Abstract
Patient: Female, 60
Final Diagnosis: Corneal ulceration
Symptoms: Blurred vision
Medication: Abatacept
Clinical Procedure: —
Specialty: Ophthalmology
Objective:
Management of emergency care
Background:
To report a case of a patient with rheumatoid arthritis (RA) and associated peripheral corneal ulceration.
Case Report:
A 60-year-old woman with RA diagnosed 15 years ago, under immunosuppressive therapy (IV abatacept 250 mg/month), demonstrated blurring of vision in her RE (right eye). Visual acuity was 6/10 in the RE and 10/10 in the LE. Slit lamp examination revealed a paracentral superior corneal melt in the RE. Anterior chamber reaction was 2+. Laboratory investigations revealed positive anti-Ro and anti-La, anti-Extractable Nuclear Antigens (anti-ENA, ELISA), while anti-Sm, anti-Rnp, anti-Jo1 and anti-Scl70 were found negative. IgG and IgA serum immunoglobulins were found elevated, but IgE and IgM were within normal levels. Further evaluation for the underlying disease revealed highly elevated rheumatoid factor and C-reactive protein. The patient, who had been receiving anti-TNF during the last 6 months, underwent treatment with topical tobramycin and lubricants and oral prednisone 60 mg/day with tapering doses, to which methotrexate p.os. 15 mg/week was added.
The condition improved within a few days after the initiation of prednisone treatment. Re-epithelization occurred 1 week after the onset of the immunosuppressive treatment. Only punctate fluorescein dye uptake was detected in the margins of the lesion.
Conclusions:
The effective control of the underlying disease and early diagnosis of the dry eye syndrome in RA patients may prevent serious corneal complications such as corneal ulceration. The initiation of treatment with steroids and immunosuppresants was found to halt the progression of keratolysis, and assisted re-epithelization.
Keywords: keratolysis, rheumatoid arthritis, peripheral ulceration, immunosuppressive therapy, ant-TNF, anti-ENA
Background
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease of unknown origin. RA primarily manifests as an erosive, symmetric synovitis and frequently has multiregional, extra-articular manifestations [1,2]. Generally, the eye, and the ocular surface in particular, is one of the regions affected by this disease, since 90% of patients with RA present with dry eye syndrome [3]. The statistical association between the presence of dry eye syndrome and RA duration longer than 10 years has been proved [4]. The most frequent ocular manifestation of RA is keratoconjunctivitis sicca (KCS). Although not as severe, other common ocular complications are episcleritis, scleritis, corneal changes, and retinal vasculitis [5]. Furthermore, corneal lesions include marginal thinning of the cornea, with keratolysis, stromal corneal opacities with peripheral vascularization, and associated iridocyclitis [6].
RA patients manifest secondary Sjogren’s syndrome (SSII) in approximately 11% to 31% of cases, with KCS the most characteristic finding [7]. The dry eye associated with Sjogren’s syndrome is viewed as a prototype not only of the KCS associated with autoimmune diseases, but also with hyposecretory dry eye in general [8,9].
Sterile corneal ulceration is a rare complication of RA that can occur in either the central or peripheral cornea and may lead to corneal perforation. Additionally, it is well-known that the systemic immune mediated disease involves abnormal B cell – T cell interaction, with presentation of antigens by B cells to T cells via HLA-DR, eliciting T cell help; inflammation is then driven either by B cell or T cell products stimulating release of TNF and other cytokines, triggering the dryness of eyes and mouth caused by lymphocyte infiltration of lacrimal and salivary glands [10]. However, the process of corneal melting is poorly understood. Despite the availability of many different types of treatment, the prognosis is very poor.
The aim of the current study is to report a case of a RA patient with associated peripheral corneal ulceration.
Case Report
A 60-year-old female patient with RA, under immunosuppressive therapy (IV abatacept 250 mg/month), presented redness and blurring of vision in her right eye for 3 days. On examination, she had visual acuity of 20/30 in the RE and 20/20 in the LE. The slit lamp examination revealed a paracentral superior corneal melt in the RE, and anterior chamber reaction was 2+. The lesion was classified as a large sterile corneal ulcer (Figure 1). No abnormality was noticed in the fundus examination of both eyes.
Figure 1.
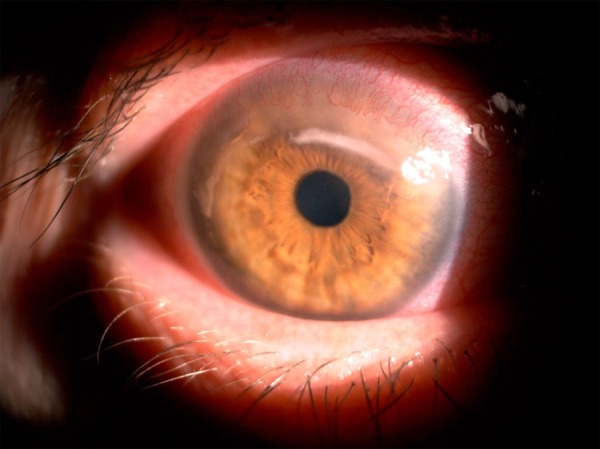
Slit-lamp examination in the right eye showing a paracentral superior corneal melt.
