Abstract
This study examined the sequences of the two rRNA (rrn) operons of pathogenic non-cultivable treponemes, comprising 11 strains of T. pallidum ssp. pallidum (TPA), five strains of T. pallidum ssp. pertenue (TPE), two strains of T. pallidum ssp. endemicum (TEN), a simian Fribourg-Blanc strain and a rabbit T. paraluiscuniculi (TPc) strain. PCR was used to determine the type of 16S–23S ribosomal intergenic spacers in the rrn operons from 30 clinical samples belonging to five different genotypes. When compared with the TPA strains, TPc Cuniculi A strain had a 17 bp deletion, and the TPE, TEN and Fribourg-Blanc isolates had a deletion of 33 bp. Other than these deletions, only 17 heterogeneous sites were found within the entire region (excluding the 16S–23S intergenic spacer region encoding tRNA-Ile or tRNA-Ala). The pattern of nucleotide changes in the rrn operons corresponded to the classification of treponemal strains, whilst two different rrn spacer patterns (Ile/Ala and Ala/Ile) appeared to be distributed randomly across species/subspecies classification, time and geographical source of the treponemal strains. It is suggested that the random distribution of tRNA genes is caused by reciprocal translocation between repetitive sequences mediated by a recBCD-like system.
Introduction
rRNA genes are co-localized in rRNA (rrn) operons. The typical bacterial rrn operon consists of 16S–23S–5S rRNA genes. In addition, rrn operons may contain tRNA genes and regulatory regions. The rrn operons are highly transcribed in bacteria (Condon et al., 1992), especially during the exponential phase of growth and in fast-growing bacteria. It is generally believed that bacteria with a short generation time have multiple rrn operons in the genome. Multiple copies of 16S and 23S rRNA genes in an organism are almost identical (Pei et al., 2009, 2010), suggesting homogenization of rRNA genes through homologous recombination (Liao, 2000). The 16S and 23S rRNA genes are widely used in bacterial phylogenetic studies, but the 5S rRNA genes are too short to be useful for this purpose.
In addition to the rRNA genes, the rrn operons contain intergenic spacer regions (ISRs). The ISRs are not involved in ribosomal function, so they are not under functional constraints, resulting in higher ISR microheterogeneity among bacterial species and strains (de Vries et al., 2006; Gürtler, 1999). The 16S–23S ISRs vary in length, tRNA composition and intragenomic nucleotide diversity (Stewart & Cavanaugh, 2007), and have been used for bacterial identification, molecular typing (Indra et al., 2010; Sadeghifard et al., 2006) and evolutionary studies (Antón et al., 1998).
In this study, we used the variation present in the rrn operons to assess evolutionary relationships among several pathogenic non-cultivable treponemes. The organisms studied comprised Treponema pallidum and Treponema paraluiscuniculi species and an unclassified simian isolate (Fribourg-Blanc). The species of T. pallidum comprised T. pallidum ssp. pallidum (TPA), T. pallidum ssp. pertenue (TPE) and T. pallidum ssp. endemicum (TEN), the aetiological agents of syphilis, yaws and endemic syphilis, respectively. T. paraluiscuniculi (TPc), the aetiological agent of rabbit syphilis, and the simian Fribourg-Blanc isolate are closely related to the T. pallidum spp. (Šmajs et al., 2011a).
Two rrn operons have been observed in pathogenic treponemes (Fukunaga et al., 1992) composed of 16S–23S–5S rRNA genes. The 16S–23S ISRs of the TPA Nichols strain (Fraser et al., 1998) contain tRNA-Ile (tRNA-Ile-1; TP_t12) and tRNA-Ala (tRNA-Ala-3; TP_t15) genes within the rrn1 and rrn2 operons, respectively. The same spacer pattern (Ile/Ala) has been observed in other complete treponemal genomes (Giacani et al., 2010; Matějková et al., 2008; Šmajs et al., 2011b). In contrast, the TPE CDC-2 and TPE Gauthier strain genomes (Čejková et al., 2012) show an Ala/Ile spacer pattern, where the TP_t12 and TP_t15 orthologues are located within the rrn2 and rrn1 operons, respectively.
Stamm et al. (2002) used the sequences of 16S–23S ISRs for molecular typing of dermatitis-associated treponemes in cattle. These treponemes are divided into three phylotypes, which cluster within the group of human saprophytic treponemes (Treponema denticola, Treponema phagedenis and Treponema vincentii). Centurion-Lara et al. (1996) examined the TPA Nichols and TPE Gauthier strains and found no difference in the 16S–23S ISRs.
Closely related spirochaetes in the genus Borrelia contain two distinct rrn operon patterns. Whereas Lyme disease agent (Borrelia burgdorferi sensu lato) harbours a unique operon composed of 16S–23S–5S–23S–5S rRNA genes, agents of relapsing fever carry an operon consisting of 16S–23S–5S rRNA genes (Fraser et al., 1997; Schwartz et al., 1992). Two typing systems have been developed using the 16S–23S ISR, which includes both the tRNA-Ala and tRNA-Ile genes (Bunikis et al., 2004; Liveris et al., 1996). The typing systems have been applied to differentiate species within B. burgdorferi sensu lato in North America (Bunikis et al., 2004), to study populations of tick- and bird-borne Borrelia garinii in Eurasia (Comstedt et al., 2009) and to study the association between the B. burgdorferi sensu stricto genotype and dissemination of infection (Hanincová et al., 2008; Wormser et al., 2008).
In this study, we compared the sequences of both rrn operons among pathogenic treponemes, comprising 11 strains of TPA, five strains of TPE, two strains of TEN, a simian Fribourg-Blanc isolate and a rabbit TPc strain. We also studied 16S–23S ISRs in 30 clinical samples positive for T. pallidum DNA.
Methods
Strains used in this study.
The rrn operon sequences were examined in 20 strains of the genus Treponema (Table 1), comprising a baboon isolate (unclassified T. pallidum strain Fribourg-Blanc), a rabbit syphilis strain (TPc) and 18 human strains (TPA, TPE and TEN). Thirty clinical samples (named 2K, 4K, 6K, 15K, 24K, 27K, 34K, 40K, 44K, 47K, 49K, 51K, 52K, 53K, 63K, 73K, 91K, 6000, 9888, 14048, 14207, 16142, RL86Z, RL89BZ, RL95B, RL102B, RL104B, RL110B, RL111B and RL116A) were tested for the presence of 16S–23S ISR sequences encoding either tRNA-Ile or tRNA-Ala, with positive detection of treponemal DNA in all samples. More detailed data on these samples were published recently (Flasarová et al., 2012).
Table 1. Treponema strains used in this study.
