Abstract
Introduction
Reintroduction of Variola major as an agent of bioterrorism remains a concern. A shortened dosing schedule of Bavarian Nordic’s (BN) IMVAMUNE® (modified vaccinia Ankara vaccine against smallpox) was compared to the currently recommended 0 and 28 day schedule for non-inferiority by evaluating the magnitude and kinetics of the immune responses.
Methods
Subjects were assigned to receive IMVAMUNE or placebo administered subcutaneously on Days 0 and 7, Days 0 and 28, or Day 0. Blood was collected for antibody and cell-mediated immune assays. Subjects were followed for safety for 12 months after last vaccination.
Results
The primary endpoint of this study was the geometric mean antibody titers (GMT) at 14 days post last vaccination. Of 208 subjects enrolled, 191 received vaccine (Group:0+7, Group:0+28 and Group:0) and 17 received placebo. Moderate/severe systemic reactogenicity after any vaccination were reported by 31.1%, 25.4%, and 28.6% of the subjects for Group:0+7, Group:0+28, and Group:0, respectively (Chi-square test P value=0.77). Based on BN’s Plaque Reduction Assay GMTs, Group:0+7 was non-inferior to Group:0+28 at Day 4, 180, and 365 after the second vaccination. On Day 14, Group:0+7 and Group:0+28 GMT were 10.8 (CI:9.0;12.9) and 30.2 (CI:22.1;41.1), respectively. Based on BN’s Enzyme-linked immunosorbent assay, the proportion of subjects with positive titers for Group:0+28 was significantly greater than that for Group:0+7 after second vaccination at Day 4 and 180. By Day 14 after the second dose, the IFN-y Enzyme-linked immunosorbent spot (ELISPOT) responses were similar for Group:0+28 and Group:0+7.
Conclusion
Overall, a standard dose of IMVAMUNE (0.5mL of 1×108 TCID/mL) administered subcutaneously was safe and well tolerated. A second dose of IMVAMUNE at Day 28 compared to Day 7 provided greater antibody responses and the maximal number of responders. By Day 14 after the second dose, IFN-y ELISPOT responses were similar for Group:0+28 and Group:0+7.
Keywords: MVA, IMVAMUNE, smallpox, vaccine, bioterrorism, post-event
INTRODUCTION
Bioterrorism remains a concern and release of Variola major, smallpox virus, could be catastrophic. Only 20% of the population has some immune response against smallpox, due to previous vaccination [1,2]. Current therapy against smallpox is limited, and preexposure vaccination is not recommended. Although the United States government replaced Dryvax® with ACAM2000 [3-5], a purified clone of Dryvax, ACAM2000 has the same contraindications for use as Dryvax.
MVA (modified vaccinia Ankara), a highly attenuated vaccinia virus, does not replicate in human cells. Mayr [6] derived MVA from dermal vaccinia strain Ankara (CVA) by completing over 500 continuous passages in primary chicken embryo fibroblasts cells in Germany, between 1960 and 1974. Further work in animals [6-9] and humans [9] led to the official registration of MVA in Germany in 1976 for two-stage smallpox vaccination of children, consisting of MVA, followed by the Lister-Elstree strain up to six months later [10]]. More recently, several clinical trials including challenge studies [11,12,13] to develop IMVAMUNE® for licensure as a vaccine against orthopox infections have been completed.
Currently, the planned pre-event vaccination schedule using IMVAMUNE® is a prime-boost vaccination regimen with IMVAMUNE® administered on Days 0 and 28; however, an effective shorter vaccination interval could limit casualties during a post-event scenario. The purpose of this trial was to determine whether an accelerated dosing schedule, Days 0 and 7, was non-inferior to the currently recommended Days 0 and 28 schedule. The kinetics and the magnitude of the immune response following vaccination were evaluated. Additionally, a single dose group provided information regarding duration and level of immunogenicity responses should only one dose be received during a post event scenario.
METHODS
Vaccine
IMVAMUNE is manufactured by Impfstoffwerk Dessau-Tornau GmbH (Rosslau, Germany) for Bavarian Nordic (BN) A/S (Copenhagen, Denmark). The vaccine is supplied as liquid-frozen aliquots of 0.65 mL. One dose of 0.5 mL liquid-frozen vaccine contains 1×108 TCID50 Modified Vaccinia virus Ankara. Saline was used as the placebo.
Study design
One hundred ninety-five subjects (60 vaccine recipients and 5 saline placebo recipients per arm) were to be randomly assigned to 3 Groups to exclusively receive IMVAMUNE or placebo on Days 0 and 7, Days 0 and 28 and Day 0, respectively. Injections were administered subcutaneously. Clinical laboratory testing was done at screening and 14 days after the last dose. Blood samples were collected prior to each vaccination and at specified time points following the last vaccination for plaque reduction neutralizing antibody titer (PRNT), ELISA, and IFN-γ ELISPOT assays. Both BN and Saint Louis University (SLU) performed PRNT and ELISA assays. Subjects were followed for 12 months after the last vaccination.
Institutional Review Boards representing each site approved the study and all subjects provided informed consent. Healthy, adult subjects, born after 1971, were eligible if they had an acceptable medical history and physical exam; negative ELISA (screen only) for HIV and hepatitis C virus antibody, and hepatitis B surface antigen; normal liver and kidney function, urine glucose, and hematologic parameters; and an electrocardiogram (ECG) (screen only) without clinically significant findings as assessed by a single study cardiologist.
Subjects were excluded if they had a previous smallpox vaccination or scar consistent with smallpox vaccination; military service prior to 1991 or after January 2003; greater than 10% risk of developing a myocardial infarction or coronary death within the next 10 years using the National Cholesterol Education Program’s risk assessment tool (http://hp2010.nhlbihin.net/atpiii/calculator.asp) received inactivated vaccine within 14 days or live attenuated vaccine within 30 days of vaccination; received blood products or immunoglobulin within six months prior to vaccination; atopic dermatitis; or were pregnant or lactating.
Subjects collected reactogenicity information using a memory aide for 15 days (Days 0-14). Adverse events and serious adverse events were collected for 28 days after each vaccination and for the duration of the study, respectively. Reactogenicity events included fever, pain at injection site, erythema, induration, muscle aches, chills, headache, nausea, feeling tired, underarm pain, underarm swelling, itchiness at vaccination site, change in appetite, and joint pain. Reactogenicity and unsolicited events were graded as mild, moderate and severe (Figure 1). Erythema or induration was measured in millimeters (Figure 1).
Figure 1.
