Abstract
Using our tridentate NHC-amidate-alkoxide Pd(II) complex, we developed a catalytic method for oxidative C-C bond cleavage of glycerol. The glycerol was degraded exclusively to formic acid and CO2. Two possible degradation pathways were proposed through 13C labeled studies.
Keywords: NHC amidate ligand, Palladium catalyst, Degradation of glycerol, Formic acid, Oxidative catalysis
Glycerol, also called glycerin, is a main by-product of biodiesel production and traditional soap manufacturing processes.1 A rapid increase in biomass conversion has produced a massive stockpile of glycerol, and its transformation to value-added chemicals has been in great demand. A number of methods have been reported to provide C2 ~ C3 chemical products such as glyceraldehyde, glyceric acid, hydroxypyruvic acid, tartronic acid, glycolic acid, and oxalic acid.2 Because these C2 ~ C3 products have been short of practical use, the formation of C1 products such as formic acid has attracted much attention for future energy applications.3 There are few examples have been introduced through hydrothermal oxidation, heterogeneous catalysts, and electrocatalytic oxidation.4
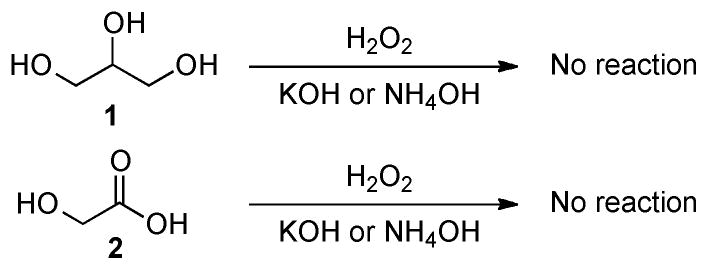
One noteworthy application of formic acid is the DFAFC (direct formic acid fuel cell), which has been of increasing popularity compared with hydrogen and methanol based fuel cells because of their ease of refuelling, efficiency, and safety. As an emerging technology, DFAFC is currently being tested by major producers of portable electronics in phones, laptops, and computers.5 In an effort to find a potential source of formic acid, we embarked on the development of new oxidative carbon-carbon bond cleavage methods of glycerol mainly because the previously reported conditions failed to degrade glycerol as shown in scheme 1.
Scheme 1.
Limitations of known conditions on glycerol degradation.
Representative examples included Isbell’s alkaline hydrogen peroxide and our hydrogen peroxide/ammonia water conditions.6 Under these conditions, various aldoses were oxidatively transformed to formic acid whereas glycerol (1) barely reacted. In addition, these procedures converted ketoses to both formic and glycolic acids (2), while glycolic acid was resistant to further degradation. These shortcomings prompted us to undertake studies on oxidative degradation using organometallic catalysts.
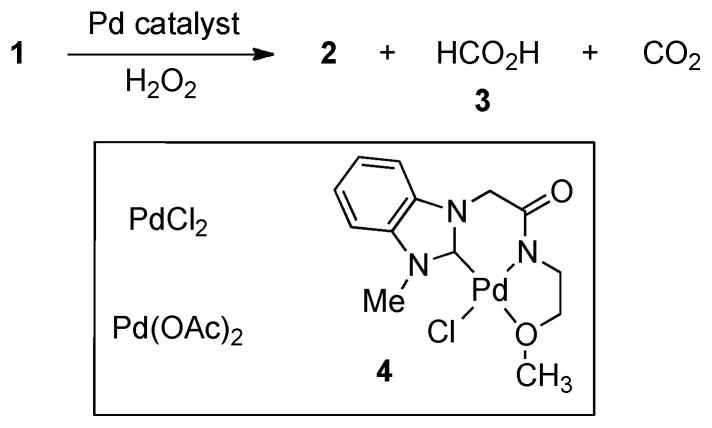
Recently, we reported that NHC-palladium complexes including 4 were highly stable and still reactive enough to facilitate C-H activation of relatively unreactive hydrocarbons.7 Their high stability in nucleophilic solvents such as water and alcohol could allow for conditions amenable to oxidative carbon-carbon bond cleavage of glycerol. These processes could provide C1 ~ C2 products encompassing carbon dioxide, formic acid (3), and glycolic acid (2). Using known palladium catalysts and our Pd(II) catalyst 4, we evaluated the feasibility of oxidative degradation of glycerol (table 1).