–, Not known.
| Strain | Treponema (sub)species | Place of isolation | Date of isolation | Reference | Source of material* |
| Bal 73-1 | TPA | Baltimore, USA | 1968 | Hardy et al. (1970) | David L. Cox, CDC, Atlanta, GA, USA |
| Bosnia A | TEN | Bosnia | 1950 | Turner & Hollander (1957) | Sylvia M. Bruisten, PHL, Amsterdam, NL |
| CDC-1 | TPE | Dersuso, Ghana | 1980 | Liska et al. (1982) | David L. Cox, CDC, Atlanta, GA, USA |
| CDC-2 | TPE | Akorabo, Ghana | 1980 | Liska et al. (1982) | Steven J. Norris, UT, Houston, TX, USA |
| Cuniculi A | paraluiscuniculi | – | pre-1957 | Turner & Hollander (1957) | Steven J. Norris, UT, Houston, TX, USA |
| DAL-1 | TPA | Dallas, USA | 1991 | Wendel et al. (1991) | David L. Cox, CDC, Atlanta, GA, USA |
| Fribourg-Blanc | Simian isolate | Guinea | 1966 | Fribourg-Blanc & Mollaret (1969) | David L. Cox, CDC, Atlanta, GA, USA |
| Gauthier | TPE | Brazzaville, Congo | 1960 | Gastinel et al. (1963) | Steven J. Norris, UT, Houston, TX, USA |
| Grady | TPA | Atlanta, USA | 1980s | – | David L. Cox, CDC, Atlanta, GA, USA |
| Haiti B | TPA | Haiti | 1951 | Turner & Hollander (1957) | David L. Cox, CDC, Atlanta, GA, USA |
| Iraq B | TEN | Iraq | 1951 | Turner & Hollander (1957) | Kristin N. Harper, Emory University, Atlanta, GA, USA |
| Madras | TPA | Madras, India | 1954 | Laboratory notebook of Rob George CDC | David L. Cox, CDC, Atlanta, GA, USA |
| Mexico A | TPA | Mexico City, Mexico | 1953 | Turner & Hollander (1957) | David L. Cox, CDC, Atlanta, GA, USA |
| MN-3 | TPA | Minnesota, USA | – | – | David L. Cox, CDC, Atlanta, GA, USA |
| Nichols | TPA | Washington, DC, USA | 1912 | Nichols & Hough (1913) | Steven J. Norris, UT, Houston, TX, USA |
| Philadelphia 1 | TPA | Philadelphia, USA | 1988 | – | David L. Cox, CDC, Atlanta, GA, USA |
| Philadelphia 2 | TPA | Philadelphia, USA | – | – | David L. Cox, CDC, Atlanta, GA, USA |
| Samoa D | TPE | Samoa | 1953 | Turner & Hollander (1957) | Steven J. Norris, UT, Houston, TX, USA |
| Samoa F | TPE | Samoa | 1953 | Turner & Hollander (1957) | Steven J. Norris, UT, Houston, TX, USA |
| SS14 | TPA | Atlanta, USA | 1977 | Stamm et al. (1983) | Steven J. Norris, UT, Houston, TX, USA |
CDC, Centers for Disease Control and Prevention; PHL, Public Health Laboratory; UT, University of Texas.
Isolation of treponemal DNA.
TPA Nichols and SS14, TPE Samoa D and CDC-2, and TPc Cuniculi A chromosomal DNA was prepared as described previously by Fraser et al. (1998) by extracting DNA from experimentally infected rabbits. Treponemes were purified by Hypaque gradient centrifugation (Baseman et al., 1974). Because a high input of DNA was required for the sequencing approach, whole-genome amplification (WGA) (REPLI-g Midi kit; Qiagen) was performed for TPA Nichols DNA according to the manufacturer’s instructions. In addition, non-WGA DNAs from TPA Nichols and SS14, TPE Samoa D and CDC-2, and TPc Cuniculi A were used. The Philadelphia 1, Philadelphia 2, DAL-1, Mexico A, Bal 73-1, Grady, MN-3, Madras and Haiti B (TPA), CDC-1, CDC-2, Gauthier and Samoa F (TPE), Bosnia A and Iraq B (TEN), and Fribourg-Blanc (a simian T. pallidum) strains were obtained as rabbit testicular tissues containing treponemal cells. After brief centrifugation of the samples at 100 g for 5 min, the DNA enriched for bacterial cells was amplified using the REPLI-g Midi kit.
PCR amplification.
The primer pairs RNA1F (5′-GTGTGTGAGTCTGGCAGGAA-3′) and RNA1R (5′-TTATTGCTGTGCGCATCTTC-3′), and RNA2F (5′-ACAAGTGAGCGAAGCGTTTT-3′) and RNA2R (5′-CCAAGAGAGCTACCCGTCTG-3′), were used for amplification of the rrn operons from treponemal strains. These primer pairs produced extra-large PCR (XL-PCR) products of 5.85 and 5.92 kb, respectively. To obtain these XL-PCR amplicons, a GeneAmp XL PCR kit (Roche Molecular Systems) was used as described by Strouhal et al. (2007). XL-PCR products were purified using a QIAquick PCR Purification kit (Qiagen) or ExoSAP-IT kit (GE Healthcare) according to the manufacturer’s instructions.
DNA sequencing.
DNA sequencing of the XL-PCR products was carried out with a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) using a primer-walking approach. Additional internal oligonucleotide sequencing primers (see Table S1, available in JMM Online) were designed using Primer3 software (Rozen & Skaletsky, 2000). The lasergene program package (DNASTAR) was used to assemble the consensus sequences.
Phylogenetic analyses.
In addition to the rrn operons investigated in the 20 strains (Table 1), the rrn operons of TPA Chicago (GenBank accession no. CP001752; Giacani et al., 2010) was included in the evolutionary analysis. Concatenated sequences of rrn1 and rrn2 operons (Table S2) were used for the construction of evolutionary trees using the neighbour-joining method (Saitou & Nei, 1987) in mega4 software (Tamura et al., 2007). The bootstrap consensus trees were determined from 1000 bootstrap resamplings. Branches with <50 % bootstrap support were collapsed.
Detection of recombination.
To identify genomic rearrangements, rrn operons were analysed using the Recombination Detection Program package (version rdp3; Martin et al., 2010). Four methods, including rdp, geneconv (Sawyer, 1989), MaxChi (Smith, 1992) and chimaera (Posada & Crandall, 2001), implemented in the rdp3 package, were applied using default settings.
Analysis of clinical specimens.
