4, 0.1 g KHPO4, and 0.6 g KNO3 was added to 100 g sterilized rice (pH 6.0) containing 28.5% moisture and mixed. The sterilized rice along with the various salts was inoculated with 10% (v/w) 4-d-old liquid culture. The aerial conidial production process was conducted at 25℃ and 60% or 75% RH, and the conidial number/g rice was determined after 12 d of cultivation.
Counting conidia
Two grams of conidiated rice was mixed with 18 ml of 0.02% Tween 80. We confirmed that 0.02% Tween 80 was not harmful to aphids or conidial germination in previous experiments. After 60 min of agitation in a 100 ml flask at 200 rpm, the mixture was filtered through three layers of cheesecloth. The number of conidia at the desired dilution was determined using a haemocytometer (Neubauer improved, Superior Marienfeld, Germany).
Statistical program
Data was analyzed using one and two way analyses of variance (ANOVA) (∝ = 0.05) followed by comparison of means using the Duncan's multiple range test (SAS Institute, Cary, NC, USA).
Results and Discussion
Selection of solid substrate
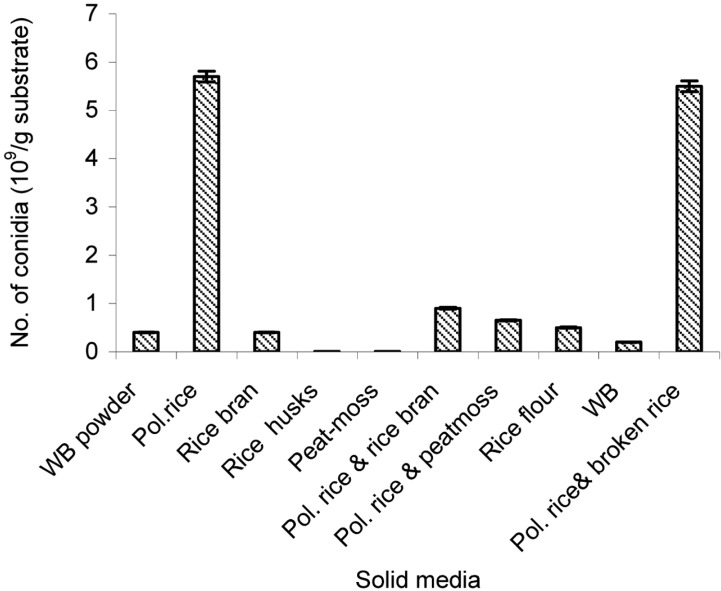
After 12 d of cultivation, the highest amount of aerial conidia was produced using steamed polished rice (5.7 × 109 conidia/g substrate) followed by polished rice mixed with broken rice (4.5 × 109 conidia/g substrate) (Fig. 1). Conversely, the results showed that both rice husk and peat moss were not suitable for production of aerial conidia of L. lecanii 41185. According to Feng et al. (2000), the conidial yield of V. lecanii produced on cooked rice and rice bran was 1.5 × 109 and 1.4 × 109 conidia/g solid culture, respectively. These yields were significantly higher than those on the mixture of rice, rice bran, and rice husk as well as those on rice husk under the same culture condition. The conidia yield was 2.9 × 109 conidia/g dry powder from B. brongniartii grown in mixtures of 70% cotton seed-shell powder, 25% wheat bran, and 5% corn flour (Lin et al., 1988). B. brongniartii was also shown to produce 2 × 109 conidia/g dry powder on mixtures of shelled barley, sunflower oil, and water after 42 days of cultivation (Aregger, 1992). Therefore, the favorable substrate or favorable mixture of substrates for high yield of conidia varied depending on the fungal species.
Fig. 1.
The aerial conidia production of L. lecanii 41185 on different solid culture media. WB, wheat bran; Pol. rice, polished rice. Various steamed substrates with about 30% moisture content were incubated at 25℃ and 50~60% RH for 10 days.
By far the most commonly selected substrate for the production of fungal conidia has been white rice (Alves and Pereira, 1989; Mendonca, 1992; Ibrahim and Low, 1993; Feng et al., 2004). This is probably due to a combination of factors, including nutritional balance, cost, worldwide availability, physical characteristics such as grain size and shape, hydration properties, and its structural integrity even after colonization by fungi (Jenkins et al., 1988).
