Abstract
Background
Gestation is a critical window for neurodevelopmental vulnerability. This study examined whether the presence of trophoblast inclusions (TIs) in the placenta could serve as a predictor for children at elevated risk for autism spectrum disorder (ASD).
Methods
Placentas were obtained from 117 births in the MARBLES (Markers of Autism Risk in Babies—Learning Early Signs) cohort of families who have one or more previous biological children with ASD, placing their newborn at elevated risk for neurodevelopmental compromise. Control samples were obtained from 100 uncomplicated term pregnancies of multiparous women with one or more typically developing biological children. Frequency of TIs was compared across the two groups.
Results
Placentas from at-risk pregnancies had an eightfold increased odds of having two or more TIs compared with control samples (odds ratio: 8.0, 95% confidence interval: 3.6–18.0). The presence of ≥2 TIs yielded a sensitivity of 41% and a specificity of 92% for predicting ASD risk status, whereas ≥4 TIs yielded a sensitivity of 19%, a specificity of 99.9%, and a positive likelihood ratio of 242 and conservatively predicted an infant with a 74% probability of being at risk for ASD.
Conclusions
Our findings suggest that the placentas from women whose fetuses are at elevated risk for autism are markedly different from control placentas. These differences are manifested histologically as TIs. Their identification has the possibility of identifying newborns at risk for ASD who might benefit from targeted early interventions aimed at preventing or ameliorating behavioral symptoms and optimizing developmental outcomes.
Keywords: ASD, autism, genetics, pathology, placenta, trophoblast inclusions
Autism spectrum disorders (ASDs) are increasingly common, with the Centers for Disease Control and Prevention estimating the prevalence to be approximately 1 in 88 and the diagnosis five times more common among boys (1 in 54) than among girls (1 in 252) (1). Most scientists consider gene × environment interaction to play a prominent role in the etiology of ASDs, with genetic vulnerability (2) setting the stage for environmental influences (3,4). The recurrence risk of ASDs among children with an older affected sibling was recently estimated to be 18.7%, with an almost threefold increased risk for male subjects and an additional twofold increased risk if there was >1 older affected sibling (5). Neurobiological findings suggest that the pathophysiology of ASD originates during fetal development (6,7).
The human placenta mediates interactions between mother and fetus throughout gestation and provides a historical record of maternal physiologic influences on the fetus (8). Its genetic composition is fetal, and thus it provides a proxy for evaluation of morphological patterns in the fetus. Moreover, through its role in regulating the passage of substances between maternal and fetal compartments and production of bioactive proteins and small molecules, the placenta is central to fetal health, survival, and programming (9).
The serendipitous observation of an increased frequency of trophoblast inclusions (TIs) in two placenta consult cases by the corresponding author (H.J.K.), which coincidentally were associated with children with ASD, led to the hypothesis that TIs might be a marker of ASD—much as TIs are a marker for a number of other genetic abnormalities. Trophoblast inclusions are the result of abnormal infoldings of the trophoblast bilayer, either due to an increased number of cytotrophoblasts or a decreased fusion rate of cytotrophoblasts into the overlying syncytial trophoblast layer (10–12). They were first described as a marker of triploid gestations (13) but are now recognized to be common in a wide range of karyotypically abnormal gestations (14) and spontaneous pregnancy losses (15–17). They have not been described in common placental pathologies, including chorioamnionitis, decreased maternal placental perfusion, or chronic villitis. As TIs age, they become calcified and are referred to as calcified TIs (18). Thus, uncalcified TIs are by convention simply referred to as TIs. Our initial retrospective case-control study in 2007 validated the hypothesis and identified a threefold increase in the rate of TIs among children with ASD in comparison with children from the general population (18).
The goal of this study was to determine in a blinded case-control study whether TIs are more common among placentas from newborns at elevated risk for developing ASDs in comparison with those from children born from the general population without a familial predisposition to ASD.