Skin and mucosal swabs were placed in a tube containing 1.5 ml sterile water and agitated for 5 min at room temperature. The swab was withdrawn and the supernatant was used for DNA isolation. Swab supernatant (0.2–0.4 ml) and whole blood (0.2–0.8 ml) were used for DNA isolation using a QIAamp DNA Mini kit (Qiagen) according to the manufacturer’s Blood and Body Fluid Spin Protocol. To detect the presence of treponemal DNA in swab and whole-blood samples, a diagnostic PCR assay amplifying five different Treponema-specific genes including polA (TP0105 locus), tmpC (TP0319), TP0136, TP0548 and the 23S rRNA gene was performed. Amplification and subsequent sequencing of TP0136, TP0548 and the 23S rRNA gene have been used, although not for diagnostic purposes, for molecular typing of treponemal strains (Flasarová et al., 2006, 2012; Liu et al., 2001; Matějková et al., 2009; Woznicová et al., 2007).
The composition of 16S–23S ISR sequences in the rrn1 and rrn2 operons, encoding either tRNA-Ile or tRNA-Ala, was determined by another nested PCR. In the first step, each clinical isolate was tested in four parallel reactions with the following primer pairs (Fig. 1 and Table S3): RNA1Fb and RNA1-tRNA-Ile (first reaction), RNA1Fb and RNA2-tRNA-Ala (second reaction), RNA2Fc and RNA1-tRNA-Ile (third reaction) and RNA2Fc and RNA2-tRNA-Ala (fourth reaction). Using these primer sets, the PCR products revealed the position (rrn1 or rrn2) and composition (tRNA-Ile or tRNA-Ala) of the amplified rrn operon. In the second step of the nested PCR, the PCR product of the rrn1 (from the first and second reactions) region was amplified using TP0225-6aF and TP0225-6bR primers, whilst the PCR product of the rrn2 (from the third and fourth reactions) region was amplified with RNA2Fa and TP0225-6bR. The second step was not specific for the Ile/Ala or Ala/Ile rrn spacer pattern but improved the sensitivity of detection of the PCR product from the first step. Each PCR contained 0.4 µl 10 mM dNTP mix, 2 µl 10× ThermoPol Reaction buffer (New England BioLabs), 0.1 µl each primer (100 pmol µl−1), 0.1 µl Taq DNA polymerase (5000 U ml−1; New England BioLabs), 1 µl test sample and 16.3 µl PCR-grade water, giving 20 µl in total. PCR amplification was performed using a GeneAmp 9800 thermocycler (Applied Biosystems) with the following cycling conditions: 94 °C for 5 min; 40 cycles of 94 °C for 60 s, 72 °C for 20 s and 72 °C for 150 s; and a final extension at 72 °C for 10 min. The second step of the nested PCR used the same conditions but a lower annealing temperature of 67 °C.
Fig. 1.
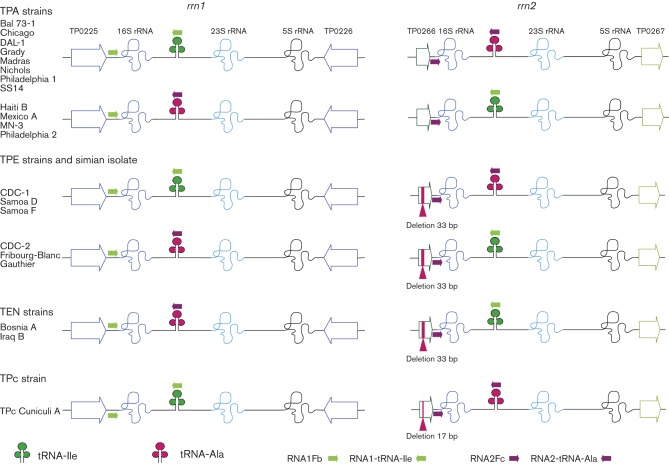
A schematic representation of the treponemal rrn operons consisting of 16S–23S–5S rRNA genes, intergenic regions and a 212 bp DNA sequence upstream of 16S rRNA gene. The positions of the 33 and 17 bp deletions in the non-TPA strains are shown. Please note that both spacer patterns of the 16S–23S ISR encoding either tRNA-Ile or tRNA-Ala were present among TPA and TPE strains in the rrn1 and rrn2 regions. Green symbols represent tRNA-Ile, whilst red symbols represent tRNA-Ala. Small coloured arrows in green and purple represent primers used in the clinical samples examined.
Results
Amplification and sequencing of the rrn operons
Two rrn operons (16S–23S–5S) have been described in pathogenic Treponema genomes with the 16S–23S ISR comprising genes encoding tRNA-Ala or tRNA-Ile (Fraser et al., 1998; Fukunaga et al., 1992; Giacani et al., 2010; Šmajs et al., 2011b). Using XL-PCR, we amplified the rrn operons in 20 treponemal strains (Tables 1 and S2) comprising 11 strains of TPA, five strains of TPE, an unclassified simian isolate, two strains of TEN and a rabbit TPc isolate. XL-PCR products were obtained for all 40 investigated regions. However, the assembled sequence of the rrn2 operon of Iraq B (TEN) was repeatedly ambiguous at several positions, probably due to low DNA quality, so the Iraq B sequences were excluded from the construction of phylogenetic trees.
Sequence analysis of rrn operons
In the individual TPA genomes, the amplified rrn1 and rrn2 regions were identical for 5141 bp (Tables 2 and S2, Fig. 1) including the DNA regions 212 bp upstream of the 16S rRNA, the 16S rRNA (1537 bp), 23S rRNA (2951 bp), 5S rRNA (110 bp) and 23S–5S ISR (50 bp), and a region of 54 bp downstream of the 5S rRNA. Additional identical sequences were located within the 16S–23S ISR downstream of the 16S rRNA (120 bp) and upstream of the 23S rRNA (118 bp) genes (Fig. 2, Table 2). Alternative sequences within the 16S–23S ISR, encoding tRNA-Ile or tRNA-Ala, comprised an additional 64 or 74 bp, respectively (Fig. 2). To extend the comparative analysis over all available data, the TPA Chicago sequences of the rrn operons (GenBank accession no. CP001752; Giacani et al., 2010) were added to the sequences of the 20 strains used in this study.
Table 2. DNA sequence polymorphisms found among 20 pathogenic Treponema strains at the rrn operons.