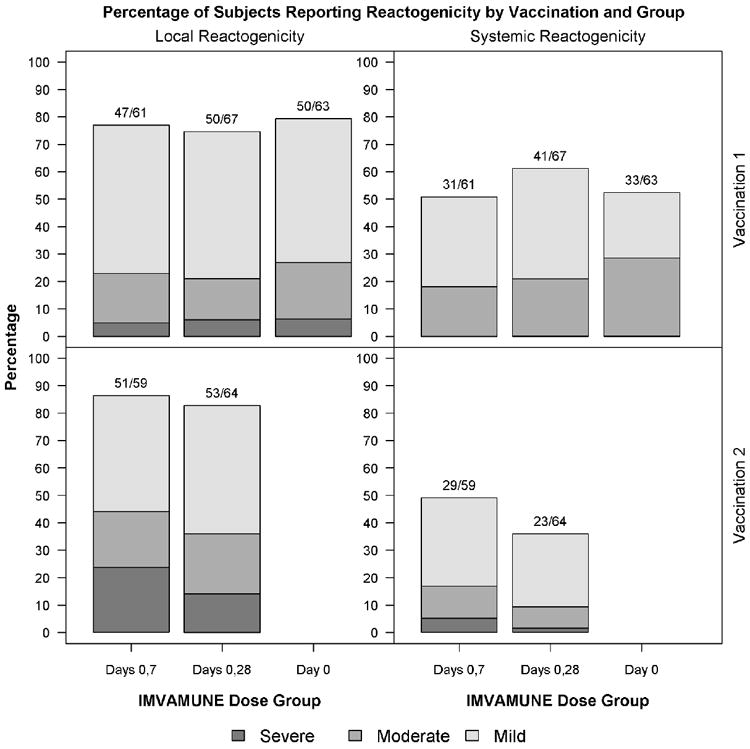
Percent of Subjects Reporting by Vaccination and Group: The percentage of subjects with local and systemic reactogenicity is shown by vaccination group and vaccination number. The highest grade for each event is reported. Subjects were vaccinated on Days 0 and 28. Placebo recipient data is not shown.
Solicited and unsolicited events were graded according to the following scale: Grade 0= Not present: Grade 1= Mild (present, but easily tolerated); Grade 2= Moderate (able to tolerate routine activity with effort); Grade 3= Severe (unable to continue routine activity) and Grade 4 = Life-threatening.
Erythema or induration at the vaccination site was measured in millimeters: Grade 1= Mild (≤15mm); Grade 2= Moderate (>15-≤30mm) and Grade 3= Severe (>30mm).
Immunogenicity assays
The immunogenicity assays are described elsewhere.
The BN PRNT assay [11] and ELISA [11] assay antigen were Western Reserve and BN MVA, respectively; positive titers were ≥15 and ≥50. The SLU PRNT assay [14] and ELISA [15] assay antigen was ATCC MVA (VR-1508); positive titers were ≥20 and >50, respectively.
The IFN-γ ELISPOT [16] assay antigen was Vaccinia virus (Western Reserve). Cryopreserved PBMC were used for the testing performed at CTL. Positive results were greater than the mean number of IFN-γ spot-forming cells at baseline plus 2 times standard deviation.
DATA ANALYSIS
Sample Size
The sample size had at least 80% power to test if the PRNT GMT (primary objective) and ELISA titers (secondary objective) at Day 14 following the 2nd dose for Group:0+7 were non-inferior to Group:0+28 titers with a margin of 2-fold based on an one-sided test at Type I error rate of 2.5%.
Statistical Analysis Plan
The primary outcomes for this study were safety measures and immunogenicity (neutralizing antibody) responses in Group:0+7 and Group:0+28. For each type of reactogenicity, each subject’s results were summarized using the most severe response recorded during the 15-day follow-up period and proportions were reported. Immune responses were summarized by computing the difference in log2 GMTs between Group:0+7 and Group:0+28 and its associated two-sided 95% confidence interval (CI). If the upper limit of the 95% CI was less than 1, Group:0+7 was considered non-inferior to Group:0+28. The difference in proportions of subjects considered positive was determined along with the 95% CI; however, a margin for evaluating non-inferiority was not defined in the protocol so the computations were intended only to suggest a degree of significance and correspondence with the conclusions based on GMT. Each antibody assay was analyzed as a dichotomous variable, responders versus non-responders, and as a continuous variable using the assay results directly. Dichotomous outcomes were summarized using response rates and their exact 95% confidence intervals, and compared by Chi-square (or Fisher’s exact or Fisher-Freeman-Halton) test between groups. Continuous outcomes were summarized using GMTs and their 95% CI or means, and compared by two sample t test or Wilcoxon rank sum test between groups.
No imputations were performed to account for missing data.
Only MVA recipients were included in the analysis unless otherwise stated in the results.
RESULTS
Demographics and Characteristics
The study enrolled 208 subjects; of these 191 and 17 subjects received vaccine and placebo, respectively (Tables 1 and 2). In subjects receiving vaccine, there was no significant difference in gender among the 3 groups (Chi-square test P value = 0.33). The mean age was 24.7 years (range 18 to 35 years). One hundred seventy-eight (85.6%), 15 (7.2%), and 5 (2.4%) subjects were white, black, and Asian, respectively, with 9 (4.3%) identified as Hispanic.
Table 1.
Vaccination Schedule
| Vaccination Schedule1 | ||||
|---|---|---|---|---|
| Group | Vaccination Days | Vaccinees N | Placebo Recipients N | Total number of subjects in each group |
| 0+7 | 0 and 7 | 61 | 6 | 67 |
| 0+28 | 0 and 28 | 67 | 4 | 71 |
| 0 | 0 | 63 | 7 | 70 |
Vaccine: Imvamune 0.5 mL of 1× 108 cfu/mL administered subcutaneously
Placebo: Saline 0.5 mL administered subcutaneously
Vaccinees and placebo recipients were administered only vaccine or placebo at each time point for a given group, respectively.
Table 2.