Effect of rice water content on aerial conidia production
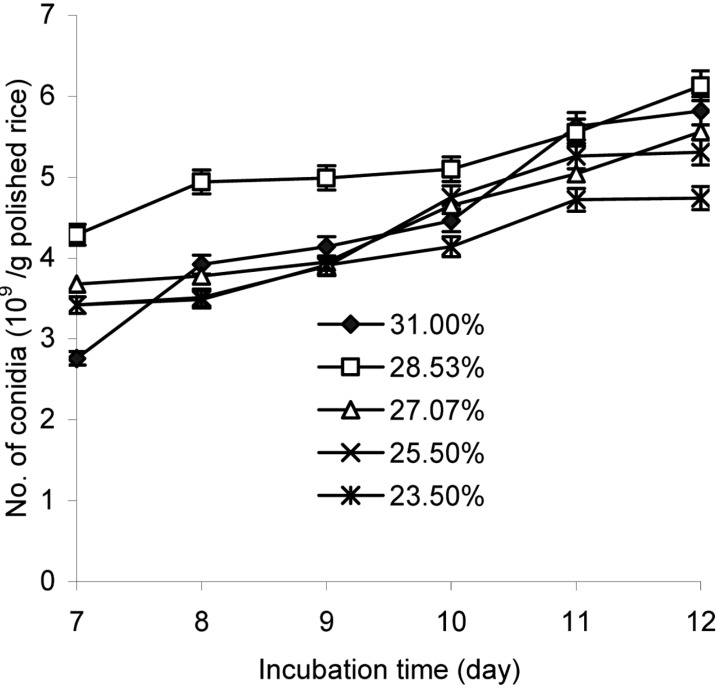
Moisture content in the substrate plays a significant role in the final yield of conidia (Jenkins et al., 1998). Most mitosporic fungi prefer humid conditions; however, different substrates vary in their moisture sorption curves and will reach maximum adsorption at different moisture content levels. In this study, moisture content in rice was depended on the draining time of water from the soaked substrate; where less draining time resulted in increased water content of the rice. The production of aerial conidia of L. lecanii 41185 drastically depended on both moisture content and culture time (P < 0.001) for the duration of conidia production, as depicted in Fig. 2. The conidial yield increased with the length of incubation time. The conidia number produced on rice containing 28.5% moisture was higher than those at other moisture contents, over any culture time, and the highest amount of conidia was 6.13 × 109 conidia/g polished rice at 12 days after inoculation. The amount of conidia was the lowest on the rice containing 23.5% moisture.
Fig. 2.
Effect of rice moisture content on aerial conidia production. The inoculated steamed polished rice with a pH of 6.5 and 28.5% moisture content was incubated at 25℃ and 50~60% RH for 7~12 days.
The moisture content of the medium changes during fermentation as a result of evaporation and metabolic activities, and thus the optimum moisture level of the substrate is very important (Baysal et al., 2003). In another aspect, there is a close relationship between moisture content and oxygen availability; increases in the moisture content of the substrate tend to decrease oxygen availability as the interparticular spaces become filled with water and air is forced out (Moo et al., 1983; Jenkins, 1998).