Methods and Materials
Subjects and Study Design
For this analysis, our exposure was defined as presence or absence of enhanced risk for ASD in the offspring of a multiparous woman on the basis of the neurodevelopmental status of one or more previous births. High-risk women were drawn from the MARBLES (Markers of Autism Risk in Babies – Learning Early Signs) Study, a prospective cohort that enrolls mothers of children with ASD in a subsequent pregnancy who are thus at high risk for delivering a child who develops ASD (http://marbles.ucdavis.edu). The MARBLES families are recruited from lists of children receiving services for autism through the California Department of Developmental Services, from other studies at the Medical Investigations of Neurodevelopmental Disorders Institute, and by self-referral. Inclusion criteria for the MARBLES Study included: 1) maternal age at least 18 years; 2) planning a pregnancy or pregnant; 3) one or both parents of the current or planned pregnancy being the biological parent of a child with ASD; and 4) residence within the specified catchment area in California. All 117 archived MARBLES placental specimens (39 ± 1.5 weeks gestation; range 36 + 1 to 41 + 6 weeks) of 146 births between January 11, 2008 and January 10, 2011 were processed. We did not have placental samples from 29 MARBLES births, because either study staff were not able to attend the delivery or placentas were retained by the hospital. Women in the low-risk control group were drawn from a convenience sample of 100 multiparous women delivering at the University of California Davis Medical Center between December 6, 2010 and January 29, 2011 with the following inclusion criteria: 1) at least 18 years of age; 2) a singleton gestation; 3) at term between 39 and 40 6/7 weeks; 4) without medical complications of pregnancy; and 5) with one or more other biological children who were developing typically. Due to limitations imposed by the University of California Davis Medical Center institutional review board, no additional clinical information was permitted to be collected from the control group mothers. The institutional review board of the University of California at Davis and the State of California Committee for the Protection of Human Subjects approved the study, and informed consent was obtained at enrollment.
Placentas were obtained shortly after birth, chilled, and maintained until processing, with a time interval between birth and fixation of 1–24 hours. Randomly selected 3 × 3 cm full-thickness samples were taken, de-identified, and placed in 10% phosphate-buffered formalin for between a few days to 3 years before they were batched and sent for histopathologic evaluation at the Yale Reproductive and Placental Research Unit. This aspect of the study was approved by the Yale University Human Investigation Committee (HIC protocol #1003006495). The pathology team was blinded to risk status. Each placental sample was cut into five equal slices, four of which were placed into 25 × 30 mm cassettes, processed in the Yale Dermatopathology Histology laboratory, embedded in paraffin, microtomed at 5 μm, placed on routine glass slides, and stained with hematoxylin and eosin. The final observable cross-sectional area averaged 315 ± 90 mm2. The 868 resulting slides were randomized (http://randomizer.org) and read in numeric order by an experienced placental pathologist (H.J.K.).
The outcomes of interest were the frequencies of TIs, calcified TIs, and total TIs. As described previously (11,12,18,19), TIs were identified by the presence of central syncytiotrophoblast nuclei surrounded by one or more cytotrophoblasts, always away from the villus edge (Figure 1A–D), whereas TIs with a calcified core were considered calcified TIs (Figure 1E–H). Calcification is a proxy for TI age, because calcified TIs are likely older than TIs. All the chorionic villi on each slide were scanned systematically in a row × row (raster) pattern. The TIs and calcified TIs were counted in each slide and added together to obtain a total TI count. Poor fixation was defined both in a binary fashion as zero versus one or more poorly fixed slides and also as the number of slides (zero to four) that were poorly fixed.
Figure 1.
Trophoblast inclusions (TIs) and calcified TIs (hematoxylin and eosin). Representative progression of TIs and calcified TIs identified in the chorionic villi examined for this study. (A) Single TI with central syncytial trophoblast nuclei (arrow) and circumferential cytotrophoblast (arrow head). Villus core (V) and intervillus space (I). (B) Multiple TIs in same field, some with clearly defined syncytiotrophoblast centers (long arrows) and surrounding cytotrophoblasts (arrow heads) and others with poorly formed centers (short arrows), possibly due to tangential sectioning of the TIs. (C) A TI with dense syncytial trophoblast core (arrow) surrounded by multiple cytotrophoblasts (arrow heads). (D) Very dense TI (arrow) with only a remnant of cytotrophoblast (arrow head). This is the step just before a TI becoming calcified. Fetal vessel containing erythrocytes (R). (E) Central syncytiotrophoblast core has become so dense that only a few nuclear profiles are recognizable (arrow), whereas the surrounding cytotrophoblasts have matured and have begun to fuse and form another layer of syncytiotrophoblasts (arrow heads). Intervillus space (I). (F) A TI with obvious lines of calcification but a central core with syncytiotrophoblast nuclei can still be identified (arrow). Cytotrophoblast remnant (arrow head). Fetal vessel containing erythrocytes (R). (G) A TI with mostly calcified core (arrow), with a few residual syncytiotrophoblast nuclei (arrow head). (H) Almost completely calcified TI with loss of calcified material likely secondary to histologic sectioning (arrow) but still with a few visible syncytiotrophoblast nuclei (arrow heads). Tangential section through a more recently formed TI (*).