Demographic Data by Study Group
| Category | Sub-category | Group 0+7 IMVAMUNE1 Days 0, 7 (N=61) | Group 0+28 IMVAMUNE1 Days 0, 28 (N=67) | Group 0 IMVAMUNE1 Day 0 (N=63) | Placebo2 (N=17) | All Groups (N=208) |
|---|---|---|---|---|---|---|
| GENDER | Male | 36 (59.0%) | 31 (46.3%) | 31 (49.2%) | 9 (52.9%) | 107 (51.4%) |
| Female | 25 (41.0%) | 36 (53.7%) | 32 (50.8%) | 8 (47.1%) | 101 (48.6%) | |
| RACE | White | 52 (85.2%) | 57 (85.1%) | 54 (85.7%) | 15 (88.2%) | 178 (85.6%) |
| Black | 5 (8.2%) | 3 (4.5%) | 6 (9.5%) | 1 (5.9%) | 15 (7.2%) | |
| Asian | 0 | 2 (3.0%) | 2 (3.2%) | 1 (5.9%) | 5 (2.4%) | |
| Multiracial | 4 (6.6%) | 3 (4.5%) | 1 (1.6%) | 0 | 8 (3.8%) | |
| Not Reported | 0 | 2 (3.0%) | 0 | 0 | 2 (1.0%) | |
| ETHNICITY | Non-Hispanic/Latino | 59 (96.7%) | 64 (95.5%) | 60 (95.2%) | 16 (94.1%) | 199 (95.7%) |
| Hispanic/Latino | 2 (3.3%) | 3 (4.5%) | 3 (4.8%) | 1 (5.9%) | 9 (4.3%) | |
| AGE | Mean (S.D.) | 24.7 (4.6) | 25.0 (3.9) | 24.3 (3.9) | 25.4 (4.5) | 24.7 (4.2) |
| Median | 23 | 24 | 24 | 25 | 24 | |
| Range [Min., Max.] | [18, 35] | [18, 35] | [18, 35] | [19, 35] | [18, 35] |
Vaccine: IMVAMUNE 0.5 mL of 1× 108 cfu/mL administered subcutaneously on Days 0 and 7 (Group 0+ 7), Days 0 and 28 (Group 0+28) and Day 0 (Group 0).
Placebo: Saline 0.5 mL administered subcutaneously on Days 0 and 7 (Group 0+ 7, n= 6), Days 0 and 28 (Group 0+28, n=5) and Day 0 (Group 0, n=7).
Disposition of Subjects
There were 9 early terminations due to voluntary withdrawal (2 in Group:0+7 and 1 in Group:0+28), and lost to follow-up (4 in Group:0+28 and 2 in group 0]. Five subjects’ (2 in Group:0+7 and 3 in Group:0+28) vaccinations were discontinued due to inclusion criterion error, blood pressure elevation, rash and 2 for refusal.
Adverse Events
Local Reactogenicity (Figure 1)
The proportions of subjects with moderate/severe local symptoms after vaccination 1 were 23.0% (14/61), 20.9% (14/67) and 27.0% (17/63) for Group:0+7, Group:0+28 and Group:0, respectively (Chi-square test P value = 0.71). The proportions of subjects with moderate/severe local symptoms after vaccination 2 were 44.1% (26/59) and 35.9% (23/64) for Group:0+7 and Group:0+28, respectively (Fisher’s exact test P value = 0.46). Thirty-two IMVAMUNE recipients and one placebo recipient reported severe erythema and/or induration. Severe erythema (>30mm) was reported by 23.0%, 13.4% and 6.3% (Fisher-Freeman-Halton test P value=0.03) and severe induration (>30mm) was reported by 11.5%, 11.9% and 1.6% (Fisher-Freeman-Halton test P value = 0.04 in Group:0+7, Group:0+28, and Group:0 post any vaccination, respectively). Other local reactogenicity symptoms (pain at injection site, itchiness at vaccination site, underarm pain, underarm swelling, and rash) ranged from mild to moderate.
Systemic Reactogenicity (Figure 1)
During the 15-day post vaccination period, 4 subjects reported severe systemic events following vaccination 2, 3 in Group:0+7 (joint pain associated with an upper respiratory tract infection, nausea associated with gastroenteritis, and headache) and one in Group:0+28 (nausea and feeling tired) and none in the placebo group. Moderate systemic events were reported by 16 (26.2), 16 (23.9%), and 18 (28.6%) of subjects in Group: 0+7, Group:0+28 and Group:0 after any vaccination, respectively. The most common moderate reactions were feeling tired, headache and muscle aches. Only 1 subject (Group:0+7) reported fever (38.6°C). The proportions of subjects with moderate/severe systemic symptoms after any vaccination were 31.1% (19/61), 25.4% (17/67), and 28.6% (18/63) for Group:0+7, Group:0+28, and Group:0, respectively. There were no significant differences among these groups (Chi-square test P value = 0.77).
Serious Adverse Events
Seven serious adverse events (SAE), all unrelated to vaccination, occurred in 6 vaccine recipients including cervical adenocarcinoma, sepsis with urinary tract infection and right ureteral duplication resection, appendectomy, deep vein thrombosis and complicated migraine occurring several months after last vaccination, and breast reduction. There were no vaccine-related cardiac events.
Immunogenicity results
The primary immunogenicity objective was the comparison of PRNT between Group:0+7 and Group:0+28 at 14 days after the second vaccination. All other analyses were exploratory. Blood was collected for PRNT, ELISA and IFN-γ production on Days 0, 4, 8, 14, 28,180 and 365 after final vaccination.
Bavarian Nordic PRNT (Figure 2, Table 3)
Figure 2.
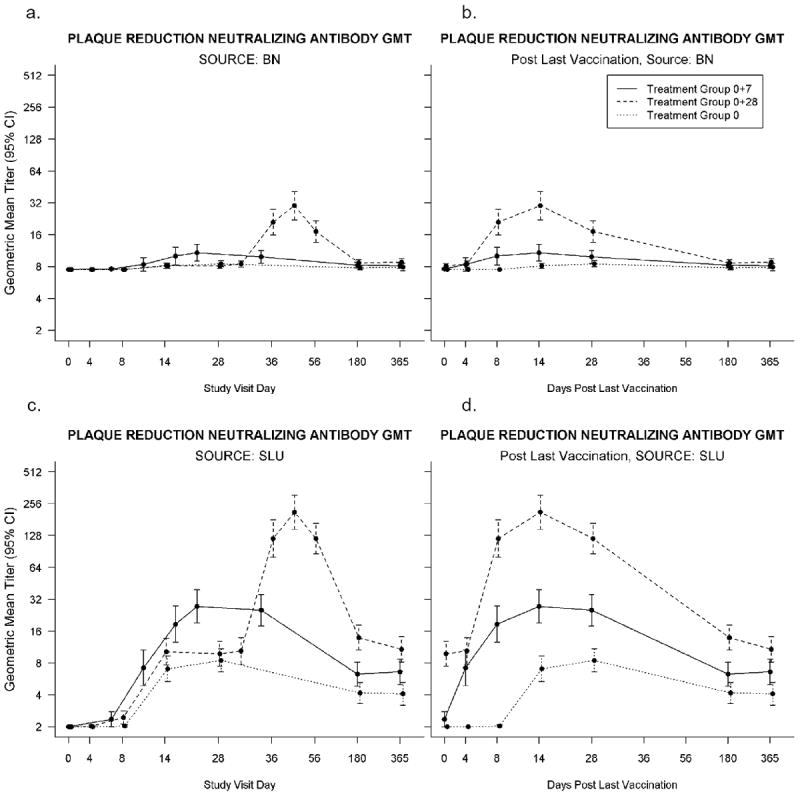
a-d. PRNT Assays: 2.a-b. Plaque Reduction Neutralizing Antibody Geometric Mean Titers (GMT) conducted at Bavarian Nordic using Bavarian Nordic’s MVA as the assay antigen, positive titer ≥15. Time points represent the number of days after the first vaccination for all three groups (2.a.) and after last vaccination for all three groups, i.e. after the single vaccination in Group:0, and after the second vaccination for the other two groups (Group:0+7 and Group:0+28) (2.b.). Placebo recipient data is not shown. 2.c-d. Plaque Reduction Neutralizing Antibody Geometric Mean Titers (GMT) conducted at Saint Louis University using ATCC MVA (VR-1508) as the assay antigen, positive titer ≥20. Time points represent the number of days after the first vaccination for all three groups (2.c.) and after last vaccination for all three groups, i.e. after the single vaccination in Group:0, and after the second vaccination for the other two groups (Group:0+7 and Group:0+28) (2.d.). Placebo recipient data is not shown.