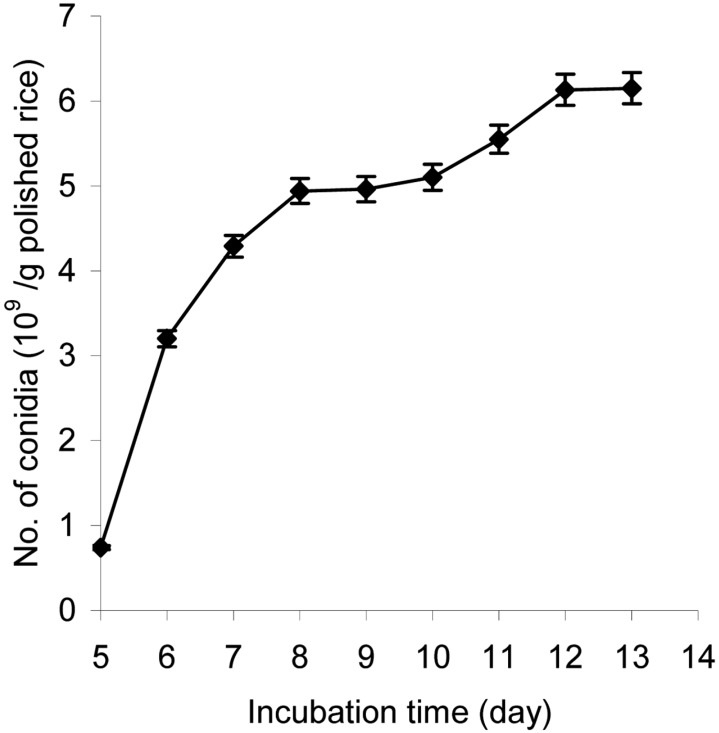
Effect of culture time on aerial conidia production
The conidial yield significantly depended on the culture time as shown in Fig. 3. The longer the incubation time, the higher the conidia yield. Thus by using steamed polished rice containing 28.5% moisture, the conidia yield was 6.13 × 109 conidia/g polished rice after 12 d of cultivation. Further cultivation after 12 d did not increase the conidial number.
Fig. 3.
Effect of cultivation time on aerial conidia production. The inoculated steamed polished rice with a pH of 6.5 and 28.5% moisture content was incubated at 25℃ and 50~60% RH for 7~12 days.
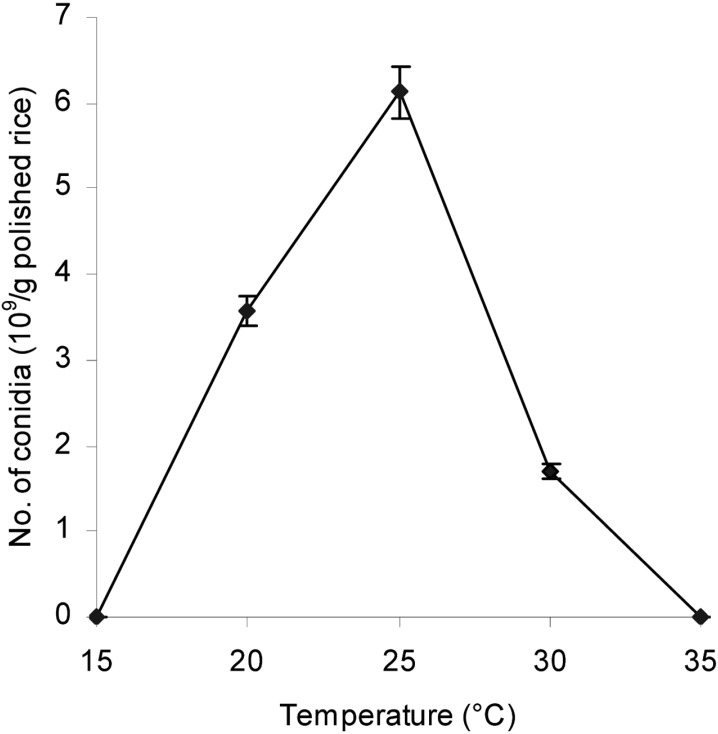
Effect of incubation temperature on aerial conidia production
The result depicted in Fig. 4 indicated that incubation temperature markedly affected the conidial yield. The optimal temperature for conidia production was 25℃, where the conidial number produced on rice was 6.13 × 109 conidia/g polished rice.
Fig. 4.
Effect of temperature on aerial conidia production. The inoculated steamed polished rice with a pH of 6.5 and 28.5% moisture content was incubated at various temperatures and 50~60% RH for 12 days.
Incubation temperatures other than 25℃, as in a low temperature range (15~20℃) or a high temperature range (30~35℃), were not favourable for high conidia production and caused a sharp decline in yields. In this study, the optimal temperature (25℃) for high conidial production matched the optimal temperature for growth of L. lecanii 41185 (Vu et al., 2007). Thomas and Jenkins (1997) have also shown that incubation temperature for the liquid and solid production stages matched the optimal temperature for the germination and mycelial growth of Metarhizium flavoviride.