Statistical Analysis
We examined the frequency of TIs in the MARBLES group compared with the control group. The TIs were counted and summed across all four slides and analyzed both as a continuous variable and a dichotomous one, with zero to one total TIs being considered a negative placenta and two or more total TIs being considered a positive placenta. This dichotomous cut-point was set a priori on the basis of previous research (18).
Proportions of positive placentas in the at-risk versus control groups were compared with two-tailed Fisher exact probability test. Additional exploratory analyses examined different TI cutoffs, the use of TIs only, calcified TIs only, and total TIs—which were then used to estimate sensitivity and specificity of alternative cutoffs for discriminating between MARBLES and control placentas.
Intra-rater test-retest reliability was established by rereading all four slides from a 10% random subset of the samples (22 of 217 cases, yielding 88 of the original 868 slides) and analyzing for two or more TIs only, calcified TIs only, and total TIs/sample as well as poor fixation between reads. The observed percent agreement across all slides with two or more total TIs/slide was 95% (84 of 88) and per sample was 82% (18 of 22), with per slide and per sample κ values of .58 and .54, respectively, indicating good agreement. The Spearman correlation coefficient for total TI counts between the two reads in a slide-to-slide comparison was .64, indicating that they were moderately to strongly correlated. The intra-rater test-retest reliability for poor fixation was good (κ .59).
Poisson distribution data fitting was performed initially with the population means of TIs in the control and MARBLES groups. The data were fit to a mixture of two Poisson distributions (20), to improve fitting for the MARBLES group. Bayes theorem was then used to update the probability of being in the at-risk population (21).
Logistic regression was performed to determine the level of association between autism risk status and total TIs/placenta, controlling for fixation quality and duration. A mixed-effects multinomial logistic regression model was used to assess whether autism risk status was associated with the relative timing of TI formation, as judged by the presence or absence of calcification, while adjusting for fixation quality for individual slides (up to four/placenta). The three-level categorical dependent variable classified the slide as containing inclusions, calcified inclusions, or no inclusions. Confidence intervals (CIs) for the mixed-effects model were based on the robust sandwich covariance estimator, with a small sample adjustment to ensure accurate coverage (22).
Analyses were carried out with SAS (version 9.3; SAS Institute, Cary, North Carolina) and R software (version 2.14.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
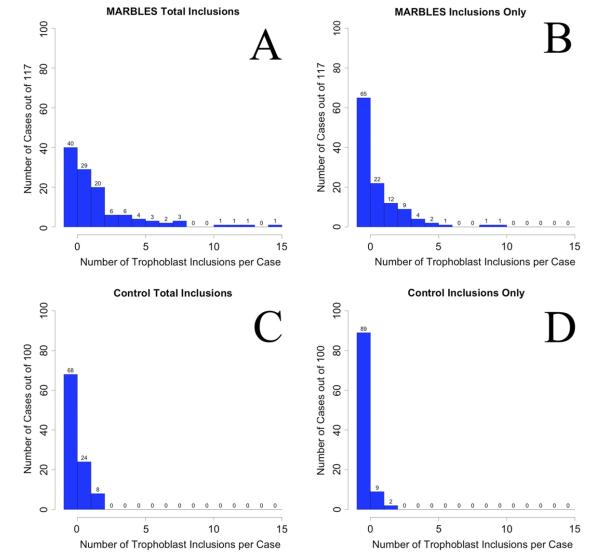
At-risk MARBLES placentas had up to 15 total TIs, whereas control placentas had no more than two TIs, calcified TIs, or total TIs, with 92% having zero or one total TI, which was considered negative by our a priori criteria (Figure 2). The proportion of positive MARBLES cases was significantly higher than that of the control group (41% vs. 8%, odds ratio [OR]: 8.0, 95% CI: 3.6–18.0). The presence of two or more total TIs had a sensitivity of 41% and specificity of 92% for identifying the at-risk MARBLES group. Exploratory analyses were performed with various post hoc definitions of a positive case (Table 1).
Figure 2.
Frequencies of total trophoblast inclusions (TIs) (A, C) and TIs (B, D) in the MARBLES (Markers of Autism Risk in Babies-Learning Early Signs) (A, B) and control (C, D) placentas. There were always more TIs in the MARBLES placentas compared with the control subjects. There were no control cases with 3 or more TIs, whereas there were 28 cases with three or more total TIs, and 1 case with a total of 15 TIs, from the MARBLES placentas.