Table 3.
Geometric Mean Titers (GMT) and Confidence Intervals (CI) and Number of Positive Responders/Number of Subjects Tested (%) for PRNT and ELISA and Mean Number of IFY-N Gamma Producing Cells and Standard Deviation (SD) by ELISPOT
| Prevaccination | Days after last vaccination | ||||||
|---|---|---|---|---|---|---|---|
| Days | 0 | 4 | 8 | 14 | 28 | 180 | 365 |
| Assay and Group (or day of vaccination) | GMT (CI) for PRNT/ELISA, Mean (SD) for IFN-γ no. positive/no. tested (%) (CI of %) | ||||||
| BN PRNT | |||||||
| 0,7 | 7.5 (-, -) | 8.4 (7.2, 9.7) | 10.0 (8.3, 12.2) | 10.8 (9.0, 12.9) | 9.9 (8.6, 11.3) | 8.2 (7.7, 8.8) | 8.1 (7.7, 8.6) |
| 0/60 (0.0) | 4/59 (6.8) | 14/59 (23.7) | 23/59 (39.0) | 19/58 (32.8) | 8/59 (13.6) | 7/59 (11.9) | |
| (0.0, 6.0) | (1.9, 16.5) | (13.6, 36.6) | (26.5, 52.6) | (21.0, 46.3) | (6.0, 25.0) | (4.9, 22.9) | |
| 0,28 | 7.5 (-, -) | 8.4 (7.9, 9.0) | 21.0 (15.8, 27.8) | 30.2 (22.1, 41.1) | 17.2 (13.6, 21.7) | 8.7 (8.1, 9.3) | 8.8 (8.2, 9.5) |
| 0/67 (0.0) | 11/64 (17.2) | 44/63 (69.8) | 52/63 (82.5) | 47/64 (73.4) | 13/63 (20.6) | 14/60 (23.3) | |
| (0.0, 5.4) | (8.9, 28.7) | (57.0, 80.8) | (70.9, 90.9) | (60.9, 83.7) | (11.5, 32.7) | (13.4, 36.0) | |
| 0 | 7.5 (-, -) | 7.5 (-, -) | 7.5 (-, -) | 8.1 (7.7, 8.6) | 8.5 (7.9, 9.1) | 7.8 (7.5, 8.1) | 7.9 (7.3, 8.6) |
| 0/63 (0.0) | 0/63 (0.0) | 0/63 (0.0) | 7/62 (11.3) | 11/62 (17.7) | 3/61 (4.9) | 2/61 (3.3) | |
| (0.0, 5.7) | (0.0, 5.7) | (0.0, 5.7) | (4.7, 21.9) | (9.2, 29.5) | (1.0, 13.7) | (0.4, 11.3) | |
| SLU PRNT | |||||||
| 0,7 | 2.0 (-,-) | 7.2 (4.9, 10.6) | 18.6 (12.6, 27.6) | 27.4 (19.1, 39.3) | 25.2 (17.8, 35.5) | 6.3 (4.8,8.1) | 6.6 (5.0, 8.7) |
| 0/60 (0) | 13/59 (22.0) | 31/59 (52.5) | 35/59 (59.3) | 33/58 (56.9) | 7/59 (11.9) | 10/59 (16.9) | |
| (0.0, 6.0) | (12.3, 34.7) | (39.1, 65.7) | (45.7, 71.9) | (43.2, 69.8) | (4.9, 22.9) | (8.4, 29.0) | |
| 0,28 | 2.0 (-,-) | 10.4 (7.8, 13.9) | 119.6 (79.5, 179.9) | 212.2 (146.6, 307.2) | 119.7 (86.0, 166.7) | 13.9 (10.6, 18.2) | 10.8 (8.2, 14.2) |
| 0/68 (0) | 22/64 (34.4) | 54/63 (85.7) | 60/63 (95.2) | 60/64 (93.8) | 26/63 (41.3) | 20/60 (33.3) | |
| (0.0, 5.3) | (22.9, 47.3) | (74.6, 93.3) | (86.7, 99.0) | (84.8, 98.3) | (29.0, 54.4) | (21.7, 46.7) | |
| 0 | 2.0 (-,-) | 2.0 (-, -) | 2.0 (2.0, 2.1) | 7.0 (5.3, 9.3) | 8.5 (6.6, 10.9) | 4.2(3.3,5.2) | 4.1 (3.2,5.3) |
| 0/63 (0) | 0/63 (0) | 0/63 (0) | 12/62 (19.4) | 11/62 (17.7) | 6/61 (9.8) | 5/61 (8.2) | |
| (0.0, 5.7) | (0.0, 5.7) | (0.0. 5.7) | (10.4, 31.4) | (9.2, 29.5) | (3.7, 20.2) | (2.7, 18.1) | |
| BN ELISA | |||||||
| 0,7 | 25.3 (24.7, 25.9) | 43.1 (35.6, 52.2) | 74.0 (60.5, 90.7) | 108.7 (87.7, 134.8) | 99.3 (81.0, 121.7) | 37.6 (32.7. 43.3) | 40.6 (33.1, 49.9) |
| 1/60 (1.7) | 30/59 (50.8) | 48/59 (81.4) | 54/59 (91.5) | 54/58 (93.1) | 27/59 (45.8) | 26/59 (44.1) | |
| (0.0, 8.9) | (37.5, 64.1) | (69.1, 90.3) | (81.3, 97.2) | (83.3, 98.1) | (32.7, 59.2) | (31.2, 57.6) | |
| 0,28 | 26.3 (24.6, 28.2) | 63.7 (52.2, 77.8) | 269.2 (195.1, 371.4) | 501.7 (364.1, 691.3) | 311.8 (231.7, 419.7) | 57.4 (47.8, 68.9) | 50.3 (41.9, 60.2) |
| 3/67 (4.5) | 46/64 (71.9) | 57/63 (90.5) | 61/63 (96.8) | 60/64 (93.8) | 46/63 (73.0) | 37/60 (61.7) | |
| (0.9, 12.5) | (59.2, 82.4) | (80.4, 96.4) | (89.0, 99.6) | (84.8, 98.3) | (60.3, 83.4) | (48.2, 73.9) | |