Table 1.
Catalytic oxidative carbon-carbon bond cleavage of glycerol with various oxidizing agents in the presence of Pd catalysts at room temperature.a
| entry | catalyst | oxidant | yield (%)d | 2/3 (× 10−5 mol)e |
|---|---|---|---|---|
| 1 | PdCl2 | H2O2 | trace | trace/trace |
| 2 | Pd(OAc)2 | H2O2 | 6 | 0.16/0.20 |
| 3 | 4 | H2O2 | 41 | 1.50/8.05 |
| 4 | 4 | t-BuO2H | trace | -/- |
| 5b | 4 | Oxone | 10 | 0.39/1.52 |
| 6 | 4 | K2S2O8 | - | -/- |
| 7c | 4 | O2 | - | -/- |
All reactions were performed with glycerol (10 mg, 10.8 × 10−5 mol), Pd catalyst (5 mol%). Entry 1–3: 30% H2O2 (0.4 mL) in H2O (0.1 mL) was added. In the entry 4, tBuOOH (70% in H2O, 0.4 mL) was used. Entries 5~6: 10.8 × 10−5 mol of oxidant (entry 5: oxone, entry 6: K2S2O8) in 0.3 mL of H2O was used. Entry 7: O2 was bubbled in 0.3 mL of H2O solution. All reactions were performed at room temperature for 6 hours..
Run in H2O (0.4 mL).
Continuous flow with O2.
Conversion yield of glycerol.
The amount of each product was determined by 1H NMR spectral analysis using MeOH as the internal standard.8
Regarding oxidative degradation by hydrogen peroxide, commercially available Pd complexes including PdCl2 and Pd(OAc)2 didn’t offer meaningful improvement over KOH or NH4OH (entries 1 and 2).6 However, NHC-Pd complex 4 exhibited significant consumption of glycerol at room temperature to furnish formic acid as the major product (entry 3). We also noticed that the ratio of formic acid to glycolic acid produced was much higher than that of the reaction with Pd(OAc)2 despite its low yield. These results might indicate our catalyst degraded both glycerol and glycolic acid unlike other Pd salts or basic conditions. We screened other oxidants such as tert-butyl peroxide, oxone, K2S2O8, and molecular oxygen, most of which were ineffective (entries 4 ~ 7). Similarly to hydrogen peroxide, oxone provided a higher ratio of formic acid to glycolic acid compared to the Pd(OAc)2 case (entries 2 and 5).
In the catalytic processes using 4, one equivalent of glycerol can produce either three equivalents of formic acid or one equivalent of formic acid and glycolic acid each. To understand how many equivalents of formic acid can be produced from glycerol, it was necessary to understand degradation pathways. In this context, we carried out the oxidative cleavage reaction using 1,3-13C-labeled glycerol and 2-13C-labeled glycerol in the presence of NHC-Pd complex 4 and hydrogen peroxide at 60 °C for 6 hours, under which conditions we tried to consume most of glycerol (cf. table 2, entry B).
Table 2.
Yields of reactions in the figure 3
| entry | glycerol (%)a | glycolic acid (%)b | formic acid (%)c |
|---|---|---|---|
| B | trace | 11 | 39 |
| C | trace | 20 | 50 |
| D | 19 | 28 | 57 |
| E | 0 | 0 | 61 |
Remaining glycerol/added glycerol × 100.
Moles of glycolic acid/moles of added glycerol × 100.
(Moles of formic acid/moles of glycerol × 100)/3, assuming one mole of glycerol produced 3 mole of formic acid. Moles of each product were calculated by using methanol as an internal NMR reference.