SNPs are indicated by underlining, whereas translocation of tRNA is shown in bold. IGR, Intergenic region.
| Species | Strain (operon) | Treponemal homologous sequences of rRNA operons, and position downstream (D), upstream (U) or within the RNA gene | ||||||||||||||||||
| IGR (212 bp) | 16S rRNA (1537 bp) | IGR (117 or 116 bp)* | tRNA (74 bp)* | IGR (111 or 122 bp)* | 23S rRNA (2951 bp) | IGR (50 bp) | 5S rRNA (110 bp) | |||||||||||||
| 171–167 U | 96 U | 93 U | 647 | 1134 | 1375 | 1441 | 71 D | 21 U | 458 | 763 | 766 | 1092 | 1359 | 1546 | 2104 | 47 D | 81 | |||
| TPA | Bal 73-1 (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C |
| Bal 73-1 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Chicago (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Chicago (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| DAL-1 (rrn1) | GGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| DAL-1 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Grady (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Grady (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Haiti B (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Haiti B (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Madras (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Madras (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Mexico A (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Mexico A (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| MN-3 (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| MN-3 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Nichols (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Nichols (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Philadelphia 1 (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| Philadelphia 1 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Philadelphia 2 (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | A | C | C | |
| Philadelphia 2 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | A | C | C | |
| SS14 (rrn1) | GGGGG | A | A | G | G | G | C | G | tRNA-Ile | G | G | G | G | G | A | A | G | C | C | |
| SS14 (rrn2) | GGGGG | A | A | G | G | G | C | G | tRNA-Ala | G | G | G | G | G | A | A | G | C | C | |
| TPE | CDC-1 (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C |
| CDC-1 (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| CDC-2 (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| CDC-2 (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| Gauthier (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| Gauthier (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| Samoa D (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| Samoa D (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| Samoa F (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| Samoa F (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| Simian isolate | Fribourg-Blanc (rrn1) | GGGGG | A | G | G | G | G | C | G | tRNA-Ala | G | A | G | A | G | A | A | A | C | C |
| Fribourg-Blanc (rrn2) | GGGGG | A | G | G | G | G | C | G | tRNA-Ile | G | A | G | A | G | A | A | A | C | C | |
| TEN | Bosnia A (rrn1) | GGGGG | A | A | G | G | A | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C |
| Bosnia A (rrn2) | GGGGG | A | A | G | G | A | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| Iraq B (rrn1) | GGGGG | A | A | G | G | A | C | G | tRNA-Ala | G | G | G | A | G | A | A | A | C | C | |
| Iraq B (rrn2) | GGGGG | A | A | G | G | A | C | G | tRNA-Ile | G | G | G | A | G | A | A | A | C | C | |
| TPc | Cuniculi A (rrn1) | GGGGG | G | A | A | A | G | T | A | tRNA-Ile | A | G | A | A | A | G | G | A | T | A |
| Cuniculi A (rrn2) | GGGGG | G | A | A | A | G | T | A | tRNA-Ala | A | G | A | A | A | G | G | A | T | A | |
The size of sequence between the 16S and 23S rRNA genes (both excluded) varied based on the presence of the tRNA-Ile (117+74+111, in total 302 bp) or tRNA-Ala (116+74+122, in total 312 bp) gene.
Fig. 2.

Alignment of 16S–23S ISRs in TPA Nichols rrn operons. The gene encoding tRNA-Ile (TP_t12) is shown in red, whilst the gene encoding tRNA-Ala-3 (TP_t15) is in blue.
When compared with the TPA strains, a deletion of 33 bp was found in homologous regions of the rrn2 region in the TPE, TEN and simian strains (Fig. 1), whilst the TPc strain contained a 17 bp deletion at the same position (Fig. 1). These deletions resulted in shortening (33 bp deletion) or truncation (17 bp deletion) of TP0266 orthologues. Among all investigated strains, in addition to the observed deletions, we found only 17 heterogeneous sites within the entire region, excluding the 16S–23S ISR encoding tRNA-Ile or tRNA-Ala. Sixteen sites were single nucleotide changes and one was a single base-pair deletion (Table 2). The rrn1 operon of the reference TPA Nichols genome (GenBank accession no. AE000520.1; Fraser et al., 1998) showed a deletion within the 16S rRNA gene (data not shown), whereas all other strains, including the Nichols strain examined in our study, did not. This deletion may represent a sequencing error present in the reference Nichols genome, as dozens of such sequencing errors have already been confirmed (Giacani et al., 2012; Matějková et al., 2008). In contrast, a 1 bp deletion in the TPA DAL-1 genome, upstream of the 16S rRNA gene in the rrn1 operon, was repeatedly confirmed by Sanger sequencing. The identified nucleotide change at position 2104 of the 23S rRNA gene (differentiating the SS14 strains from other investigated strains) corresponded to the mutation causing macrolide resistance in treponemal strains (Stamm & Bergen, 2000).
All TPA strains differed from the other pathogenic treponemes by a nucleotide change at position 766 of the 23S rRNA gene. The TPE strains and the simian isolate Fribourg-Blanc could be distinguished from the other pathogenic treponemes by a single-nucleotide polymorphism (SNP) localized 93 bp upstream of the 16S rRNA genes. The TPE strains could be differentiated from the simian isolate by a nucleotide sequence change in the 23S rRNA gene (nt 458). The TEN showed a nucleotide change in the 16S rRNA gene, and TPc showed 12 nt changes in the investigated rrn sequences (Table 2).
Reciprocal translocation of tRNA genes
In contrast to the phylogenetically conserved SNP distribution in the repetitive sequences of the rrn operons, the genes coding for tRNA did not show the same evolutionary pattern (Table 2, Fig. 1). In this study, we observed two 16S–23S ribosomal ISR patterns. The spacer pattern Ile/Ala included the tRNA-Ile gene within the rrn1 region and the tRNA-Ala gene within the rrn2 region. The Ile/Ala pattern was observed in the following strains: TPA Nichols, Bal 73-1, Grady, SS14, Chicago, DAL-1, Philadelphia 1 and Madras; TPE Samoa D, CDC-1 and Samoa F; and TPc Cuniculi A. The reverse ISR pattern Ala/Ile consisted of the tRNA-Ala gene within the rrn1 region and the tRNA-Ile gene within the rrn2 region. The Ala/Ile pattern was found in TPA Mexico A, MN-3, Philadelphia 2 and Haiti B strains; in TPE Gauthier and CDC-2; in the unclassified treponeme Fribourg-Blanc; and in TEN Iraq B and Bosnia A genomes.
The concatenated rrn operons, excluding the tRNA genes and their vicinity, clustered according to the species/subspecies classification (Fig. 3a). The TEN Iraq B strain was omitted from the analysis because we were unable to obtain an unambiguous rrn2 operon sequence. Nevertheless, the rrn1 operon was identical to another TEN strain, Bosnia A. In contrast, the trees showing concatenated rrn operons including tRNA genes (Fig. 3b) branched according to the composition of tRNA in the individual rrn operons, and then according to the species/subspecies classification. This phenomenon can be explained by recombination events that have occurred between rrn operons.
Fig. 3.