| 0 | 25.8 (24.9, 26.8) | 25.8 (24.9, 26.8) | 27.6 (25.6, 29.8) | 56.6 (47.0, 68.1) | 63.8 (50.2, 81.1) | 35.3 (29.1, 42.9) | 35.1 (30.2, 40.8) |
| 3/63 (4.8) | 3/63 (4.8) | 7/63 (11.1) | 44/62 (71.0) | 46/62 (74.2) | 20/61 (32.8) | 22/61 (36.1) | |
| (1.0, 13.3) | (1.0, 13.3) | (4.6, 21.6) | (58.1, 81.8) | (61.5, 84.5) | (21.3, 46.0) | (24.2, 49.4) | |
| SLU ELISA | |||||||
| 0,7 | 50.0 (-, -) | 54.0 (46.3, 62.9) | 54.8 (47.1, 63.7) | 60.5 (51.0, 71.7) | 59.3 (52.7, 66.6) | 54.0 (48.7, 59.9) | 60.0 (50.4, 71.5) |
| 0/60 (0.0) | 1/59 (1.7) | 2/59 (3.4) | 7/59 (11.9) | 8/58 (13.8) | 3/59 (5.1) | 6/59 (10.2) | |
| (0.0, 6.0) | (0.0, 9.1) | (0.4, 11.7) | (4.9, 22.9) | (6.1, 25.4) | (1.1, 14.1) | (3.8, 20.8) | |
| 0,28 | 50.0 (-, -) | 54.4 (50.4, 58.8) | 280.1 (203.9, 384.7) | 510.9 (373.0, 699.7) | 319.6 (244.2, 418.3) | 60.4 (54.1, 67.5) | 59.3 (53.8, 65.4) |
| 0/67 (0.0) | 5/64 (7.8) | 47/63 (74.6) | 55/63 (87.3) | 54/64 (84.4) | 11/63 (17.5) | 11/60 (18.3) | |
| (0.0, 5.4) | (2.6, 17.3) | (62.1, 84.7) | (76.5, 94.4) | (73.1, 92.2) | (9.1, 29.1) | (9.5, 30.4) | |
| 0 | 50.0 (-, -) | 50.0 (-, -) | 50.0 (-, -) | 50.0 (-, -) | 52.7 (50.0, 55.5) | 50.0 (-, -) | 50.8 (49.2, 52.5) |
| 0/63 (0.0) | 0/63 (0.0) | 0/63 (0.0) | 0/62 (0.0) | 4/62 (6.5) | 0/61 (0.0) | 1/63 (1.6) | |
| (0.0, 5.7) | (0.0, 5.7) | (0.0, 5.7) | (0.0, 5.8) | (1.8, 15.7) | (0.0, 5.9) | (0.0, 8.8) | |
| IFN-γ ELISPOT | |||||||
| Days | 0 | 4 | 8 | 14 | 28 | 180 | 365 |
| 0,7 | 0.55 (0.95) | 348.51 (287.41) | 305.85 (279.13) | 279.20 (250.48) | 173.79 (211.90) | 96.68 (116.38) | 58.97 (81.12) |
| 0/59 (0.0) | 50/58 (86.2) | 48/59 (81.4) | 46/57 (80.7) | 37/59 (62.7) | 31/57 (54.4) | 25/59 (42.4) | |
| (0.0, 6.1) | (74.6, 93.9) | (69.1, 90.3) | (68.1, 90.0) | (49.1, 75.0) | (40.7, 67.6) | (29.6, 55.9) | |
| 0,28 | 6.40 (39.32) | 97.95 (156.58) | 202.34 (243.37) | 243.66 (274.06) | 185.66 (185.83) | 121.04 (113.05) | 73.14 (79.75) |
| 1/65 (1.5) | 26/63 (41.3) | 42/62 (67.7) | 42/67 (67.7) | 38/61 (62.3) | 41/62 (66.1) | 26/59 (44.1) | |
| (0.0, 8.3) | (29.0, 54.4) | (54.7, 79.1) | (54.7, 79.1) | (49.0, 74.4) | (53.0, 77.7) | (31.2, 57.6) | |
| 0 | 3.33 (17.62) | 3.84 (16.34) | 58.50 (86.54) | 297.62 (262.28) | 117.30 (178.92) | 45.20 (44.31) | 27.53 (39.30) |
| 1/63 (1.6) | 1/63 (1.6) | 20/63 (31.7) | 51/62 (82.3) | 27/62 (43.5) | 20/61 (32.8) | 8/59 (13.6) | |
| (0.0, 8,.5) | (0.0, 8.5) | (20.6, 44.7) | (70.5, 90.8) | (31.0, 56.7) | (21.3, 46.0) | (6.0, 25.0) | |
Group:0+7 GMTs were non-inferior to Group:0+28 at Days 4, 180, and 365 after second vaccination. On Day 14, Group:0+7 GMT=10.8 (CI:9.0;12.9) and Group:0+28 GMT= 30.2 (CI:22.1;41.1). The proportion of subjects with positive titers for Group:0+28 are statistically significantly greater than Group:0+7 after second vaccination at Days 8, 14 and 28 (P values < 0.0001). Day 14 responders were 23/59 (39.0%, CI:26.5,52.6) and 52/63 (82.5%, CI:70.9,90.9) for Group:0+7 and Group:0+28, respectively.
There were statistically significantly larger GMT and proportion of subjects with positive responses for Group:0+7 after vaccination 2 compared to Group:0 after a single dose on Days 8 and 14 (P values ≤0.0041 and P values ≤0.0006, respectively). Day 14 GMTs were 10.8 (CI:9.0;12.9) and 8.1 (CI:7.7;8.6) for Group 0+7 and Group 0, respectively. Day 14 responders were 23/59 (39.0%,CI:26.5,52.6) and 7/62 (11.3%,CI:4.7,21.9) for Group:0+7 and Group:0, respectively.