Table 1.
Frequencies and Statistics of TIs, Calcified TIs, and Total TIs in MARBLES and Control Placentas
| MARBLES (High Risk) |
Control Subjects |
|||||||
|---|---|---|---|---|---|---|---|---|
| M+ | M− | C+ | C− | Sens | Spec | p a | OR | |
| TIs cutoffb | ||||||||
| 1 or more | 52 | 65 | 11 | 89 | 44 | 89 | 4 × 10−8 | 6.5 |
| 2 or more | 30 | 87 | 2 | 98 | 26 | 98 | 4 × 10−7 | 16.9 |
| Calc TIs cutoff | ||||||||
| 1 or more | 55 | 62 | 24 | 76 | 47 | 76 | .0006 | 2.8 |
| 2 or more | 26 | 91 | 3 | 97 | 22 | 97 | .00003 | 9.2 |
| Total TIs cutoff | ||||||||
| 1 or more | 77 | 40 | 32 | 68 | 66 | 68 | 8 × 10−7 | 4.1 |
| 2 or morec | 48 | 69 | 8 | 92 | 41 | 92 | 2 × 10−8 | 8 |
C+, low-risk control subjects that are positive; C−, low-risk control subjects that are negative; M+, positive MARBLES (Markers of Autism Risk in Babies—Learning Early Signs) placentas; M−, negative MARBLES placentas.
p values calculated with two-tailed Fisher exact probability test.
Various cutoffs for trophoblast inclusions alone (TIs), calcified TIs alone (Calc TIs), and TIs plus calcified TIs (Total TIs) were tested to generate post hoc sensitivities (Sens), specificities (Spec), p values, and odds ratios (ORs).
Our a priori cutoff.
Because obstetrical characteristics differed between cases and control subjects, we performed additional analyses to determine the impact of these covariates on the core relationship between TIs and ASD risk. First, there was no association among cases between TI status and pregnancy complications (preeclampsia, gestational diabetes, or hypertension), either collectively (p = 1.0) or individually (preeclampsia: p = .3; gestational diabetes: p = .77; hypertension: p = 4). Second, there was no correlation between TI status among cases that delivered at <39 or ≥40 weeks (or cases where the gestational age at delivery was not known) and the absence or presence of two or more total TIs (p = .83). Finally, when the frequency of two or more total TIs in the control group was compared with the subset of MARBLES cases that exactly matched the inclusion and exclusion criteria of the control group, the association between TIs and ASD risk was stronger than in the unrestricted analysis (50% vs. 8%; OR: 11.5, 95% CI: 4.0–33.0; p = 4.9 × 10−6; sensitivity of 50% and specificity of 92%, resulting in a positive likelihood ratio of 6.25, 95% CI: 2.9–13.5).
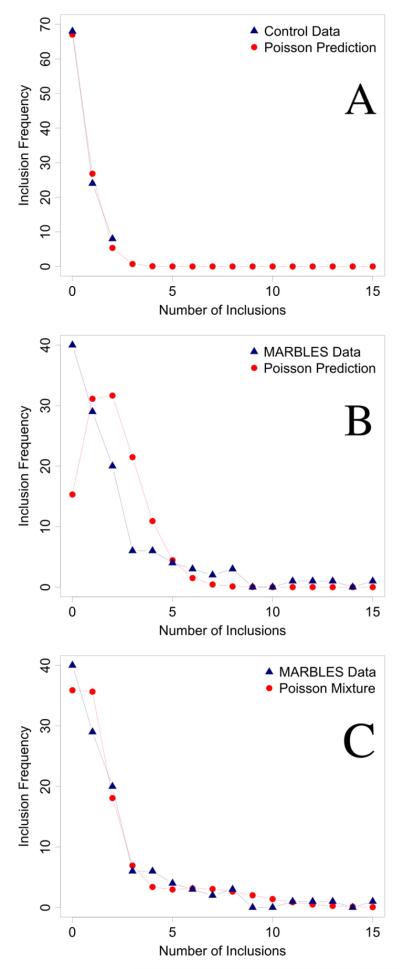
The rarity of TIs in the control group suggested that these data might be best fit to a Poisson distribution. The mean number of total TIs in the control group was calculated to be .4 TIs/case, which generated a Poisson distribution that showed a very close fit to the control population (Figure 3A). However, when the mean number of total TIs in the MARBLES population (2.03 TIs/case) was used to estimate expected frequencies for each value of TIs on the basis of the assumption of a Poisson distribution, the fit was poor (Figure 3B), likely reflecting the heterogeneous nature of the at-risk group. This was validated when we successfully fit the MARBLES data to a mixture of two Poisson distributions with distinct means (Figure 3C).
