There are very few things in medicine which are simple. Birth and mortality are relatively straightforward to define….but after that the trouble begins. To the casual observer the etiology of myocardial infarction (MI) is relatively obvious: prolonged myocardial ischemia. However, as Aldrovandi et al1 have taught us in this edition of Circulation, we still have a lot to learn about this disease. Indeed, earlier this year the updated Third Universal Definitions of Myocardial Infarction were published.2 Of course, the tacit fact underlying this publication is that even the definition of an MI remains a major ‘work in progress. ’
Nevertheless, it cannot be argued that we have come a very long way in our knowledge of plaque biology and MI. In particular, the last 2–3 decades has seen a revolution in our understanding of this disease. Thanks to a series of pivotal observations, we now appreciate that MI rarely arises due to progressive vessel narrowing that culminates in a critical flow-limiting stenosis. Rather, it is now understood that an atherosclerotic plaque can become unstable, exposing lipid, debris or other material to the blood and triggering an acute coronary artery thrombosis, which then leads to vessel occlusion and MI. As investigators we were privileged to have played a small part in elucidating the pathology of the ‘unstable plaque’ that causes MI, when in 1988 we showed with Ambrose et al that MI frequently develops from previously non-severe lesions.3 In a retrospective study of 38 patients, progression of coronary stenoses between two cardiac catheterization procedures was compared among patients presenting with MI versus stable coronary occlusion at the time of second catheterization. In the group presenting with MI the stenosis severity at the initial angiogram of the subsequent infarct related artery was significantly less severe than culprit lesions in the other group of patients that later became the site of a new total occlusion. In other words, atherosclerotic lesions causing acute MI were less severe than those progressing to stable occlusion. Soon after this study, pathological examinations of sudden coronary death victims demonstrated that plaque rupture with associated thrombosis may be present in up to 73% of cases.4 Yet another major advance was an improved histopathologic characterization and microscopic understanding of the non-obstructive lesions that cause MI, which was summarized in 2000 by Virmani et al with their paper “Lessons From Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions.”5 Here, Virmani et al described 7 categories of atherosclerotic lesions: intimal xanthoma, intimal thickening, pathological intimal thickening, fibrous cap atheroma, thin fibrous cap atheroma, calcified nodule, and fibrocalcific plaque. Of relevance, they also clarified that either rupture or erosion were the main mechanisms of plaque disturbance that could culminate in arterial thrombosis and MI. More recently, plaque biology has moved to the cellular and sub-cellular level, progressively unraveling the complex inflammatory and molecular signaling pathways that govern atherosclerosis and vascular injury.
However, despite this impressive progress, as every cardiologist will attest all too often we have been confronted with the diagnostic and management dilemma of a “troponin positive” acute MI patient with angiographically “normal coronary arteries”. The magnitude of this problem is significant. Data pooled from three Thrombolysis in Myocardial Infarction (TIMI) trials on patients presenting with non-ST-segment elevation acute coronary syndromes identified that 4–5% of patients had “normal coronary arteries”, while a similar proportion had mild or “non-obstructive” disease.6 While some of these patients may ultimately be diagnosed with conditions such as demand ischemia, myocarditis or Stress (Takotsubo) cardiomyopathy, an unsatisfying number are ultimately discharged from hospital without a clear etiology for their MI-like presentation. Or, perhaps even more unsatisfactorily, patients with a classic atherosclerotic risk factor profile and typical MI presentation but angiographically “normal coronary arteries” may be ascribed with unusual diagnoses such as coronary artery embolism or regional myocarditis. While these conditions certainly exist, our experience has been that these ‘other’ diagnoses are evoked far more frequently than appears reasonable. In short, there has long been a population of “missing MI” patients with what appears to be clinically typical atherosclerotic MI, but a remarkable absence of angiographic coronary artery lesions.
In this issue of Circulation, Aldrovandi et al have eloquently closed the loop on a proportion of these “missing MI” patients.1 In a simple but effective study, they evaluated the presence and morphological characteristics of coronary lesions using CT coronary angiography (CTCA) in patients with acute MI documented by late gadolinium enhancement cardiac MRI (LGE-MRI), but without significant coronary lesions at angiography (<50% angiographic stenosis). In what was a relatively small final sample of 50 patients with acute MI confirmed by LGE-MRI, half had angiographically “normal coronary arteries”, while the remainder had non-obstructive disease. A total of 101 plaques were identified in these 50 patients, with 61 plaques (60%) being in the coronary vessel which subtended the MI territory. By comparison to plaques in the non-infarct vessels, those in the infarct-related artery displayed less calcification, greater plaque area and a greater extent of positive remodeling – features that generally characterize ‘vulnerable’ atherosclerotic lesions. Taking this data as a whole, Aldrovandi et al have essentially identified a “smoking gun” in a proportion of “missing MI” patients which may be the causative factor in their infarction, being an increased burden of vulnerable-type plaques in infarct-related vessels. In our opinion, this amounts to reasonably strong (albeit circumstantial) evidence implicating angiographically non-obstructive but unstable plaques as being causative in a sub-set of patients with unexplained MI. Furthermore, these data are intuitive and fit comfortably with our current paradigms for plaque biology.