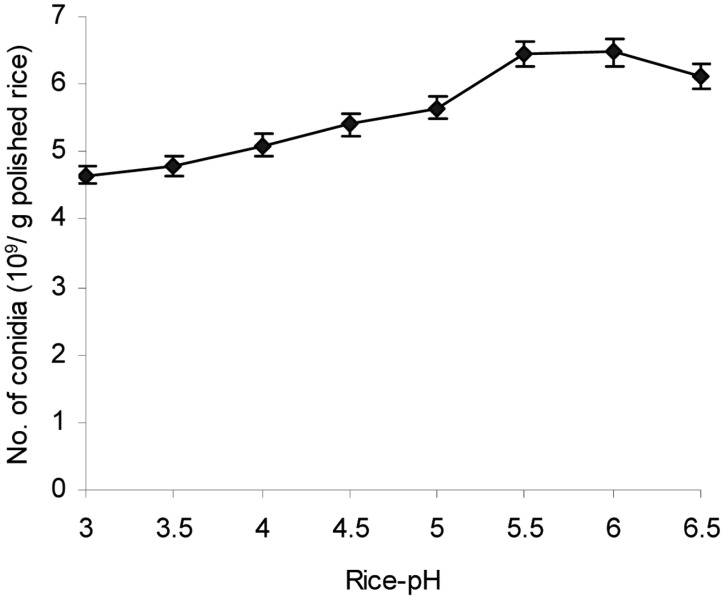
Effect of rice pH on aerial conidia production
pH is one of the factors that influences microbial development during SSF (Doelle, 1985). The results depicted in Fig. 5 indicated that rice pH markedly affected aerial conidia production of L. lecanii 41185 (P < 0.01). Rice pH from 5.5 to 6.0 was optimal for the highest conidia production at 6.45 × 109 and 6.47 × 109 conidia/g polished rice, respectively, after 12 d of cultivation at 25℃. Beyond the range of optimal pH (5.5~6.0) the production of aerial conidia of L. lecanii 41185 declined sharply.
Fig. 5.
Effect of rice pH on aerial conidia production. The inoculated steamed polished rice with different pHs and 28.5% moisture content was incubated at 25℃ and 50~60% RH for 12 days.
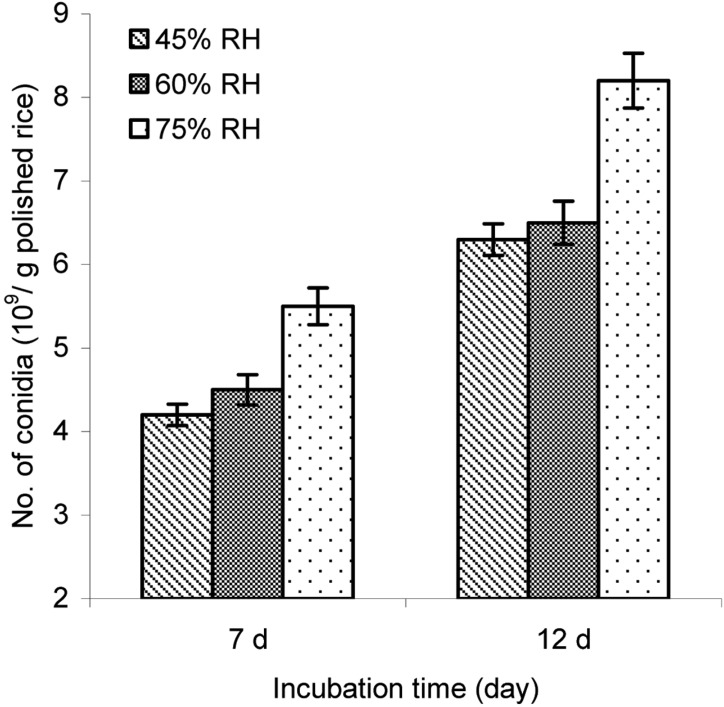
Effect of ambient RH on aerial conidia production
The production of aerial conidia was examined at different ambient RHs, such as 45%, 60%, and 75%, at a rice pH of 6.0. The results in Fig. 6 indicated that the amount of aerial conidia significantly depended on the ambient RH and cultivation time. Amount of conidia produced at 75% RH was significantly higher than that either at 45% or 60% RH (P < 0.05) after 12 d of cultivation.
Fig. 6.
Effect of ambient RH on aerial conidia production. The inoculated steamed polished rice with a pH of 6.0 and 28.5% moisture content was incubated at 25℃ and different percents of RHs for 7 and 12 days.