Figure 3.
(A) The frequencies of total trophoblast inclusions (TIs) in the control placentas was very closely fit by a single Poisson distribution with the sample mean of .4 TIs/case (χ2 = .86, p = .65, 2 degrees of freedom with counts at and above two combined together). (B) The frequencies of TIs in the MARBLES (Markers of Autism Risk in Babies-Learning Early Signs) placentas did not fit a single Poisson distribution when using the sample mean of 2.03 TIs/case (χ2 = 71.4, p < .001, 5 degrees of freedom with counts at and above five combined together). (C) When a two-component mixture of Poisson distributions was fit (with mixing weights of .82 and .18 and means of .99 and 6.88 TIs/case for the two component distributions, respectively) a much closer fit was achieved (χ2 = 4.07, p = .25, 3 degrees of freedom with counts at and above five combined together). All estimates were maximum likelihood.
We were able to estimate, with the Poisson distribution for the control placentas, the expected number of cases with total TI cutoffs of three and four and compute specificities (Table 2). These higher cutoffs yielded lower sensitivities to identify the at-risk population but significantly higher specificities (99% and 99.9%) and positive likelihood ratios (30.2 and 242) for cutoffs of three and four total TIs, respectively.
Table 2.
Total TI Frequencies in MARBLES Placentas Compared With Poisson Predicted Frequencies in Control Placentas
In a logistic regression analysis controlling for quality and duration of fixation, placentas from children with an older sibling with ASD were at much higher risk of having two or more total TIs compared with control placentas (adjusted odds ratio [aOR]: 10.37, 95% CI: 3.86–27.87). Although there were more poorly fixed placentas in the control group (43%) compared with the MARBLES group (2%), the frequency of two or more total inclusions in the poorly fixed control placentas (4 of 43 = 9%) was similar to the frequency in the normally fixed control placentas (4 of 57 = 7%), confirming that inclusions could be identified equally well in poorly versus normally fixed placentas. In the multinomial logistic regression analysis assessing the influence of TI age (adjusted for fixation quality), placentas from the at-risk births had higher odds than those from the control births for one or more calcified TIs (aOR: 5.58, 95% CI: 2.30–13.52) and for one or more TIs (aOR: 3.81, 95% CI: 1.21–11.97).
Discussion
One in 88 children has ASD in the United States (1), underscoring the need to understand pathophysiologic mechanisms and initiate primary and secondary prevention programs for this growing population. Trophoblast inclusions, which are the result of an abnormal balance of placental cytotrophoblast proliferation and differentiation (11,12), were five times more frequent among MARBLES placentas than control placentas, suggesting a role for increased cellular proliferation during fetal life that could result in neurodevelopmental changes. Furthermore, the finding of an increase in both TIs and calcified TIs—which form months before delivery—suggests that at-risk pregnancies are likely influenced by factors that foster TI development throughout gestation. It is important to restate that this study associated TIs with risk for ASD rather than ASD itself. Furthermore, TI presence was not found to be better than family history, and our study was not designed to address families without a prior affected child. However, our previous retrospective study showed a significantly greater frequency of TIs in placentas from validated ASD children compared with control subjects (18). Taken together with our previous work, our current findings open the door to potential primary prevention mechanisms perhaps aimed at maternal metabolic and dietary factors, should such mechanisms be further elucidated. More importantly, the finding that four or more total TIs in a four-slide review of placental histology accurately predicts children at increased risk for ASD with a specificity of 99.9% provides a simple test available at birth that could allow for identification of these at-risk children and implementation of targeted early interventions to maximize functional outcomes.