SLU PRNT (Figure 2; Table 3)
Based on GMTs, there was insufficient evidence to conclude Group:0+7 was non-inferior to Group:0+28 at any of the 6 time points after second vaccination. The Day 14 GMT (95% CI) were 27.4 (CI:19.1;39.3) and 212.2 (CI:146.6;307.2)] for Group:0+7 and Group:0+28, respectively. The proportion of subjects with positive titers for Group:0+28 were statistically significantly greater than Group:0+7 after second vaccination at Days 8, 14, 28 and 180 (P values≤0.0003) but not Day 4 (P value = 0.16). The difference on Day 365 approached significance (P=0.06).
There were statistically significantly larger GMTs and proportion of subjects with positive responses for Group:0+7 after vaccination 2 compared to Group:0 after a single vaccination on Days 4, 8, 14, and 28 (P< 0.0001). On Day 14, Group:0+7 GMT=27.4 (CI:19.1;39.3) and Group:0 GMT=7.0 (CI:5.3;9.3). On Day 14, Group:0+7 responders = 35/59 (59.3%, CI:45.7,71.9) and Group:0 = 12/62 (19.4%, CI:10.4,31.4). On Days 180 and 365, the difference in GMT remained significant (P values ≤ 0.0192), however, the difference in proportion positive is not significant on Days 180 and 365 (P values=0.78 and 0.18, respectively).
Bavarian Nordic ELISA (Figure 3, Table 3)
Figure 3.
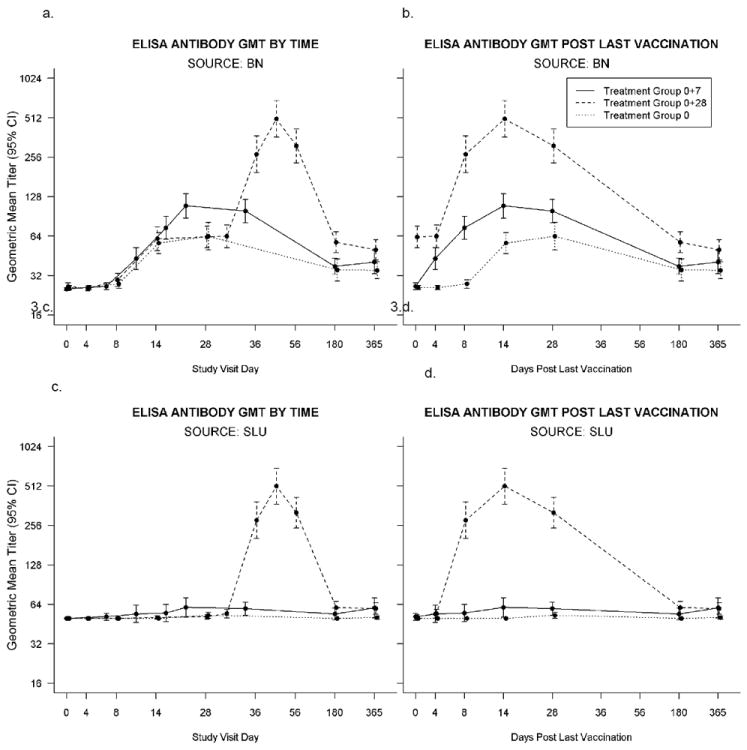
a-d. ELISA: 3.a-b. ELISA conducted at Bavarian Nordic using BN MVA as the assay antigen, positive titer ≥50. Time points represent the number of days after the first vaccination for all three groups (3.a.) and after last vaccination for all three groups, i.e. after the single vaccination in Group:0, and after the second vaccination for the other two groups (Group:0+7 and Group:0+28) (3.b.) vaccinations. Placebo recipient data is not shown. 3.c-d. ELISA Antibody conducted at Saint Louis University using ATCC MVA (VR-1508) as the assay antigen, positive titer > 50. Time points represent the number of days after the first vaccination for all three groups (3.c.) and after last vaccination for all three groups, i.e. after the single vaccination in Group:0, and after the second vaccination for the other two groups (Group:0+7 and Group:0+28) (3.d.). Placebo recipient data is not shown.
Based on GMTs, Group:0+7 was non-inferior to Group:0+28 at Days 4, 180, and 365 after vaccination 2. Day 14 GMT = 108.7 (CI:87.7,134.8) and GMT = 501.7 (CI:364.1,691.3) for Group:0+7 and Group:0+28, respectively. The proportion of subjects with positive titers for Group:0+28 was significantly greater than Group:0+7 after second vaccination at Days 4 and 180 (P values ≤ 0.02). Day 14 responders were 54/59 (91.5%) and 61/63 (96.8%) for Group:0+7 and Group:0+28, respectively.
There were statistically significantly larger GMT and proportion of subjects with positive responses for Group:0+7 after vaccination 2 compared to Group:0 after one vaccination on Days 4, 8, 14 and 28 (P values all ≤0.0061 and P values all ≤0.0066, respectively). Day 14 GMT were 108.7 (CI:87.7;134.8) and 56.6 (CI:47.0;68.1) for Group:0+7 and Group:0 respectively. Day 14 responders were 54/59 (91.5%) and 44/62 (71.0%) for Group:0+7 and Group:0, respectively.
The GMT (and proportions with titers ≥50) for Group:0+7, Group:0+28 and Group:0 were 37.6 (45.8), 57.4 (73.0) and 35.3 (32.8) at 6 months and 40.6 (44.1), 50.3 (61.7) and 35.1 (36.1) at 12 months, respectively.
SLU ELISA (Figure 3, Table 3)
Based on GMT, Group:0+7 was non-inferior to Group:0+28 at Days 4, 180, and 365 after second vaccination. Day 14 GMT were 60.5 (CI:51.0;71.7) and 510.9 (CI:373.0;699.7] for Group:0+7 and Group:0+28, respectively. The proportion of subjects with positive titers for Group:0+28 are statistically significantly greater than Group:0+7 after second vaccination at Days 8, 14, 28, and 180 (P values ≤0.045). Day 14 responders were 7/59 (11.9%, CI:4.9,22.9) and 55/63 (87.3%, CI:76.5,94.4) for Group:0+7 and Group:0+28.