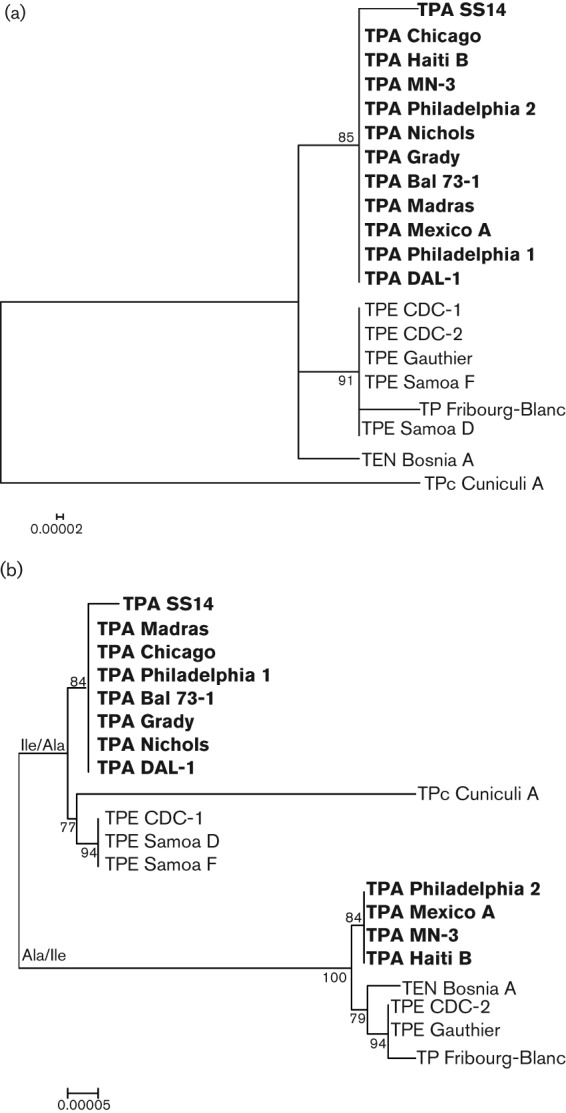
(a) An unrooted tree constructed from the concatenated sequences of the rrn operons excluding the heterologous tRNA genes. The rrn operons clustered according to the species/subspecies classification of treponemes. Bar, 0.00002 nt substitutions per site. (b) An unrooted tree constructed from the sequences of the rrn operons including heterologous tRNA genes. The rrn operons clustered according to the ISR pattern. Bar, 0.00005 nt substitutions per site. Bootstrap values based on 1000 replications are shown next to the branches. The TPA strains causing syphilis are shown in bold.
To predict recombination hot-spot sites within the rrn operons, four methods from the rdp3 program were applied. All four methods predicted four recombination sites (Table 3), two sites in each rrn operon. The predicted sites corresponded to the same positions within the 16S (nt 783) and 23S (nt 324) rRNA genes in both rrn operons.
Table 3. Predicted recombination hot-spot sites using the rdp3 program.
| Predicted recombination hot-spot site* | Prediction algorithm used in the rdp3 program (P value) | ||||
| Start | End | RDP | geneconv | MaxChi | chimaera |
| 231656 | 233036 | 1.33E−58 | 2.20E−55 | 8.06E−13 | 7.80E−13 |
| 280058 | 281448 | 7.22E−20 | 1.12E−20 | 5.91E−04 | 1.33E−03 |
Whole-genome TPE Samoa D coordinates are shown (Čejková et al., 2012; GenBank accession no. CP002374).
Structure of the rrn operons in clinical isolates containing T. pallidum DNA
The composition of the 16S–23S ribosomal ISR (Ile/Ala or Ala/Ile spacer pattern in the rrn operons) was tested in 30 recently isolated clinical samples (Flasarová et al., 2012). The results are summarized in Table 4. Only the Ile/Ala pattern was identified in all clinical samples tested, despite the fact that the clinical samples belonged to five different genotypes (Table 4), as revealed by CDC and sequencing-based typing (Flasarová et al. 2012; Pillay et al. 1998). Nevertheless, all clinical strain genotypes were similar to the SS14 strain genotype.
Table 4. Composition of the 16S–23S ribosomal ISR in clinical samples containing TPA DNA.
| Clinical sample | 16S–23S ISR (rrn1/rrn2) | Genotype* | Subtype† |
| 2K | Ile/Ala | SU2R8 | 14d |
| 4K | Ile/Ala | SSS | 14d |
| 6K | Ile/Ala | SSS | 14d |
| 15K | Ile/Ala | SSS | 14d |
| 24K | Ile/Ala | SSS | 14d |
| 27K | Ile/Ala | SSS | 14d |
| 34K | Ile/Ala | SSS | 14d |
| 40K | Ile/Ala | SSR8 | 14d |
| 44K | Ile/Ala | SSS | 14d |
| 47K | Ile/Ala | SSS | 12d |
| 49K | Ile/Ala | SSR8 | 14d |
| 51K | Ile/Ala | SSS | 14d |
| 52K | Ile/Ala | SSS | 14d |
| 53K | Ile/Ala | SSS | 14d |
| 63K | Ile/Ala | SSR9 | 15d |
| 73K | Ile/Ala | SU2R8 | 14d |
| 91K | Ile/Ala | SSS | 14d |
| 6000 | Ile/Ala | SU2R8 | 14d |
| 9888 | Ile/Ala | SSS | 14d |
| 14048 | Ile/Ala | SSS | 14d |
| 14207 | Ile/Ala | SSS | 14d |
| 16142 | Ile/Ala | SU2R8 | 14d |
| RL86Z | Ile/Ala | SU2R8 | 14d |
| RL89BZ | Ile/Ala | SSS | 14d |
| RL95B | Ile/Ala | SSS | 14d |
| RL102B | Ile/Ala | SSS | 14d |
| RL104B | Ile/Ala | SU2R8 | 14d |
| RL110B | Ile/Ala | SSS | 14d |
| RL111B | Ile/Ala | SU2R8 | 14d |
| RL116A | Ile/Ala | XXR8 | 14e |
Identified according to the method of Flasarová et al. (2012).
Subtype according to the method of Pillay et al. (1998).
Discussion
In this study, we examined the rrn operons in 20 pathogenic treponemal strains and 30 clinical isolates. All investigated strains contained two copies of the rrn operons. Two rrn operons with the same composition have also been described in other human and animal treponemes except for T. vincentii containing only one rrn operon (Fraser et al., 1998; Matějková et al., 2008; Seshadri et al., 2004; Stamm et al., 2002).
Our results confirmed that there is little diversity within rRNA genes and ISRs. However, our data showed that the rrn operon structure displayed blocks of conserved and polymorphic sites. The TPA DAL-1 strain showed a 1 bp deletion upstream of the 16S rRNA gene in the rrn1 operon. It is known that TPA DAL-1 grows more rapidly in rabbits than other pathogenic strains (Wendel et al., 1991), and it is possible that the different promoter DNA conformation may affect expression of the rrn1 operon.