Currently, children who develop ASD are not identifiable in utero or early in infancy but instead manifest a progressive and changing constellation of signs and symptoms with variable patterns of onset that by age 3 years meet ASD criteria on the basis of DSM or ICD codes. Heterogeneity in onset patterns, behaviors, and cognitive abilities complicates the search for consistent and reliable prodromal signs (23,24). Presently, the best early marker of ASD risk is family history, with the recurrence rate for couples with one or more affected children recently ascertained prospectively to be 18.7% (5). Higher risk was established for two subgroups, with male infants having nearly a threefold increased chance of developing ASD and children with multiple older affected siblings having an additional two fold risk amplification. Families with a hereditary predisposition for ASD can consider various options when completing their families, including carefully monitoring at-risk subsequent children and employing early intervention strategies to maximize outcomes. Regrettably, couples without known genetic susceptibility must rely on identification of prodromal preclinical signs or indicators that might not manifest until the second or third year of life of the child. Practice guidelines from the American Academy of Pediatrics recommend that all children undergo screening for autism at 18 months of age (25), by which time a considerable amount of neurological plasticity and opportunities for intervention to maximize outcome has been lost.
The primary motivation for identifying enhanced risk and early signs of ASD emanate from the wish to employ interventions that might change the course of the emerging developmental abnormality (26,27). Early enthusiasm for behavioral interventions in children with ASD intensified after a small nonrandomized treatment trial reported that 47% of those who received rigorous intervention achieved normal educational and intellectual functioning in comparison with 2% in the control group (28). Methodological concerns hampered the interpretation of many subsequent studies of early intensive behavioral intervention variations, with improvements in cognitive performance, language skills, and adaptive behavior skills observed in some young children with ASD (29,30). Recent evidence from a randomized controlled trial in the greater Seattle area reported that comprehensive early intervention for toddlers with ASD resulted in substantial gains in cognitive and adaptive behavior and reduced severity of ASD diagnosis (26).
Our initial 2007 retrospective study demonstrating an association of ASD with an increased frequency of placental TIs held out the potential for an ASD diagnosis at the time of birth (18). Findings from our current blinded prospective study validate these earlier results, although the prediction in this case relates to risk for ASD rather than the actual diagnosis of ASD. Once all the children in our MARBLES group are given final diagnoses, we will be able to compare TI frequency with specific neurodevelopmental outcomes. Nevertheless, our current study did demonstrate that even with as few as four total TIs a clinically strong positive likelihood ratio of 242 for ASD risk is calculated (Table 2). If we conservatively assume, for example, that the pretest probability of being in the at-risk group equals the ASD prevalence of 1.1% (1 of 88 or odds of 1:87), the posttest probability of being in the ASD at-risk population is effectively increased to 74% (posttest odds = pretest odds × likelihood ratio [1:87 × 242 = 242:87], which corresponds to a posttest probability of 242/[242+87] .74) (21).
The association of increased TIs in the placentas of ASD (18) and at-risk newborns suggests a possible common abnormality that is manifested by increased cellular growth and tissue folding in both the placentas (18) and brains of these children (31,32). In children with ASD, the finding of early enhanced brain growth is demonstrated by a steeper than average trajectory in head circumference enlargement during the first year of life (33,34), which might reflect augmented generalized growth processes (35). In addition to increased global growth, there also is clear evidence for abnormal brain folding in ASD, both at the macroscopic (31,36,37) and microscopic levels (32).
Our results suggest that examination of the genes, metabolic, and developmental pathways that regulate cell proliferation, tissue folding, and branching morphogenesis might be fruitful lines to investigate potential etiologic factors for ASD (38–41). Correlating known causes for tissue dysmorphisms, either secondary to endogenous (genetic) (42) or exogenous (environmental or maternal pregnancy related) (43,44) developmental regulators, might be a good starting point for such studies.
For example, abnormal folding in fetal tissues might reflect alterations in the neurohormone serotonin (5-hydroxytryptamine [5-HT]), which has been implicated in autism etiology (45) and targeted in therapeutic strategies (46). Increased circulating 5-HT levels have been identified in approximately 30% of subjects with ASD (45,47) and are associated with repetitive behaviors (48). Linkage studies among individuals with ASD have focused on the 17q11.2 region containing SLC6A4 (49,50) and identified five rare coding variants that seem to confer increased 5-HT transport in multiplex families (51,52). The most common of these variants, Ala56, is associated with both rigid-compulsive behavior and sensory aversion (50,53). Studies in transgenic mice confirm that 5-HT transport variant Ala56 causes hyperserotonemia as well as alterations in social function, communication, and repetitive behavior (54). Coupling these findings back to our study, 5-HT has been shown to be synthesized in the placenta from a maternal tryptophan precursor (55) and to exert a potent mitogenic effect on trophoblasts in the placenta (56). Thus, because increased cytotrophoblast proliferation is likely the key etiology for TI formation (11,19), increased exposure to 5-HT might be involved in cytotrophoblast proliferation in at-risk placentas. Epidemiologic evidence supports these basic science findings. For example, increased placental 5-HT production (57) or exaggeration of placental 5-HT concentrations as the result of maternal use of selective serotonin reuptake inhibitors (58) can alter subtle facets of cortical cell proliferation, migration, and circuit wiring (59). This, together with evidence for a potential role of the functional monoamine oxidase A promoter alleles in ASD (60), suggests a direct role for placental metabolic pathways in modulating fetal brain development and an important function for maternal-placental-fetal interactions and 5-HT in the fetal programming of neurodevelopmental disorders.