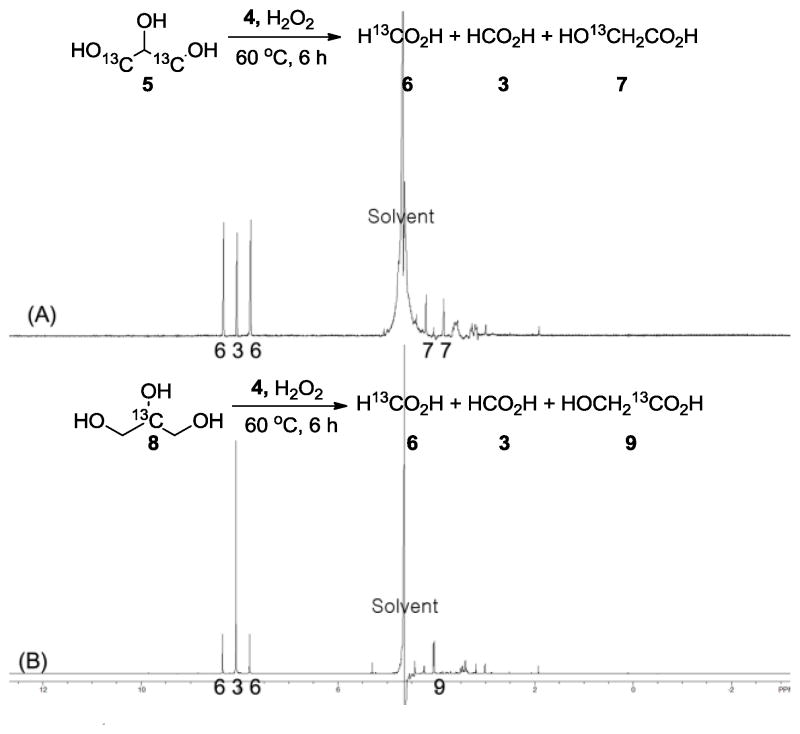
As shown in figure 1, the reaction of 1,3-13C-labeled glycerol (5) afforded both 13C-labeled formic acid (6) and unlabeled formic acid (3) in a 2 to 1 ratio, as well as a small amount of 13C-labeled glycolic acid on the β-carbon (7). In the case of 2-13C-labeled glycerol (8), a 1 to 2.5 ratio of 13C-labeled formic acid (6) to unlabeled formic acid (3), as well as 1-13C-labeled glycolic acid (9) were observed. In addition, 13C-NMR analysis further confirmed the assignment by 13C-12C coupling for 13C-labeled glycolic acid (7) (δ= 60.6 Hz at 176.0 ppm). Therefore, these results indicated that the formic acid produced contained both the secondary and primary carbons of glycerol. On the other hand, the carbonyl carbon of glycolic acid would stem only from the secondary carbon of glycerol while the carbinol carbon would originate from the primary carbons.
Figure 1.
1H-NMR spectra for the oxidative degradation reactions of 1,3-13C-glycerol (A) and 2-13C-glycerol (B).
Since the observed products didn’t satisfy the mass balance, we supected that we lost some carbons in the form of carbon dioxide, and examined such possibility (scheme 3). In the presence of NHC-Pd complex 4, both acids gradually disappeared over time to form carbon dioxide. For example, glycolic acid led to formic acid in 10% yield after 3 hours while formic acid gave no detectable products except carbon dioxide. In addition, unlabeled formic acid (3), but no 13C labeled formic acid (6) was detected when (1-13C)glycolic acid (9) was reacted with hydrogen peroxide. It was evident that 2-12C and 1-13C in glycolic acid were incorporated into formic acid and 13CO2, respectively. These results were consistent with the aforementioned glycol oxidation patterns.
Scheme 3.