Gürtler & Stanisich (1996) used the 16S–23S ribosomal ISR for classification of bacteria. 16S–23S ISRs have been used in several studies (de Vries et al., 2006; Lan & Reeves, 1998; Lebuhn et al., 2006), including for treponemal (Centurion-Lara et al., 1996; Stamm et al., 2002) and borrelian samples (Bunikis et al., 2004; Comstedt et al., 2009). Centurion-Lara et al. (1996) examined the TPA Nichols and TPE Gauthier strains, and no difference was found. However, they did not examine the genomic positions of individual 16S–23S ISRs. Interestingly, the 16S–23S ISR typing of Borrelia burgdorferi sensu stricto is in accordance with ospC gene typing (Hanincová et al., 2008; Wormser et al., 2008). The ospC gene, encoding a protein involved in the initiation of infection in warm-blooded animals, is located on plasmid DNA, whilst the rrn operon is on chromosomal DNA. Moreover, different 16S–23S ISR genotypes are associated with different degrees of invasivity (Wormser et al., 2008).
Despite the low heterogeneity in the rrn operons, two different ISR patterns were observed in the pathogenic treponemal samples. Whereas detection of specific nucleotide changes may be of interest in identification of treponemal diseases, the detection of tRNA genes in the 16S–23S ribosomal ISR appears to be of limited use in typing of clinical samples. All clinical samples showed the Ile/Ala spacer pattern in rrn operons, so the tRNA-Ile and tRNA-Ala genes are not useful for molecular typing of clinical strains, at least for treponemes present in the population of the Czech Republic.
Due to the conserved machinery of protein synthesis, rRNA genes are expected to be under strong purifying selection and are exposed to the intragenomic homogenization process via gene conversion (Liao, 2000; Nei & Rooney, 2005). Several studies (Acinas et al., 2004; Pei et al., 2009, 2010) have shown that homogenization of multiple rRNA genes is common among bacteria. In addition, Harvey & Hill (1990) successfully constructed several Escherichia coli strains with recombined inverted rrn operons; however, the recombinants tended to recover the original configuration. The rrn operons of treponemal strains are direct repeats: the tRNA-Ala gene is replaced by tRNA-Ile (and vice versa), and the recombination is a common event with no correlation to the otherwise-determined phylogenetic relationship among tested treponemes. It has been postulated that recombination between direct repeats leads to the duplication or deletion of a repeat (Petes & Hill, 1988; Petit, 2005). Whereas tRNA-Ile (TP_t12) is a unique gene in sequenced treponemal genomes, there are three predicted tRNA-Ala genes (TP_t15, TP_t41 and TP_t45; Fraser et al., 1998). As both tRNA-Ile (TP_t12, GenBank accession no. AE000520.1) and tRNA-Ala (TP_t15, AE000520.1) genes need to be maintained in the genomes of pathogenic treponemes, reciprocal translocation, rather than gene conversion, appears to be the mechanism for the observed rrn heterogenity among tested strains. Such a process would require double cross-overs in both rrn operons, and therefore is much less common than insertion/deletion or gene-conversion events (Harvey & Hill, 1990; Hashimoto et al., 2003). Predicted recombination hot-spot sites were located in the 16S and 23S rRNA genes, genes with two identical copies within every strain examined in our study.
During replication of direct-repeat regions, DNA polymerase might lead to strand slippage, thus collapsing a replication fork formation, and recombination enzymes are involved in the DNA repair machinery (Darling et al., 2008; Santoyo & Romero, 2005). Although only the recF recombination pathway was predicted in the TPA Nichols genome (Fraser et al., 1998), the recF pathway suggests the gene-conversion mechanism (Kobayashi, 1992; Takahashi et al., 1992). Therefore, the reciprocal recombination in pathogenic treponemes may be accompanied by crossing-over, a repair mechanism implemented by the recBCD pathway in E. coli (Kobayashi, 1992). Recently, recBCD orthologues (addA and addB) were predicted for several investigated treponemal genomes (Čejková et al., 2012; Giacani et al., 2012; Šmajs et al., 2011b), composed of TP0898 and fused TP0899–TP0900 orthologues. However, it would be extremely difficult to prove experimentally the recBCD-mediated crossing-over mechanism in T. pallidum.
In summary, two different rrn spacer patterns (Ile/Ala and Ala/Ile) seem to be distributed randomly across the time and place of original isolation of treponemal strains (e.g. Philadelphia 1 vs Philadelphia 2, CDC-1 vs CDC-2) and the laboratory that provided the treponemal material (Tables 1 and 2). This random distribution of tRNA genes is probably caused by reciprocal translocation between repetitive sequences mediated by a recBCD-like system.
Acknowledgements
The authors thank Dr David Cox for providing the Bal 73-1, CDC-1, DAL-1, Fribourg-Blanc, Grady, Haiti B, Madras, Mexico A, MN-3, Philadelphia 1 and Philadelphia 2 strains, Dr Steven Norris for the CDC-2, Gauthier, Nichols, Samoa D, Samoa F and SS14 strains, Dr Kristin Harper for the Iraq B strain, Dr Sylvia Bruisten for the Bosnia A strain, and Dr Elizabeth A. Lobos for help with manuscript preparation. This work was supported by grants from the Grant Agency of the Czech Republic (P302/12/0574) and the Ministry of Health of the Czech Republic (NT11159-5/2010) to D. S.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- ISR
intergenic spacer region
- SNP
single-nucleotide polymorphism
- TEN
T. pallidum ssp. endemicum
- TPA
T. pallidum ssp. pallidum
- TPc
T. paraluiscuniculi
- TPE
T. pallidum ssp. pertenue
- WGA
whole-genome amplification
- XL-PCR
extra-large PCR
Footnotes
Three supplementary tables are available with the online version of this paper.