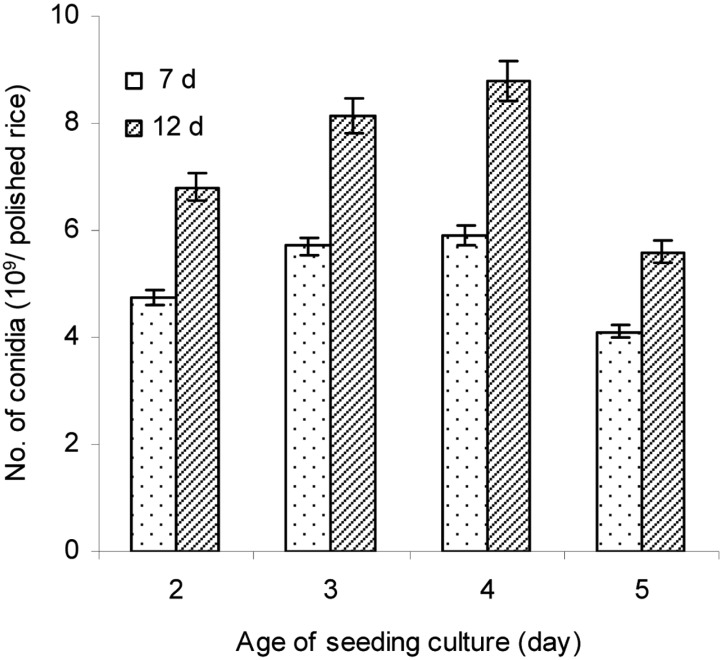
Effect of liquid seeding culture age on aerial conidia production
Different ages of liquid culture were inoculated onto steamed rice (pH 6.0), and then the conidia production was performed at 25℃ and 75% RH. The results in Fig. 7 showed that conidial production significantly depended on both the age of liquid culture and culture time (P < 0.05). The rice inoculated with 4-d-old liquid culture produced significantly higher conidial number (8.79 × 109 conidia/g polished rice) than those of other ages (P < 0.05). The conidial production on rice inoculated with liquid culture of 2-d or 3-d-old culture was similar, but rice inoculated with 5-d-old liquid culture produced the lowest number of aerial conidia. Moreover, the amount of aerial conidia produced on rice after 12 d of cultivation was significantly higher than that at 7 d for all ages of liquid culture, which also supports that conidia yield depended on culture time.
Fig. 7.
Effect of seeding culture age on aerial conidia production. Ten percent (v/w) seeding cultures at different ages were inoculated into steamed polished rice with a pH of 6.0 and 28.5% moisture content, and the inoculated rices were then incubated at 25℃ and 75% RH for 7 and 12 days.
Effect of inorganic salts added to the substrate on aerial conidia production
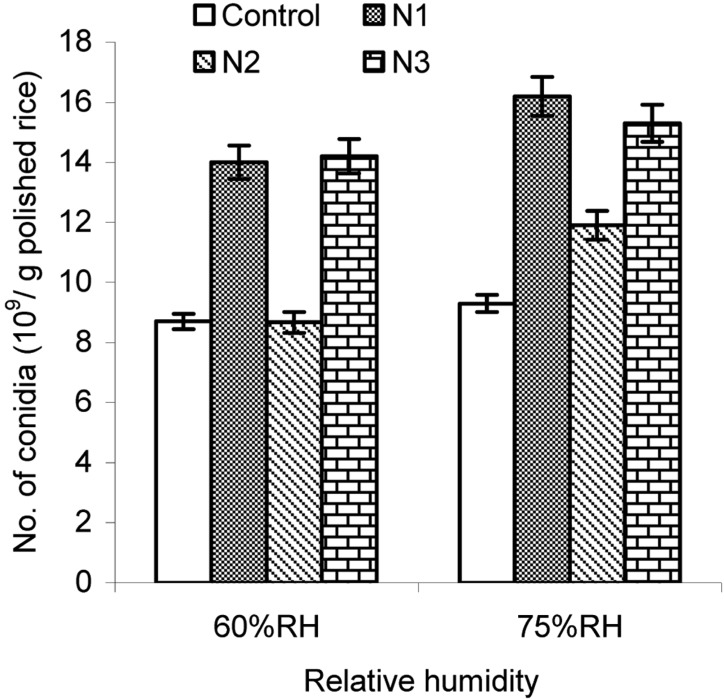
The results showed that the conidia number per gram of polished rice markedly depended on inorganic salts (P < 0.001) (Fig. 8). After 12 d of cultivation at 60% RH, conidial number on steamed rice containing inorganic salts N1 (0.6% of KNO3) or N3 (0.02% of MgSO4, 0.1% of KHPO4, and 0.6% KNO3) was 14.0 × 109 and 14.2 × 109 conidia/g polished rice, respectively. Moreover, conidia yields produced on steamed rice supplemented with either N1 or N3 was significantly higher than that of control (8.7 × 109 conidia/g polished rice) as well as that of polished rice supplemented with N2 (0.02% of MgSO4 and 0.1% of KHPO4) at 8.67 × 109 conidia/g polished rice. The same trend was found at 75% RH where conidia numbers on un-supplemented rice and supplemented rice were higher than those at 60% RH. At this RH, conidial yields on rice supplemented with N1 and rice supplemented with N3 were 16.2 × 109 and 15.3 × 109 conidia/g polished rice, respectively; while control and rice supplemented with N2 the conidial yield was 9.3 × 109 and 11.9 × 109 conidia/g polished rice, respectively.
Fig. 8.
Effect of inorganic salts added to the polished rice on aerial conidia production. N1, N2, and N3 indicate sterilized rice supplemented with 0.6% of KNO3; 0.02% MgSO4 and 0.1% of KHPO4; or 0.02% of MgSO4, 0.1% of KHPO4, and 0.6% KNO3; respectively. Ten percent (v/w) 4-d-old seeding culture was inoculated into steamed polished rice with a pH of 6.0 at 28.5% moisture content in combination with different inorganic salts, and the inoculated rice was then incubated at 25℃ and 60~75% RH for 12 days.