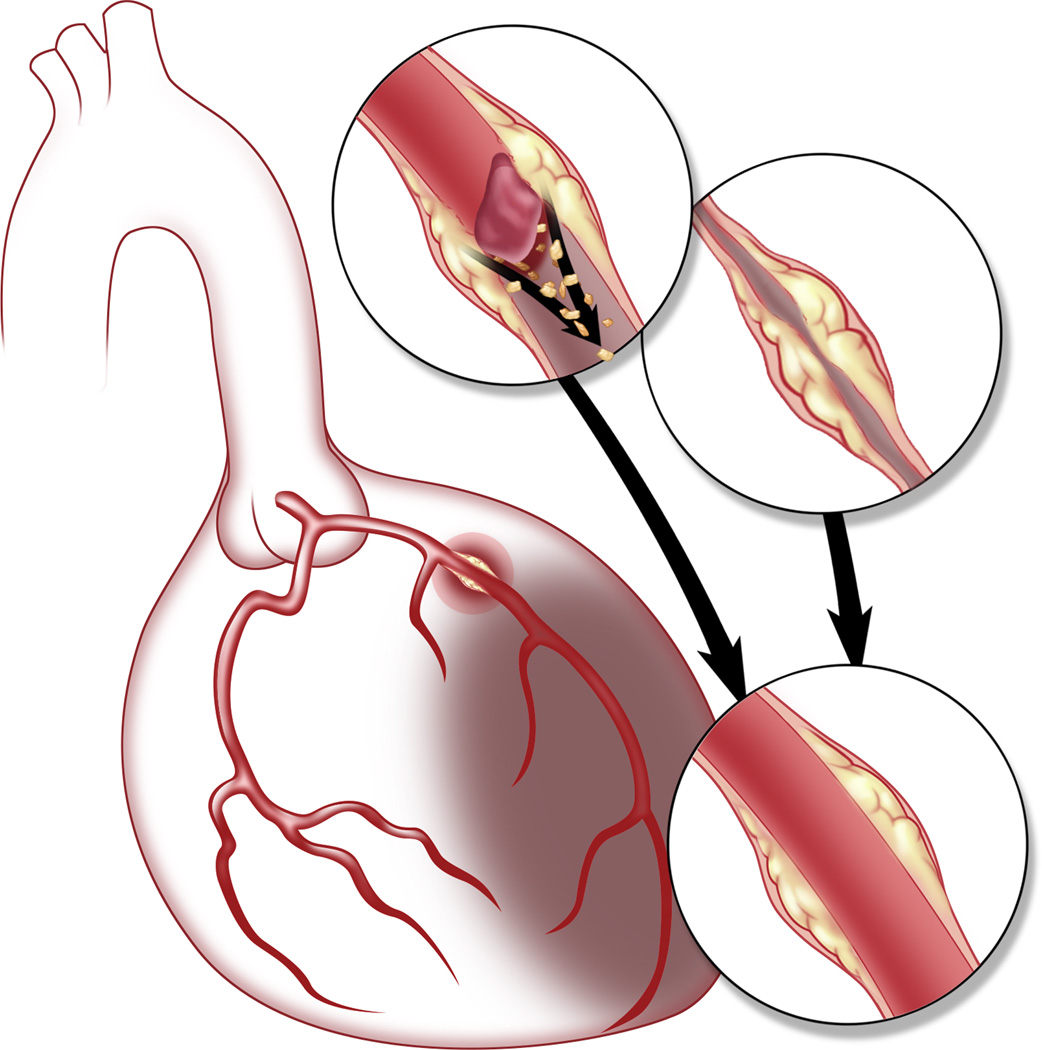
Importantly, this study only identified a “smoking gun”. The investigators did not document the “gun being fired”, that is, they did not image or observe what actually occurs in these non-obstructive lesions at the point in time when the MI is in evolution. As regards for what may be occurring to cause acute MI in these patients, vast clinical and post-mortem experience has demonstrated that thrombotic vessel occlusion is the principal substrate causing the majority of MIs. Taking this body of evidence into account, until proven otherwise we concur with Aldrovandi et al that transitory thrombotic occlusion (with thrombus resolution) remains a likely mechanism to explain their observations. However, we hypothesize that distal embolization of plaque-derived lipid, debris and other material may also be involved. It is known that embolization of debris is a factor in acute MI, and is implicated in the ‘no reflow’ phenomenon.7 Indeed, the embolization of plaque contents into the distal microcirculation might not only explain the MI presentation, but may also account for the fact that there was minimal encroachment of the (remaining) plaque on the vessel lumen, because much of plaque may have embolized downstream (Figure). This would be akin to the situation in pulmonary embolism when no thrombus can be found in the lower limbs because it has already embolized from the leg veins and travelled to the lungs.
Figure.