References
- Acinas S. G., Marcelino L. A., Klepac-Ceraj V., Polz M. F. (2004). Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J Bacteriol 186, 2629–2635 10.1128/JB.186.9.2629-2635.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón A. I., Martínez-Murcia A. J., Rodríguez-Valera F. (1998). Sequence diversity in the 16S–23S intergenic spacer region (ISR) of the rRNA operons in representatives of the Escherichia coli ECOR collection. J Mol Evol 47, 62–72 10.1007/PL00006363 [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. (1974). Purification of Treponema pallidum from infected rabbit tissue: resolution into two treponemal populations. Infect Immun 10, 1062–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A. G. (2004). Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150, 1741–1755 10.1099/mic.0.26944-0 [DOI] [PubMed] [Google Scholar]
- Čejková D., Zobaníková M., Chen L., Pospíšilová P., Strouhal M., Qin X., Mikalová L., Norris S. J., Muzny D. M. & other authors (2012). Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl Trop Dis 6, e1471 10.1371/journal.pntd.0001471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion-Lara A., Castro C., van Voorhis W. C., Lukehart S. A. (1996). Two 16S–23S ribosomal DNA intergenic regions in different Treponema pallidum subspecies contain tRNA genes. FEMS Microbiol Lett 143, 235–240 10.1111/j.1574-6968.1996.tb08486.x [DOI] [PubMed] [Google Scholar]
- Comstedt P., Asokliene L., Eliasson I., Olsen B., Wallensten A., Bunikis J., Bergström S. (2009). Complex population structure of Lyme borreliosis group spirochete Borrelia garinii in subarctic Eurasia. PLoS ONE 4, e5841 10.1371/journal.pone.0005841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon C., Philips J., Fu Z. Y., Squires C., Squires C. L. (1992). Comparison of the expression of the seven ribosomal RNA operons in Escherichia coli. EMBO J 11, 4175–4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A. E., Miklós I., Ragan M. A. (2008). Dynamics of genome rearrangement in bacterial populations. PLoS Genet 4, e1000128 10.1371/journal.pgen.1000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M. C., Siezen R. J., Wijman J. G., Zhao Y., Kleerebezem M., de Vos W. M., Vaughan E. E. (2006). Comparative and functional analysis of the rRNA-operons and their tRNA gene complement in different lactic acid bacteria. Syst Appl Microbiol 29, 358–367 10.1016/j.syapm.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Flasarová M., Šmajs D., Matějková P., Woznicová V., Heroldová-Dvoráková M., Votava M. (2006). [Molecular detection and subtyping of Treponema pallidum subsp. pallidum in clinical specimens]. Epidemiol Mikrobiol Imunol 55, 105–111 (in Czech). [PubMed] [Google Scholar]
- Flasarová M., Pospíšilová P., Mikalová L., Vališová Z., Dastychová E., Strnadel R., Kuklová I., Woznicová V., Zákoucká H., Šmajs D. (2012). Sequencing-based molecular typing of Treponema pallidum strains in the Czech Republic: all identified genotypes are related to the sequence of the SS14 strain. Acta Derm Venereol 92, 669–674 [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Casjens S., Huang W. M., Sutton G. G., Clayton R., Lathigra R., White O., Ketchum K. A., Dodson R. & other authors (1997). Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Norris S. J., Weinstock G. M., White O., Sutton G. G., Dodson R., Gwinn M., Hickey E. K., Clayton R. & other authors (1998). Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281, 375–388 10.1126/science.281.5375.375 [DOI] [PubMed] [Google Scholar]
- Fribourg-Blanc A., Mollaret H. H. (1969). Natural treponematosis of the African primate. Primates Med 3, 113–121 [PubMed] [Google Scholar]
- Fukunaga M., Okuzako N., Mifuchi I., Arimitsu Y., Seki M. (1992). Organization of the ribosomal RNA genes in Treponema phagedenis and Treponema pallidum. Microbiol Immunol 36, 161–167 [DOI] [PubMed] [Google Scholar]
- Gastinel P., Vaisman A., Hamelin A., Dunoyer F. (1963). [Study of a recently isolated strain of Treponema pertenue]. Prophyl Sanit Morale 35, 182–188 (in French). [PubMed] [Google Scholar]
- Giacani L., Jeffrey B. M., Molini B. J., Le H. T., Lukehart S. A., Centurion-Lara A., Rockey D. D. (2010). Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J Bacteriol 192, 2645–2646 10.1128/JB.00159-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L., Chattopadhyay S., Centurion-Lara A., Jeffrey B. M., Le H. T., Molini B. J., Lukehart S. A., Sokurenko E. V., Rockey D. D. (2012). Footprint of positive selection in Treponema pallidum subsp. pallidum genome sequences suggests adaptive microevolution of the syphilis pathogen. PLoS Negl Trop Dis 6, e1698 10.1371/journal.pntd.0001698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler V. (1999). The role of recombination and mutation in 16S–23S rDNA spacer rearrangements. Gene 238, 241–252 10.1016/S0378-1119(99)00224-3 [DOI] [PubMed] [Google Scholar]
- Gürtler V., Stanisich V. A. (1996). New approaches to typing and identification of bacteria using the 16S–23S rDNA spacer region. Microbiology 142, 3–16 10.1099/13500872-142-1-3 [DOI] [PubMed] [Google Scholar]
- Hanincová K., Liveris D., Sandigursky S., Wormser G. P., Schwartz I. (2008). Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl Environ Microbiol 74, 5008–5014 10.1128/AEM.00479-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. B., Hardy P. H., Oppenheimer E. H., Ryan S. J., Jr, Sheff R. N. (1970). Failure of penicillin in a newborn with congenital syphilis. JAMA 212, 1345–1349 10.1001/jama.1970.03170210051008 [DOI] [PubMed] [Google Scholar]
- Harvey S., Hill C. W. (1990). Exchange of spacer regions between rRNA operons in Escherichia coli. Genetics 125, 683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto J. G., Stevenson B. S., Schmidt T. M. (2003). Rates and consequences of recombination between rRNA operons. J Bacteriol 185, 966–972 10.1128/JB.185.3.966-972.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra A., Blaschitz M., Kernbichler S., Reischl U., Wewalka G., Allerberger F. (2010). Mechanisms behind variation in the Clostridium difficile 16S–23S rRNA intergenic spacer region. J Med Microbiol 59, 1317–1323 10.1099/jmm.0.020792-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I. (1992). Mechanisms for gene conversion and homologous recombination: the double-strand break repair model and the successive half crossing-over model. Adv Biophys 28, 81–133 10.1016/0065-227X(92)90023-K [DOI] [PubMed] [Google Scholar]
- Lan R. T., Reeves P. R. (1998). Recombination between rRNA operons created most of the ribotype variation observed in the seventh pandemic clone of Vibrio cholerae. Microbiology 144, 1213–1221 10.1099/00221287-144-5-1213 [DOI] [PubMed] [Google Scholar]
- Lebuhn M., Bathe S., Achouak W., Hartmann A., Heulin T., Schloter M. (2006). Comparative sequence analysis of the internal transcribed spacer 1 of Ochrobactrum species. Syst Appl Microbiol 29, 265–275 10.1016/j.syapm.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Liao D. (2000). Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J Mol Evol 51, 305–317 [DOI] [PubMed] [Google Scholar]
- Liska S. L., Perine P. L., Hunter E. F., Crawford J. A., Feeley J. C. (1982). Isolation and transportation of Treponema pertenue in golden hamsters. Curr Microbiol 7, 41–43 10.1007/BF01570978 [DOI] [Google Scholar]