These results indicated that the inorganic salt supplements had an important role in increasing the yield of aerial conidia. In particular, KNO3 played the most important role in stimulating the production of aerial conidia, whereas MgSO4 and KHPO4 has less of an effect on increasing conidial production. This finding was similar to that reported by Thomas et al. (1978), where a nitrogen source like nitrate salts (e.g., KNO3) played the most crucial role in increasing conidia yield.
The aerial conidia production of L. lecanii 41185 at optimal conditions
Aerial conidia production of L. lecanii 41185 was performed under optimal conditions, as established in this study, including a 28.5% moisture content in the polished rice, a 25℃ culture temperature, a rice pH of 6.0, 75% ambient RH, 4-d-old seeding culture, 10% inoculumn size, 0.6% KNO3 as culture additive, and 12 d of culture time. Under these conditions the amount of aerial conidia produced was 18.2 × 109 conidia/g polished rice, which is more than 3-times higher than before optimization. This is also a higher yield than the 1.5 × 109 spores/g cooked rice reported by Feng et al. (2000). If 100 g of conidiated rice containing 18.2 × 109 conidia/g polished rice obtained in the present study is extracted using a suspending solution, a 182-liter conidia suspension containing 1 × 107 conidia/ml can be produced. Before optimization, 100 g of 5.7 × 109 conidia/g rice could produce only 57 liters of conidia suspension at 1 × 107 conidia/ml. This high yield highlights the major advantage of L. lecanii 41185 compared to other Lecanicillium sp., because only 1 × 107 conidia/ml of L. lecanii 41185 is required to effectively control aphids (Vu et al., 2007), while more than 1 × 108 conidia/ml of other Lecanicillium strains are required (Kim, 2004; Kim et al., 2007).
In conclusion, the use of L. lecanii 41185 is very advantageous when compared to other entomopathogenic fungal strains, since it requires a lower concentration of aerial conidia to obtain desirable and fast control of pests. SSF has been considered the appropriate system for mass production of conidia for oil formulation (Jenkins et al., 1988), and the aerial conidia has a higher storage viability than blastospores produced via submerged liquid fermentation (Rombach, 1989; Kleespies and Zimmermann, 1992). SSF also has several additional advantages compared to submerged fermentation (Pandey, 1994; Babu and Satyanarayana, 1995). From the selected solid substrate and optimized conditions in this study, high-yield production of aerial conidia can be achieved by SSF, which makes L. lecanii 41185 an attractive mycoinsecticide.