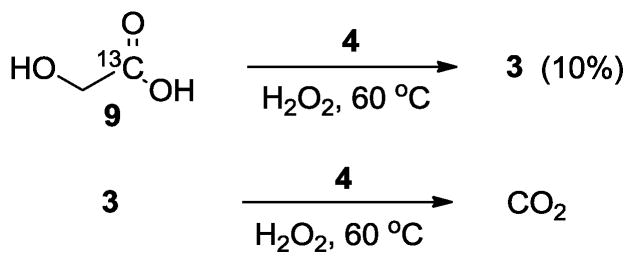
Potential degradation of glycolic acid and formic acid in the presence of NHC-Pd(II) catalyst 4.
These labeled experiments suggested two possible degradation pathways. If formic acid was derived equally from all three carbons in gylcerol, the ratio of 13C-labeled formic acid (6) and unlabeled formic acid (3) should be 2 to 1 in figure 1 (A), and 1 to 2 in figure 1 (B). Even though the observed ratios were close to the theoretical ones, these ratios were still different from the expected ones by 10–25%. Thus, we assumed a major pathway would oxidize all three carbons to formic acid. Since small amounts of glycolic acid was detected, we considered that another pathway through glycolic acid was active concomitantly. In fact, both C-1 and C-3 in glycerol could be converted to formic acid whereas the C-2 would fail to give formic acid and instead furnish CO2 as aforementioned. As a consequence, one could expect more 13C-labeled formic acid than 12C-formic acid in figure 1 (A) and more 12C-formic acid than 13C-labeled one in figure 1 (B), respectively.
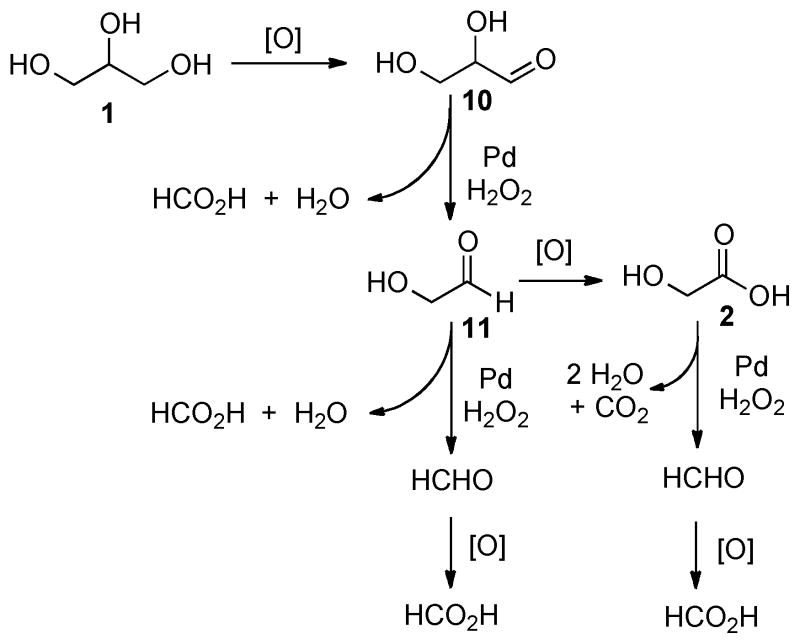
Based on these results and previously reported studies, two possible degradation pathways can be proposed as in scheme 4.3,9,10,11 One mechanistic pathway would form three equivalents of formic acid from each glycerol molecule while another mechanism would lead to two equivalents of formic acid and one part of CO2 through the glycolic acid intermediate. As the incipient product, Pd-glycerol adducts could be generated and oxidized to aldehyde 10, which would undergo rapid C-C bond cleavage to release formic acid and another aldedyde 11. Further oxidative cleavage of 11 could afford the second equivalent of formic acid and formaldehyde, which eventually would generate the third equivalent of formic acid. Meanwhile, the first oxidative C-C bond cleavage product 11 can be oxidized to yield glycolic acid 2, which subsequently can be degraded to formaldehyde and CO2, ultimately furnishing one equivalent of formic acid. In summary, dual mechanistic pathways would contribute to our catalytic processes.
Scheme 4.
Potential oxidative degradation pathways of glycerol.