Schematic representation of potential mechanisms of acute MI in patients found to have only mild obstruction or “normal coronary arteries” at coronary angiography. In this figure, a non-obstructive plaque is seen in the proximal left anterior descending coronary artery with apical left ventricular MI. The upper insets demonstrate potential mechanisms of MI, specifically transient thrombotic occlusion with or without embolization of plaque contents, and coronary artery spasm. Final inset (lower) shows restoration of normal flow with minimal residual luminal stenosis.
An additional mechanism that may be involved is coronary artery spasm (Figure). While the precise anatomical site of spasm within a vessel may differ somewhat from that of an atherosclerotic plaque,8 recent research has demonstrated that spasm and atherosclerotic disease often co-exist and that coronary spasm is responsible for a small but definite proportion of MIs.8,9 Multiple factors are thought to contribute to coronary spasm including altered environmental, genetic, inflammatory, rheologic and vascular aspects (see Yasue for review9). Several of these may have been operative in the patients described by Aldrovandi et al,1 and we believe that coronary artery spasm must be included in the differential diagnosis of any “missing MI” patient.
An important additional message remains in this study. Of the original 71 patients who presented with typical clinical features of acute MI with < 50% angiographic stenosis and who underwent MRI scanning, only 50 had evidence of MI (late enhancement). Therefore, in 30% of patients (21/71) the investigators were unable to confirm MI, let alone explain etiology.1 Furthermore, in 8 of the final 50 patients with MRI-confirmed acute MI, CTCA demonstrated no coronary plaques whatsoever. Therefore, there remain a small but not insignificant number of patients in whom we have no firm explanation for their MI-like presentation. While spasm may be implicated in a proportion of these, further innovative research is required to investigate this interesting sub-group and define the exact pathology and mechanism of their MI-like state.
Moving back to the bedside, there are clear therapeutic implications from this data. Foremost, atherosclerosis is likely to be a major factor in patients presenting with acute MI and angiographically mild coronary artery disease. While LGA-MRI and CTCA is not practical or necessary in all cases, these patients require risk factor assessment, life style modification and appropriate medical therapy.10 We suggest that in the absence of contraindications aspirin and statin therapy might be considered as the minimum therapy, but there should also be a low threshold for adding an ACE-inhibitor or beta-blocker in appropriate patients. Furthermore, current guidelines give a Class I recommendation for the additional use of clopidogrel or ticagrelor for up to 12 months in MI patients in whom an initial conservative (ie, noninvasive) strategy is selected.11 Nevertheless, while these considerations are critical, in the absence of direct evidence that is specific to the situation of MI with angiographically non-obstructive disease the management of these patients should revolve around balanced and patient-specific assessment and sound clinical judgment.
In conclusion, this editorial presents us with a timely opportunity to revisit a statement that originated from our institution over 2 decades ago. In 1988 Ambrose et al made the comment that “disruption of a mild or moderate atherosclerotic plaque with resultant thrombosis formation and total or subtotal occlusion probably explained (the) MI.”3 On the basis of the work by Aldrovandi et al and other research cited here we now suggest a revision of this comment: “disruption of a mild, moderate or angiographically insignificant atherosclerotic plaque with resultant thrombosis formation and total or subtotal occlusion, with or without spasm or embolization, probably explains most cases of MI.”
ACKNOWLEDGEMENTS
Jason C. Kovacic is supported by National Institutes of Health Grant 5K08HL111330.
No specific funding or grant was used to prepare this manuscript. Jason C. Kovacic is supported by National Institutes of Health Grant 5K08HL111330.
Footnotes
We have no financial disclosures or relationships to report.
REFERENCES
- 1.Aldrovandi A, Cademartiri F, Arduini D, Lina D, Ugo F, Maffei E, Menozzi A, Martini C, Palumbo A, Bontardelli F, Gherli T, Ruffini L, Ardissino D. Computed Tomography Coronary Angiography in Patients with Acute Myocardial Infarction without Significant Coronary Stenosis. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.117598. Current Issue. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992;85:I19–I124. [PubMed] [Google Scholar]
- 5.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 6.Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med. 2006;166:1391–1395. doi: 10.1001/archinte.166.13.1391. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz BG, Kloner RA. Coronary no reflow. Journal of Molecular and Cellular Cardiology. 2012;52:873–882. doi: 10.1016/j.yjmcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa H, Morikawa Y, Mizuno Y, Harada E, Ito T, Matsui K, Saito Y, Yasue H. Coronary spasm preferentially occurs at branch points: an angiographic comparison with atherosclerotic plaque. Circ Cardiovasc Interv. 2009;2:97–104. doi: 10.1161/CIRCINTERVENTIONS.108.803767. [DOI] [PubMed] [Google Scholar]
- 9.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kovacic JC, Fuster V. From Treating Complex Coronary Artery Disease to Promoting Cardiovascular Health: Therapeutic Transitions and Challenges, 2010–2020. Clin Pharmacol Ther. 2011;90:509–518. doi: 10.1038/clpt.2011.173. [DOI] [PubMed] [Google Scholar]
- 11.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]