References
- 1.Alves SB, Pereira RM. Production of Metarhizium anisopliae (Metsch.) Sorok and Beauveria bassiana (Bals.) Vuill. in plastic trays. Ecossistema. 1989;14:188–192. [Google Scholar]
- 2.Aregger E. Conidia production of the fungus Beauveria brongniartii on barley and quality evaluation during storage at 2℃. J Invert Patholog. 1992;5:2–10. [Google Scholar]
- 3.Babu KR, Satyanarayana T. α-Amylase production by thermophilic Bacillus coagulans in solid state fermentation. Process Biochem. 1995;30:305–309. [Google Scholar]
- 4.Bartlett MC, Jaronski ST. Mass production of entomogenous fungi for biological control of insects. In: Burge MN, editor. Fungi in Biological Control Systems. Manchester, UK: Manchester University Press; 1988. pp. 61–85. [Google Scholar]
- 5.Baysal Z, Uyar F, Aytekin C. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 2003;38:1665–1668. [Google Scholar]
- 6.Benjamin MA, Zhioua E, Ostfeld RS. Laboratory and field evaluation of the entomopathogenic fungus Metarhizium anisopliae (Deuteromycetes) for controlling questing adult Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2002;39:723–728. doi: 10.1603/0022-2585-39.5.723. [DOI] [PubMed] [Google Scholar]
- 7.Charnley AK. Entomopathogenic fungi and their role in pest control. In: Wicklow DT, Soderstrom B, editors. The Mycota IV: Environmental and Microbial Relationships. Berlin, Germany: Springer; 1997. pp. 185–201. [Google Scholar]
- 8.Cooke R. The biology of symbiotic fungi. New York: John Willey & Sons; 1977. [Google Scholar]
- 9.Cuthbertson AGS, Walters KFA. Pathogenicity of the entomopathogenic fungus, Lecanicillium muscarium, against the sweet potato whitefly Bemisia tabaci under laboratory and glasshouse conditions. Mycopathologia. 2005;160:315–319. doi: 10.1007/s11046-005-0122-2. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande MV. Mycopesticide production by fermentation: potential and challenges. Crit Rev Microbiol. 1999;25:229–243. doi: 10.1080/10408419991299220. [DOI] [PubMed] [Google Scholar]
- 11.Doelle HW. Biotechnology of solid substrate fermentation in the production of food. ASEAN Food J. 1985;1:10–14. [Google Scholar]
- 12.Feng MG, Poprawski TJ, Khachatourians GG. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: Current status. Biocontrol Sci Technol. 1994;4:3–34. [Google Scholar]
- 13.Feng KC, Liu BL, Tzeng YM. Verticillium lecanii spore production in solid-state and liquid-state fermentations. Biopro and Biosys Eng. 2000;23:25–29. [Google Scholar]
- 14.Feng MG, Chen B, Ying SH. Trials of Beauveria bassiana, Paecilomyces fumosoroseus and Imidacloprid for management of Tialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci Technol. 2004;14:531–544. [Google Scholar]
- 15.Goettel MS, Inglis GD. Fungi: Hyphomycetes. In: Lacey LA, editor. Manual of techniques in insect pathology. London: Academic Press; 1997. pp. 213–249. [Google Scholar]
- 16.Hidalgo E, Moore D, Patourel GL. The effect of different formulations of Beauveria bassiana on Sitophilus zeamais in stored maize. J Stored Prod Res. 1998;34:171–179. [Google Scholar]
- 17.Ibrahim YB, Low W. Potential of mass-production and field efficacy of isolates of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus against Plutella xylostella. Int J Pest Manag. 1993;39:288–292. [Google Scholar]
- 18.Jackson CW, Heale JB, Hall RA. Traits associated with virulence to the aphid Macrosiphoniella sanborni in eighteen isolates of Verticillium lecanii. Ann Appl Biol. 1985;106:39–48. [Google Scholar]