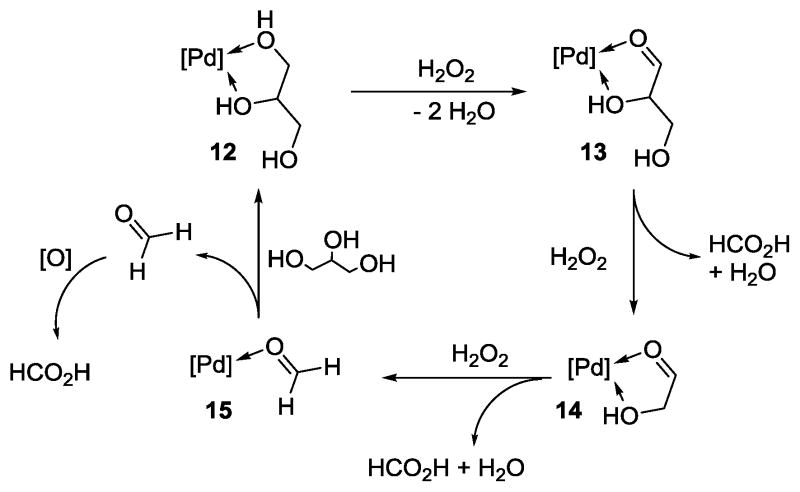
In the reaction mixture, it was unable to detect any aldehyde (10, 11, or formaldehyde). This could indicate that aldehyde compound was not completely released from Pd complex but underwent C-C bond cleavage to form formic acid and another aldehyde. Even though aldehyde was released from Pd, it could be oxidized quickly by the oxidant to form acid (2 or formic acid). Therefore the following catalytic cycle could be proposed (scheme 5). Glycerol adduct 12 could be oxidized to form 13. Subsequently, one equivalent of H2O2 could generate one equivalent of formic acid and 14. Following third oxidation, formaldehyde adduct 15 could be formed while releasing another equivalent of formic acid. The last formic acid could be produced from this formaldehyde.
Scheme 5.
Catalyic pathway for the formation of formic acid
To seek optimal conditions, we investigated various factors including amounts of hydrogen peroxide, reaction temperatures, and reaction times. As depicted in figure 2 (A), the formation of formic acid increased upon higher concentration of hydrogen peroxide. When we raised reaction temperatures gradually (25 to 60 °C), the amount of formic acid was increased until 40 °C, then decreased at higher temperatures (figure 2 (B)) presumably due to the overoxidation to carbon dioxide. Additionally, longer reaction times were not sufficient to enhance the formation of formic acid (figure 2 (C)). In addition, when the amount of Pd (4) was increased to 10 or 15% from 5%, overall yield of formic acid as well as consumption of glycerol dropped due to the degradation of formic acid and hydrogen peroxide by the catalyst.
Figure 2.
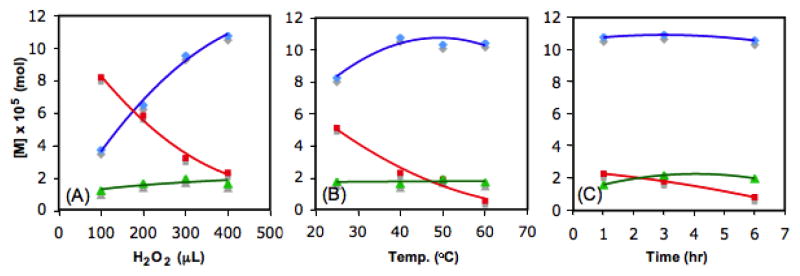
Concentration changes vs. volume of H2O2 (A), reaction temperature (B), and time (C) for the oxidative degradation of glycerol: glycerol (red square), formic acid (blue diamond), and glycolic acid (green triangle).