There were statistically significantly larger GMT and proportion of subjects with positive responses for Group:0+7 after dose 2 timepoints compared to Group:0 after a single dose on Day 14 (P values = 0.0287 and 0.0054, respectively). On Day 14, GMT were 60.5 (CI:51.0;71.7) and 50.0 (CI:N/A as all subjects = 50.0] for Group:0+7 and Group:0, respectively. Day 14 responders were 7/59 (11.9%,CI:4.9,22.9) and 0/62 (0.0%,CI:0,5.8) for Group:0+7 and Group:0+7, respectively.
IFN-γ ELISPOT (Figure 4, Table 3)
Figure 4.
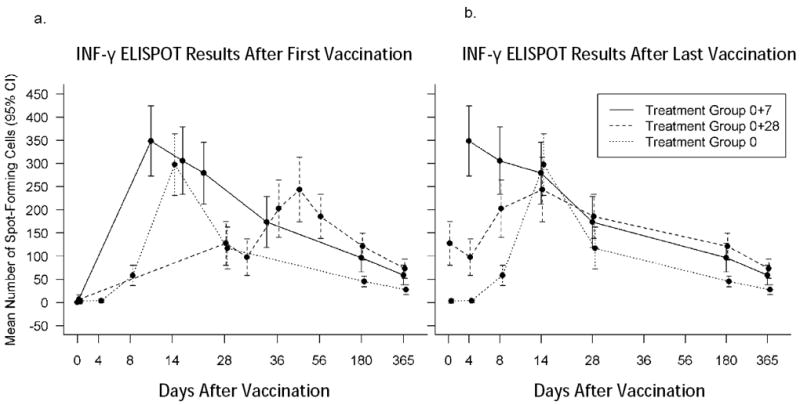
a-b. ElISPOT Results: Mean spot forming units with confidence intervals. Time points represent the number of days after the first vaccination for all three groups (4.a.) and after last vaccination for all three groups, i.e. after the single vaccination in Group:0, and after the second vaccination for the other two groups (Group:0+7 and Group:0+28) (4.b.). Vaccinia-Western Reserve was used as the assay antigen. Placebo recipient data is not shown.
The mean difference in the number of spot-forming cells was statistically significantly smaller for Group:0+28 at Days 4 and 8 after the second vaccination compared to Group:0+7 (P values ≤ 0.03). The difference in proportion of subjects with positive responses between Group:0+28 and Group:0+7 was statistically significant at Day 4 only after vaccination 2. Mean spot-forming cells on Day 14 after vaccination 2 were 279.20 (SD 250.48) and 243.66 (SD 274.06) for Group:0+7 and Group:0+28, respectively; responders were 46/57 (80.7%, CI:68.1,90.0) and 42/62 (67.7%, CI:54.7,79.1) for Group:0+7 and Group:0+28. Neither the mean difference in the number of spot forming cells nor the proportion of subjects with positive responses between Group:0 on Day 14 after one vaccination and Group:0+7 on Day 8 after vaccination 2 was significant. The number of responders at Day 28 after last vaccination was 37/59 (62.7%), 38/61 (62.3%) and 27/62 (43.5%) for Group:0+7, Group:0+28, and Group 0, respectively.
The median number of spot-forming cells/well for Group:0+7 after vaccination 2 was significantly larger than Group:0 after one vaccination at all time points (Wilcoxon rank sum test P values ≤0.0236) except on Day 14 (Wilcoxon rank sum test P value = 0.7989). The proportion of subjects with positive responses was larger for Group:0+7 at all time points (P values ≤0.0452) except on Day 14 (P value >0.9999). The median spot-forming cells on Day 14 after last dose were 246.5 (Range 1.67-939.50) and 214.7 (Range 0.09-951.33) for Group:0+7 and Group:0. Days 14 responders after last dose were 46/57 (80.7%,CI:68.1,90.0) and 51/62 (82.3%,CI:70.5,90.8) for Group:0+7 and Group:0.
DISCUSSION
This is the first clinical trial to evaluate a compressed schedule of non-replicating vaccinia virus, MVA, for use after a post-event release of smallpox virus. A postexposure immunization with modified vaccinia vaccine provided solid protection in a murine model of human smallpox [17]. Unlike replicating vaccine virus in humans, MVA requires a second dose of vaccine to mount a robust antibody response.
In this study, while the assays performed by SLU and BN to measure ELISA and PRNT titers used different antigens to compare antibody results to the vaccine antigen and a commercially available antigen, the overall immunogenicity results were similar. A second vaccination was required to mount a substantial antibody response; the 28-day rest prior to the second vaccination resulted in a greater antibody response and more responders than a 7-day rest; consistent with results from other studies [11-12]. However, based on SLU PRNT results after vaccination 2, unlike BN PRNT results, there is insufficient evidence to conclude Group:0+7 was non-inferior to Group:0+28, and in fact, Group 0+28 was at least two-fold higher than Group 0+7 at Days 8, 14, and. Interestingly, Group:0+7 reached a peak titer more rapidly than Group:0+28 post vaccination 1 and, using the BN ELISA results, there was a substantial number of seroconverters (BN ELISA) in Group:0+7. In both groups, peak titers occurred 14 days after the last vaccination and time to seroconversion was no more than 14 days.
The actual protective titer of MVA antibody in humans is not known; efficacy testing may rely on animal challenge models. However, 2 doses of MVA will mitigate the primary Jennerian response to scarification with Dryvax [10, 12]. An alternative approach is the comparison of MVA antibodies in human sera from well-controlled clinical trials, to neutralize variola and vaccinia viruses in vitro. In one such study, sera from both MVA recipients and Dryvax recipients demonstrated similarly robust 60% or 90% variola neutralization titers suggesting that the Day:0+28 MVA vaccination regimen would be as effective as a single Dryvax vaccination against smallpox [18]. Serum from the same clinical trial was used to correlate between PRNTs when different viruses and different strains of virus were used as the neutralization target. Use of Dryvax as the neutralization target resulted in significantly lower 90% PRNTs than use of variola as the neutralizing target and use of MVA resulted in significantly higher PRNTs than using variola [19].
In our study, using BN MVA as the assay antigen, the Day:0+7 MVA vaccine schedule resulted in 91% seroconverters and 39% with PRNT GMT above the cut-off compared to 97% and 82% in the Group:0+28 day group, respectively. Although the titers for the compressed schedule were less than that of Group:0+28, it is possible that CMI which occurred in the greatest proportion of responders within two weeks after last vaccination would play an added role in limiting disease.
In conclusion, IMVAMUNE is safe and well tolerated. A second dose of IMVAMUNE at Day 28, compared to Day 7 and Day 0 alone, provided a greater antibody response and the highest proportion of responders.