We have identified a marker of abnormal trophoblast proliferation that results in easily identifiable placental TIs, which might be useful to identify newborns who are at risk for ASD. Identification of these children might facilitate very early interventions and improved developmental outcomes at a time when the brain is most responsive to modification. Furthermore, once large cohorts of these infants are identified, improved early diagnostic tools can be developed and tested to hone our ability to further identify which of these at-risk children will go on to develop ASD and/or other developmental abnormalities.
Acknowledgments
This work was supported by the National Institutes of Health (1 P01 ES11269 and R01 ES 015359); the U.S. Environmental Protection Agency through the Science to Achieve Results (STAR) program (R829388 and R833292); the MIND Institute at the University of California, Davis; and the Yale University Reproductive and Placental Research Unit.
We thank the staff of the MARBLES study and all the nursing and medical staff of the participating MARBLES hospitals and University of California Davis Medical Center for their assistance in collecting, processing, storing, and shipping all the placentas collected for this study. We thank James D. Dziura, Deputy Director, Yale Center for Analytical Sciences and Herman Chernoff of the Department of Statistics, Harvard University, for their insights and assistance with the statistical analysis, and George M. Anderson of the Yale Child Study Center, Yale University, for helpful discussions. Finally, we thank the staff of the Dermatopathology Laboratory at Yale University for processing the many tissue samples analyzed in this study and for the use of their multiheaded microscope for the examination of all the resultant slides.
HJK conceived the study. CKW and HJK contributed to the study design, execution of the study, interpretation, and report and revision writing. CKW supervised collection of control and MARBLES placentas. KWA and KMM contributed to specimen processing, randomization, database entry, and data management. HJK and KWA contributed to specimen examination and results documentation. SY, DJT, HJK, and CKW contributed to statistical analysis. DJT contributed to the writing of statistical concepts. IH-P, INP, and CKW established the MARBLES Study cohort. HJK created the figures. CKW, DJT, IH-P, and HJK contributed to the writing of the report.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Centers for Disease Control and Prevention Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bello SC. Autism and environmental influences: Review and commentary. Rev Environ Health. 2007;22:139–156. doi: 10.1515/reveh.2007.22.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliman HJ. Trophoblast to human placenta. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. Academic Press; San Diego: 1999. pp. 834–846. [Google Scholar]
- 9.Longtine MS, Nelson DM. Placental dysfunction and fetal programming: The importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29:187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 11.Kliman HJ, Segel L. The placenta may predict the baby. J Theor Biol. 2003;225:143–145. doi: 10.1016/s0022-5193(03)00248-0. [DOI] [PubMed] [Google Scholar]
- 12.Rejniak KA, Kliman HJ, Fauci LJ. A computational model of the mechanics of growth of the villous trophoblast bilayer. Bull Math Biol. 2004;66:199–232. doi: 10.1016/j.bulm.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Szulman AE, Philippe E, Boue JG, Boue A. Human triploidy: Association with partial hydatidiform moles and nonmolar conceptuses. Hum Pathol. 1981;12:1016–1021. doi: 10.1016/s0046-8177(81)80259-6. [DOI] [PubMed] [Google Scholar]
- 14.Kliman HJ, McSweet JC, Franco A, Ying X, Zhao Y, Stetten G. Trophoblast inclusions are rare in elective terminations and normal deliveries, but common in cases with karyotypic abnormalities. Fertil Steril. 2003;80:88. [Google Scholar]
- 15.Honoré L, Dill FJ, Poland BJ. Placental morphology in spontaneous human abortuses with normal and abnormal karyotypes. Teratology. 1976;14:151–166. doi: 10.1002/tera.1420140206. [DOI] [PubMed] [Google Scholar]
- 16.Novak R, Agamanolis D, Dasu S, Igel H, Platt M, Robinson H, et al. Histologic analysis of placental tissue in first trimester abortions. Pediatric Pathology. 1988;8:477–482. doi: 10.3109/15513818809022303. [DOI] [PubMed] [Google Scholar]
- 17.Silvestre E, Cusí V, Borrás M, Antich J. Cytogenetic and morphologic findings in chorionic villi from spontaneous abortions. Birth Defects Orig Artic Ser. 1996;30:353–357. [PubMed] [Google Scholar]