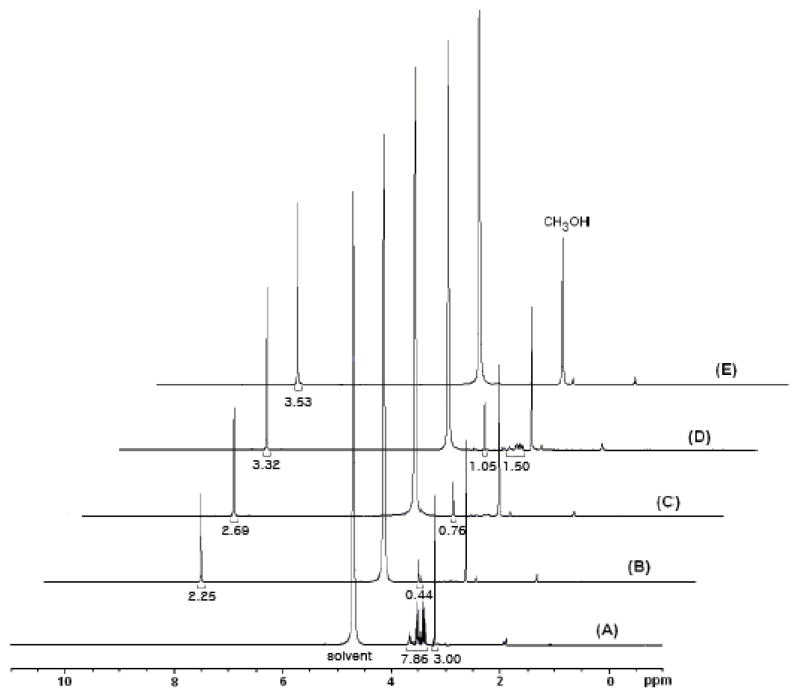
Because excess H2O2 and high reaction temperatures caused the degradation of formic acid to CO2, we decided to employ slow addition of hydrogen peroxide at mild temperatures, and evaluated the time and temperature dependence on the yields of both formic and glycolic acids using 1H-NMR techniques (figure 3 and table 2). As shown in figure 3 and table 2 (entries B and C), the formation of formic and glycolic acids was increased slightly by the slow addition of hydrogen peroxide. Although glycerol was completely consumed under these conditions, glycolic acid still remained. In efforts to avert glycolic acid and maximize the amount of formic acid, hydrogen peroxide was slowly added at 0 °C for 3 hours and stirred at room temperature over 8 hours (table 2, entry D), glycerol was not completely consumed despite higher yields of formic and glycolic acids. Finally, when we added hydrogen peroxide slowly at 0 °C for 3 hours and additional H2O2 at 60 °C for 3 hours, we observed exclusively formic acid in 61% yield (table 2, entry E).
Figure 3.
1H-NMR study for the degradation pathway of glycerol: (A) starting glycerol, (B) 0.4 mL H2O2 for 6 hours at 60 °C, (C) 6 hour slow addition of 0.4 mL H2O2 at 60 °C, (D) 3 hour slow addition of 0.4 mL H2O2 at 0 °C and 8 hour stirring at room temperature, (E) 3 hour slow addition of 0.3 mL H2O2 at 0 °C and 3 hour slow addition of 0.2 mL H2O2 at 60 °C.
In conclusion, we successfully demonstrated a method to produce formic acid as the sole product via the oxidative NHC-Pd catalyzed carbon-carbon bond cleavage of glycerol with hydrogen peroxide as an oxidizing agent. The direct conversion of glycerol into formic acid was facilitated under mild conditions. As we proposed, dual cleavage pathways were likely to be active. Based on these mechanisms, we sought optimal conditions to avoid the glycolic acid intermediate and over-oxidation of formic acid to carbon dioxide. Our catalytic conditions can be useful for the degradation of carbohydrates and biomass including starch and grass, which will be reported in due course.
Supplementary Material
Scheme 2.
Glycerol degradation in the presence of Pd(II) catalysts.
Acknowledgments
We acknowledge generous financial support from the Hydrocarbon Research Foundation and the National Institute of Health (S10 RR025432).