- 19.Jenkins NE, Heviefo G, Langewald J, Cherry AJ, Lomer CJ. Development of mass production technology for aerial conidia for use as mycopesticides. Biocontrol News and Information. 1998;19:21–31. [Google Scholar]
- 20.Kaay GP, Hassan S. Entomogenous fungi as promising biopesticides for tick control. Exp Appl Acarol. 2000;24:913–926. doi: 10.1023/a:1010722914299. [DOI] [PubMed] [Google Scholar]
- 21.Khalil SK, Bartos J, Landa Z. Effectiveness of Verticillium lecanii to reduce populations of aphid under glasshouse and field conditions. Agr Ecosys Environ. 2003;12:151–156. [Google Scholar]
- 22.Kim JJ. Pathological studies of Verticillium lacanii on the cotton aphid control. Chonnam National University; 2004. pp. 29–30. Dissertation for the degree of doctor of philosophy. [Google Scholar]
- 23.Kim JJ, Goettel MS, Gillespie DR. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca fuliginea. Biol Cont. 2007;40:327–332. [Google Scholar]
- 24.Kirkland BH, Cho EM, Keyhani NO. Differential susceptibility of Amblyomma americanum (Acari: Ixodidea) to the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. Biol Cont. 2004;31:414–421. [Google Scholar]
- 25.Kleespies RG, Zimmermann G. Production of blastospores by three strains of Metarhizium anisopliae (Metch.) Sorok. in submerged culture. Biocontrol Science Technol. 1992;2:127–135. [Google Scholar]
- 26.Lee MH, Yoon CS, Yun T, Kim HS, Yoo JK. Selection of a highly virulent Verticillium lecanii strain against Trialeurodes vaporariorum at various temperatures. J Microbiol Biotechnol. 2002;12:145–148. [Google Scholar]
- 27.Lin LX, Yu YC, Hu JW. Study on white grub control by Beauveria brongiartii in soybean field. In: Li YW, Li ZZ, Liang ZQ, Wu ZK, Xu QF, editors. Study and Application of Entomogenous Fungi in China. Vol.1. Beijing, China: Academic Periodical Press; 1988. pp. 135–139. [Google Scholar]
- 28.Mendonca AF. Mass production, application and formulation of Metarhizium anisopliae for control of sugarcane froghopper, Mahanarva posticata, in Brazil. In: Lomer CJ, Prior C, editors. Biological Control of Locusts and Grasshoppers. Wallingford, UK: CAB International; 1992. pp. 239–244. [Google Scholar]
- 29.Meyer SLF, Meyer RJ. Greenhouse studies comparing strains of the fungus Verticillium lecanii for activity against the nematode Heterodera glycines. Fund Appl Nematol. 1996;19:305–308. [Google Scholar]
- 30.Moo Y, Moreira MAR, Tengerdy RP. Principles of solid substrate fermentation. In: Smith JE, Berry DR, Kristiansen B, editors. Fungal Technology. London: Edward Arnold; 1983. pp. 117–144. [Google Scholar]
- 31.Pandey A. Solid-state fermentation. New Delhi, India: Wiley Eastern Limited; 1994. pp. 12–17. [Google Scholar]
- 32.Rombach MC. Production of Beauveria bassiana [Deuteromycotina: Hyphomycetes] sympoduloconidia in submerged culture. Entomophaga. 1989;34:45–52. [Google Scholar]
- 33.Steenberg T, Humber RA. Entomopathogenic potential of Verticillium and Acremonium species (Deuteromycotina: Hyphomycetes) J Invertebr Pathol. 1999;73:309–314. doi: 10.1006/jipa.1998.4841. [DOI] [PubMed] [Google Scholar]
- 34.Tanada Y, Kaya HK. Insect Pathology. San Diego, CA: Academic Press; 1993. pp. 318–387. [Google Scholar]
- 35.Thomas KC, Khachatourians GG, Ingledew WM. Production and properties of Beauveria bassiana conidia cultivated in submerged culture. Can J Microbiol. 1978;33:12–20. [Google Scholar]
- 36.Thomas MB, Jenkins NE. Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper Zonocerus variegatus. Mycol Res. 1997;101:1469–1474. [Google Scholar]
- 37.Vu VH, Hong SI, Kim K. Selection of entomopathogneic fungi for aphid control. J Biosci Bioeng. 2007;104:498–505. doi: 10.1263/jbb.104.498. [DOI] [PubMed] [Google Scholar]