- Liu H., Rodes B., Chen C.-Y., Steiner B. (2001). New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol 39, 1941–1946 10.1128/JCM.39.5.1941-1946.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveris D., Wormser G. P., Nowakowski J., Nadelman R., Bittker S., Cooper D., Varde S., Moy F. H., Forseter G. & other authors (1996). Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 34, 1306–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Lemey P., Lott M., Moulton V., Posada D., Lefeuvre P. (2010). rdp3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26, 2462–2463 10.1093/bioinformatics/btq467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matějková P., Strouhal M., Šmajs D., Norris S. J., Palzkill T., Petrosino J. F., Sodergren E., Norton J. E., Singh J. & other authors (2008). Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol 8, 76 10.1186/1471-2180-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matějková P., Flasarová M., Zákoucká H., Boˇrek M., Kremenová S., Arenberger P., Woznicová V., Weinstock G. M., Šmajs D. (2009). Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol 58, 832–836 10.1099/jmm.0.007542-0 [DOI] [PubMed] [Google Scholar]
- Nei M., Rooney A. P. (2005). Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 39, 121–152 10.1146/annurev.genet.39.073003.112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols H. J., Hough W. H. (1913). Demonstration of Spirochaeta pallida in the cerebrospinal fluid: from a patient with nervous relapse following the use of salvarsan. JAMA 60, 108–110 10.1001/jama.1913.04340020016005 [DOI] [Google Scholar]
- Pei A., Nossa C. W., Chokshi P., Blaser M. J., Yang L., Rosmarin D. M., Pei Z. (2009). Diversity of 23S rRNA genes within individual prokaryotic genomes. PLoS ONE 4, e5437 10.1371/journal.pone.0005437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei A. Y., Oberdorf W. E., Nossa C. W., Agarwal A., Chokshi P., Gerz E. A., Jin Z., Lee P., Yang L. & other authors (2010). Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl Environ Microbiol 76, 3886–3897 10.1128/AEM.02953-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. (1988). Recombination between repeated genes in microorganisms. Annu Rev Genet 22, 147–168 10.1146/annurev.ge.22.120188.001051 [DOI] [PubMed] [Google Scholar]
- Petit M.-A. (2005). Mechanisms of homologous recombination in bacteria. In The Dynamic Bacterial Genome, pp. 3–32 Edited by Mullany P. New York: Cambridge University Press; 10.1017/CBO9780511541544.001 [DOI] [Google Scholar]
- Pillay A., Liu H., Chen C.-Y., Holloway B., Sturm A. W., Steiner B., Morse S. A. (1998). Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis 25, 408–414 10.1097/00007435-199809000-00004 [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K. A. (2001). Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc Natl Acad Sci U S A 98, 13757–13762 10.1073/pnas.241370698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132, 365–386 [DOI] [PubMed] [Google Scholar]
- Sadeghifard N., Gürtler V., Beer M., Seviour R. J. (2006). The mosaic nature of intergenic 16S–23S rRNA spacer regions suggests rRNA operon copy number variation in Clostridium difficile strains. Appl Environ Microbiol 72, 7311–7323 10.1128/AEM.01179-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425 [DOI] [PubMed] [Google Scholar]
- Santoyo G., Romero D. (2005). Gene conversion and concerted evolution in bacterial genomes. FEMS Microbiol Rev 29, 169–183 [DOI] [PubMed] [Google Scholar]
- Sawyer S. (1989). Statistical tests for detecting gene conversion. Mol Biol Evol 6, 526–538 [DOI] [PubMed] [Google Scholar]
- Schwartz J. J., Gazumyan A., Schwartz I. (1992). rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol 174, 3757–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri R., Myers G. S., Tettelin H., Eisen J. A., Heidelberg J. F., Dodson R. J., Davidsen T. M., DeBoy R. T., Fouts D. E. & other authors (2004). Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A 101, 5646–5651 10.1073/pnas.0307639101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šmajs D., Mikalová L., Čejková D., Strouhal M., Zobaníková M., Pospisilova P., Norris S. J., Weinstock G. M. (2011a). Whole genome analyses of treponemes: new targets for strain- and subspecies-specific molecular diagnostics. In Syphilis – Recognition, Description and Diagnosis, pp. 19–34 Edited by Sato N. S. Rijeka, Croatia: InTech; 10.5772/21496 [DOI] [Google Scholar]
- Šmajs D., Zobaníková M., Strouhal M., Čejková D., Dugan-Rocha S., Pospís˜ilová P., Norris S. J., Albert T., Qin X. & other authors (2011b). Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS ONE 6, e20415 10.1371/journal.pone.0020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M. (1992). Analyzing the mosaic structure of genes. J Mol Evol 34, 126–129 10.1007/BF00182389 [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Bergen H. L. (2000). A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob Agents Chemother 44, 806–807 10.1128/AAC.44.3.806-807.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr (1983). Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun 41, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Bergen H. L., Walker R. L. (2002). Molecular typing of papillomatous digital dermatitis-associated Treponema isolates based on analysis of 16S–23S ribosomal DNA intergenic spacer regions. J Clin Microbiol 40, 3463–3469 10.1128/JCM.40.9.3463-3469.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart F. J., Cavanaugh C. M. (2007). Intragenomic variation and evolution of the internal transcribed spacer of the rRNA operon in bacteria. J Mol Evol 65, 44–67 10.1007/s00239-006-0235-3 [DOI] [PubMed] [Google Scholar]
- Strouhal M., Šmajs D., Matějková P., Sodergren E., Amin A. G., Howell J. K., Norris S. J., Weinstock G. M. (2007). Genome differences between Treponema pallidum subsp. pallidum strain Nichols and T. paraluiscuniculi strain Cuniculi A. Infect Immun 75, 5859–5866 10.1128/IAI.00709-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N. K., Yamamoto K., Kitamura Y., Luo S.-Q., Yoshikura H., Kobayashi I. (1992). Nonconservative recombination in Escherichia coli. Proc Natl Acad Sci U S A 89, 5912–5916 10.1073/pnas.89.13.5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). mega4: Molecular Evolutionary Genetics Analysis (mega) software version 4.0. Mol Biol Evol 24, 1596–1599 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Turner T. B., Hollander D. H. (1957). Biology of the treponematoses based on studies carried out at the International Treponematosis Laboratory Center of the Johns Hopkins University under the auspices of the World Health Organization. Monogr Ser World Health Organ 35, 3–266 [PubMed] [Google Scholar]
- Wendel G. D., Jr, Sánchez P. J., Peters M. T., Harstad T. W., Potter L. L., Norgard M. V. (1991). Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet Gynecol 78, 890–895 [PubMed] [Google Scholar]
- Wormser G. P., Brisson D., Liveris D., Hanincová K., Sandigursky S., Nowakowski J., Nadelman R. B., Ludin S., Schwartz I. (2008). Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis 198, 1358–1364 10.1086/592279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woznicová V., Šmajs D., Wechsler D., Matějková P., Flasarová M. (2007). Detection of Treponema pallidum subsp. pallidum from skin lesions, serum, and cerebrospinal fluid in an infant with congenital syphilis after clindamycin treatment of the mother during pregnancy. J Clin Microbiol 45, 659–661 10.1128/JCM.02209-06 [DOI] [PMC free article] [PubMed] [Google Scholar]