Reintroduction of Variola major as an agent of bioterrorism remains a concern.
A compressed schedule of MVA was evaluated for use in a post event scenario.
MVA is well tolerated when given as two standard doses at Days 0 and 28 or 0 and 7.
A 2nd dose of MVA at Day 28 compared to Day 7 provided greater antibody responses.
INF-γ expression was greatest within two weeks after last vaccination.
Acknowledgments
The authors would like to thank Robert Johnson, Carol Ostrye and Stephen Heyse at the Division of Microbiology and Infectious Diseases, NIH; Janice Tennant, Tammy Blevins, Yinyi Yu, Mahendra Mandava, Tara Giebe, Michelle Mitchell, Edwin Anderson and Irene Graham at the Saint Louis University Vaccine and Treatment Evaluation Unit at Saint Louis University; the nurses and personnel in the vaccine trials and clinical research units at the University of Iowa; Ina Adkins, Ken Bucheit, Katy Hepper, Courtney Walker at Case Western Reserve University; Robin Barnes, Melissa Billington, Brenda Dorsey, Martin Fitzgerald, Sandra Getlein, Maria Johnson, Panagiota Komninou, Mary-Lou Mullen, Gina Parsons, Mardi Reymann, Kim Rincavage, and Inna Ruslanova at the University of Maryland; Ellen Beswick, Gerianne Casey, Carrie Harrington, Mathuramany Rasiah, Victor Reyes, Richard Rupp, Karen Waterman at the University of Texas Medical Branch; Rowena Dolor, Christopher Woods, Lynn Harrington, Beth Patterson, Kathlene Chmielewski at Duke University; D’Arcy Gaisser, John Treanor, Carolyn Nolan, Patricia Smith, Diane O’Brien at the University of Rochester; Aruna Acharyya at the EMMES Corporation; Paul Lehmann, and Oleg Targoni at Cellular Technology Limited and to express our appreciation to the volunteers who made this study possible.
Funding
This project was funded fully or in part by the National Institute of Allergy and Infectious Diseases, National Institute of Health, Contract Numbers N01-AI-25464 and HHSN272200800003C (SF, FN, RB), N01-AI-80008 and UL1RR024979 (UI), 01-AI-80001 (UMD), N01-AI-25459 (UTMB), CTSA funding UL1RR024128 (Duke), NO1-AI-252160 (UR), Contract No. HHSN272200800013C (EMMES), NIAID contract N01-AI-40072 (BN), N01-AI-400948 (CTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russel PK. Vaccines in civilian defense against bioterrorism. Emerging Infectious Diseases. 1999;5:531–3. doi: 10.3201/eid0504.990413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson DA. The looming threat of bioterrorism. Science. 1999(b);283:1279–82. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 3.Artenstein AW, Johnson C, Marbury TC, Morrison D, Blum PS, Kemp T, Nichols R, et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naïve adults. Vaccine. 2005;23:3301–9. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 4.Monath TP, Caldwell JR, Mundt W, Fusco J, Johnson CS, Buller M, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) – a second-generation smallpox vaccine for biological defense. Inter J Infect Dis. 2004;8S2:S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey SE, Newman FK, Kennedy JS, Ennis FA, Abate G, Hoft DF. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax® in healthy vaccinia-naïve adults. Vaccine. 2009;27:1637–44. doi: 10.1016/j.vaccine.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 6.Mayr A, Hochstein-Mintzel V, Stickl H. Passage history, properties, and applicability of the attenuated vaccinia virus strain MVA [in German] Infection. 1975;3:6–14. [Google Scholar]
- 7.Hochstein-Mintzel V, Hanichen T, Huber HC, Stickl H. An attenuated strain of vaccinia virus (MVA). Successful intramuscular immunization against vaccinia and variola. ZentralblBakteriol. 1975;230:283–97. [PubMed] [Google Scholar]
- 8.Mayr A, Munz E. Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures. Zentralbl Bakteriol Orig. 1964;195:24–35. [PubMed] [Google Scholar]
- 9.Stickl HA. Smallpox vaccination and its consequences: first experiences with the highly attenuated smallpox vaccine “MVA”. Preventive Medicine. 1974;3:97–101. doi: 10.1016/0091-7435(74)90066-8. [DOI] [PubMed] [Google Scholar]
- 10.Mayr A, Stickl H, Müller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentralbl Bakteriol. 1978;167:375–90. [PubMed] [Google Scholar]
- 11.Von Krempelhuber A, Vollman J, Pokorny R, Rapp P, Wulff N, Petzold B, et al. A randomized, double-blind, dose-finding phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE®. Vaccine. 2010;28:1209–16. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, Hill H, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25:8562–73. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaman MS, Wilck MB, Baden LR, Walsh SR, Grandpre LE, Devoy C, Giri A, Noble LC, Kleinjan JA, Stevenson KE, Kim HT, Dolin R. Effect of vaccination with modified vaccinia Ankara (ACAM3000) on subsequent challenge with Dryvax. J Infect Dis. 2010;201:1353–60. doi: 10.1086/651560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman FK, Frey SE, Blevins TP, Mandava M, Bonifacio A, JR, Yan L, Belshe RB. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J Clin Micro. 2003;41:3154–3157. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA. 289:3295–3299. 2003. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 16.Helms T, Asaad RJ, Trezza RP, Lehmann PV, Tary-Lehmann M. Direct visualization of cytokine producing recall antigen specific memory T cells in HIV patients and healthy individuals. J Immunol. 2000;164:3723732. doi: 10.4049/jimmunol.164.7.3723. [DOI] [PubMed] [Google Scholar]
- 17.Paran N, Suezer Y, Lustig S, Israely T, Schwantes A, Melamed S, et al. Postexposure immunization with modified vaccinia virus Ankara or conventional Lister vaccine provides solid protection in a murine model of human smallpox. J Infect Dis. 2009;199:39–48. doi: 10.1086/595565. [DOI] [PubMed] [Google Scholar]
- 18.Evaluation of smallpox vaccines using variola neutralization. Damon IK, Davidson WB, Hughes CM, Olson VA, Smith SK, Holman RC, Frey SE, Newman F, Belshe RB, Yan L, Karem K. J Gen Virol. 2009;90:1962–6. doi: 10.1099/vir.0.010553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Analysis of variola and vaccinia virus neutralization assays for smallpox vaccines. Hughes CM, Newman FK, Davidson WB, Olson VA, Smith SK, Holman RC, Yan L, Frey SE, Belshe RB, Karem KL, Damon IK. Clin Vaccine Immunol. 2012;19:1116–8. doi: 10.1128/CVI.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