Footnotes
Supplementary material is available in a PDF format.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Johnson DT, Taconi KA. Environ Prog. 2007;26:338–348. [Google Scholar]; (b) Gombotz K, Parette R, Austic G, Kannan D, Matson JV. Fuel. 2002;92:9–15. [Google Scholar]
- 2.(a) Zheng Y, Chen X, Shen Y. Chem Rev. 2008;108:5253–5277. doi: 10.1021/cr068216s. [DOI] [PubMed] [Google Scholar]; (b) Pagliaro M, Ciriminna R, Kimura H, Rossi M, Pina CD. Angew Chem Int Ed. 2007;46:4434–4440. doi: 10.1002/anie.200604694. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kim HS, Morgan RB, Masel RI. J Power Sources. 2009;188:118–121. [Google Scholar]; (b) Yu X, Pickup PG. J Power Sources. 2008;182:124–132. [Google Scholar]; (c) Chetty R, Scott K. J New Mat Electrochem Systems. 2007;10:135–142. [Google Scholar]; (d) Kang S, Lee J, Lee JK, Chung SY, Tak Y. J Phys Chem B. 2006;110:7270–7274. doi: 10.1021/jp056753v. [DOI] [PubMed] [Google Scholar]; (e) Chu KL, Shannon MA, Masel RI. J Electrochem Soc. 2006;153:A1562–A1567. [Google Scholar]; (f) Rhee Y, Ha S, Masel RI. J Power Sources. 2003;117:35–38. [Google Scholar]
- 4.(a) Zhang Y-l, Zhang Min, Zheng Shen, Zhoub J-f, Zhoua X-f. J Chem Technol Biotechnol. 2013;88:829–833. [Google Scholar]; (b) McMorn P, Roberts G, Hutchings GJ. Catal Lett. 1999;63:193–197. [Google Scholar]; (c) Roquet L, Belgsir EM, Léger J-M, Lamy C. Electrochim Acta. 1994;39:2387–94. [Google Scholar]
- 5.(a) Bauskar AS, Rice CA. Electrochim Acta. 2012;62:36–41. [Google Scholar]; (b) Cai W, Yan L, Li C, Liang L, Xing W, Liu C. Int J Hydrogen Energy. 2012;37:3425–3432. [Google Scholar]; (c) Yu X, Pickup PG. J Power Sources. 2008;182:124–132. [Google Scholar]; (d) Jung WS, Han J, Ha S. J Power Sources. 2007;173:53–59. [Google Scholar]; (e) Wolfe MD, Lipscomb JD. J Biological Chem. 2003;278:829–835. doi: 10.1074/jbc.M209604200. [DOI] [PubMed] [Google Scholar]; (f) Eugeneyan Y, Schwartz FW. Environ Sci Technol. 2000;34:2535–2541. [Google Scholar]; (g) Brooks CD, Huang LC, McCarron M, Johnstone AW. Chem Commun. 1999:37–38. [Google Scholar]; (h) Stefan MI, Bolton JR. Environ Sci Technol. 1998;32:1588–1595. [Google Scholar]; (i) Sasaki K, Okamoto T, Oka S. Chem Eng Comm. 1989;83:111–116. [Google Scholar]; (l) Okamoto T, Sasaki K, Oka S. J Am Chem Soc. 1988;110:1187–1196. [Google Scholar]; (m) Kumar A. J Am Chem Soc. 1981;103:5179–5182. [Google Scholar]; (n) Venturello C, Ricci M. J Org Chem. 1986;51:1599–1602. [Google Scholar]; (o) Hockett RC, Fletcher HG., Jr J Am Chem Soc. 1944;66:469–472. [Google Scholar]