- 18.Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry. 2007;61:487–491. doi: 10.1016/j.biopsych.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 19.Kliman HJ. Placenta. In: Volkmar FR, editor. Encyclopedia of Autistic Spectrum Disorders. Springer; New York: 2012. [Google Scholar]
- 20.Schlattmann P. Medical Applications of Finite Mixture Models. Springer-Verlag; Berlin: 2009. [Google Scholar]
- 21.Deeks JJ, Altman DG. Diagnostic tests 4: Likelihood ratios. BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauermann G, Carroll RJ. A note on the efficiency of sandwich covariance matrix estimation. J Am Statist Assoc. 2001;96:1387–1396. [Google Scholar]
- 23.Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack, and the CHAT. Br J Psychiatry. 1992;161:839–843. doi: 10.1192/bjp.161.6.839. [DOI] [PubMed] [Google Scholar]
- 24.Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Dev. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 26.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Res. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J Consult Clin Psychol. 1987;55:3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, Veenstra-Vanderweele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303–e1311. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- 30.Spreckley M, Boyd R. Efficacy of applied behavioral intervention in preschool children with autism for improving cognitive, language, and adaptive behavior: A systematic review and meta-analysis. J Pediatr. 2009;154:338–344. doi: 10.1016/j.jpeds.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Awate SP, Win L, Yushkevich P, Schultz RT, Gee JC. 3D cerebral cortical morphometry in autism: Increased folding in children and adolescents in frontal, parietal, and temporal lobes. Med Image Comput Comput Assist Interv. 2008;11:559–567. doi: 10.1007/978-3-540-85988-8_67. [DOI] [PubMed] [Google Scholar]
- 32.Kemper TL, Bauman ML. Neuropathology of infantile autism. Mol Psychiatry. 2002;7(suppl 2):S12–S13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- 33.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 34.Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang J. Early generalized overgrowth in boys with autism. Arch Gen Psychiatry. 2011;68:1021–1031. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27:11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jou RJ, Minshew NJ, Keshavan MS, Hardan AY. Cortical gyrification in autistic and Asperger disorders: A preliminary magnetic resonance imaging study. J Child Neurol. 2010;25:1462–1467. doi: 10.1177/0883073810368311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, et al. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Current Biol. 2000;10:623–629. doi: 10.1016/s0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 41.Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 42.Goodman FR. Congenital abnormalities of body patterning: Embryology revisited. Lancet. 2003;362:651–662. doi: 10.1016/S0140-6736(03)14187-6. [DOI] [PubMed] [Google Scholar]
- 43.Silbergeld EK, Patrick TE. Environmental exposures, toxicologic mechanisms, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2005;192:S11–S21. doi: 10.1016/j.ajog.2004.06.117. [DOI] [PubMed] [Google Scholar]
- 44.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 46.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci. 2012;14:263–279. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Kolevzon A, Newcorn JH, Kryzak L, Chaplin W, Watner D, Hollander E, et al. Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry Res. 2010;175:274–276. doi: 10.1016/j.psychres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Molecular Genetic Study of Autism Consortium (IMGSAC) A genomewide screen for autism: Strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet. 2001;69:570–581. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R SocLond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, et al. Screening a large reference sample to identify very low frequency sequence variants: Comparisons between two genes. Nat Genet. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- 54.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fecteau KA, Eiler H. Placenta detachment: Unexpected high concentrations of 5-hydroxytryptamine (serotonin) in fetal blood and its mitogenic effect on placental cells in bovine. Placenta. 2001;22:103–110. doi: 10.1053/plac.2000.0596. [DOI] [PubMed] [Google Scholar]
- 57.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 59.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 60.Tassone F, Qi L, Zhang W, Hansen RL, Pessah IN, Hertz-Picciotto I. MAOA, DBH, and SLC6A4 variants in CHARGE: A case-control study of autism spectrum disorders. Autism Res. 2011;4:250–261. doi: 10.1002/aur.196. [DOI] [PMC free article] [PubMed] [Google Scholar]