- 6.Pullanikat P, Jung SJ, Yoo KS, Jung KW. Tetrahedron Lett. 2010;51:6192–6194. doi: 10.1016/j.tetlet.2010.09.092. and references on Isbell’s work cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Lee JH, Yoo KS, Jung KW. Bull Korean Chem Soc. 2011;32:2881–2882. [Google Scholar]; (b) Lee JH, Yoo KS, Park CP, Olsen JM, Sakaguchi S, Prakash GKS, Mathew T, Jung KW. Adv Synth Catal. 2009;351:563–568. doi: 10.1002/adsc.200800698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Schäfer A, Saak W, Detlev Haase, Müller T. Angew Chem Int Ed. 2012;51:2981–2984. doi: 10.1002/anie.201107958. [DOI] [PubMed] [Google Scholar]; (b) Wesselbaum S, Hintermair U, Leitner W. Angew Chem Int Ed. 2012;51:8585–8588. doi: 10.1002/anie.201203185. [DOI] [PubMed] [Google Scholar]; (c) Zou F, Cole JM, Jones TGJ, Jiang L. Appl Organometal Chem. 2012;26:546–549. [Google Scholar]; (d) Motokura K, Kashiwame D, Miyaji A, Baba T. Org Lett. 2012;14:2642–2645. doi: 10.1021/ol301034j. [DOI] [PubMed] [Google Scholar]; (e) Kure B, Taniguchi A, Nakajima T, Tanase T. Organometallics. 2012;31:4791–4800. [Google Scholar]; (f) Federsel C, Jackstell R, Boddien A, Laurenczy G, Beller M. Chem Sus Chem. 2010;3:1048–1050. doi: 10.1002/cssc.201000151. [DOI] [PubMed] [Google Scholar]
- 9.(a) Keith JM, Goddard WA., III J Am Chem Soc. 2009;131:1416–1425. doi: 10.1021/ja8040459. [DOI] [PubMed] [Google Scholar]; (b) Kujime M, Hikichi S, Akita M. Chemistry Lett. 2003;32:486–487. [Google Scholar]; (c) Donohoe TJ. Oxidation and Reduction in Organic Synthesis. Oxford University Press; Oxford: 2000. [Google Scholar]; (d) Nishimur T, Onoue T, Ohe K, Uemura S. J Org Chem. 1999;64:6750–6755. doi: 10.1021/jo9906734. [DOI] [PubMed] [Google Scholar]; (e) Murahashi S. Angew Chem Int Ed. 1995;34:2443–2465. [Google Scholar]; (f) Ando W. Organic Peroxides. John Wiley & Sons; Chichester: 1992. [Google Scholar]; (g) Trost BM, editor. Comprehensive Organic Synthesis: Selectivity, Strategy & Efficiency in Modern Organic Chemistry. Vol. 7. Pergamon Press; Oxford: 1991. [Google Scholar]; (h) Hosokawa T, Ataka Y, Murahashi SI. Bull Chem Soc Jpn. 1990;63:166–169. [Google Scholar]; (i) Hudlicky M. Oxidation in Organic Chemistry (ACS Monograph 186) American Chemical Society; 1990. [Google Scholar]; (j) Sheldon RA, Kochi JK. Metal-Catalyzed Oxidations of Organic Compounds. Academic Press; New York: 1981. [Google Scholar]; (k) Patai S. The Chemistry of Peroxides. John Wiley & Sons; Chichester: 1983. [Google Scholar]
- 10.Bueno AC, Gonc JA, Gusevskaya EV. Applied Catalysis A: General. 2007;329:1–6. [Google Scholar]
- 11.(a) Nishimura T, Uemura S. Synlett. 2004:201–216. [Google Scholar]; (b) Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227–8241. [Google Scholar]
- 12.Representative reaction: A mixture of glycerol (10 mg, 0.1 mmol) and catalyst 4 (5 mole%) in 0.2 mL of water was placed in a rubber stoppered vial. To this mixture, 0.3 mL of 30% H2O2 was added for 3 hours at 0 °C by using a syringe pump. After complete addition, the reaction mixture was heated to 60 °C, then 0.2 mL of 30% H2O2 was added for 3 hours. Wet 1D NMR of the crude reaction mixture showed the complete conversion of glycerol to give 0.196 mmol of formic acid. Wet 1D: δ = 8.1 (s, 1H).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.