Laboratory investigations revealed positive antinuclear antibodies (1:640 speckled) and positive anti-Ro (197 u/ml, normal range: <15 u/ml) and anti-La (68 u/ml, normal range: <15 u/ml) anti-extractable nuclear antigens (anti-ENA, ELISA). While anti-Sm, anti-Rnp, anti-Jo1 and anti-Scl70 were negative, elevated serum immunoglobulins IgG and IgA when IgE and IgM were within normal levels. Evaluation of the underlying autoimmune rheumatic disease showed elevated levels of rheumatoid factor (RF: 955 IU/ml, normal range: 0–20 IU/ml), anti-cyclic citrullinated peptide antibodies (anti-CCP) levels >300 IU/ml (normal levels <30 IU/ml) and C-reactive protein (CRP), (1.59 mg/dl, normal range 0–0.8). To further evaluate disease prognosis, HLA typing revealed B*08, DRB1*0301 “super-haplotype”, which is strongly related to autoimmune predisposition in Caucasian patients.
Our patient was treated with 60 mg/day, oral prednisone, topical tobramycin every 4 hours and lubricants (artificial tears plus carbomer eye gel). Methotrexate at a dose 15 mg/week and prednisone with tapering doses was added, while the patient continued the IV abatacept. Re-epithelization occurred 1 week after initiation of the immunosuppressive treatment.
On follow-up visits, the eye discomfort was significantly eliminated and the visual acuity improved to 20/25 in the first 2 weeks and 20/20 within 1 month. The patient was instructed to use artificial tears without interruption. A complete laboratory workup was done, which revealed normalization of both CRP and ESR levels. In addition we noted that the RF and anti-CCP levels were greatly decreased (80 IU/ml and 129.42 IU/ml, respectively). These markers play an important role in the prognostic assessment of RA and can also fluctuate with disease activity.
Discussion
Corneal ulceration and corneal melting are rare complications of established Sjogren’s syndrome (SS), usually secondary to RA. Vivino et al. reported the first case of corneal ulceration with stromal melting as the initial presentation of primary SS [11]. The ulceration required extensive treatment over several months with ocular lubricants, systemic immunosuppressants and surgical repair. Two years after the last corneal ulcer and no longer taking prednisone, the patient’s ocular disease remained quiescent, receiving only azathioprine 175 mg and hydroxychloroquine 400 mg daily.
Kervick et al. presented 6 patients with RA (8 eyes) with small paracentral perforating corneal ulcers in otherwise quiet eyes [12]. The initial management of 5 patients (7 eyes) consisted of systemic immunosuppression and therapeutic tissue adhesive with a bandage contact lens or tectonic keratoplasty. The initiation of topical cyclosporine therapy in 5 eyes with recurrent corneal ulceration was associated with arrest of keratolysis and rapid re-epithelization of the ulcer in all cases.
The exact pathogenesis of the corneal ulceration that occurs in RA is not completely defined. A study by Villani et al. evaluated the corneal thickness in patients with RA, and revealed that all patients had central corneal and stromal thicknesses that were statistically significant thinner than the control group [13]. Importantly, changes occur for KCS in general (Liu), and also for KCS combined with autoimmune disease [14,15]. Increased apoptotic and proteolytic phenomena of the stroma play a significant role in promoting corneal thinning. Corneal thinning can also be attributed to increased tangential forces acting on the epithelial surface.
Susceptibility to RA is significantly greater in individuals with the MHC class II DR4 haplotype. It has been proposed that disease risk is associated with certain hypervariable regions of the HLA-DR β-chain (“shared epitopes”), thereby suggesting a role for (auto) antigen presentation.
Other studies highlight the role of leukocyte chemotaxis (migration into inflamed tissue) release of collagenases and proteases by lysosomals, which break down connective tissue, including corneal matrix (collagen and proteoglycans) [10]. Reduced levels of tissue inhibitor of metalloproteinases (TIMP-1) expression are consistent with high collagenase activity, release of tissue-injurious mediators and tissue destruction. Epithelial – stromal cell interactions and the production of local inflammatory mediators are of major importance in the pathogenesis of corneal destruction; although the precise nature of the antigenic stimulation and/or cellular interactions remains to be elucidated [16].
Additionally, evidence of proteolytic degradation was observed in both corneas as early xerophthalmia as well as ulcerating xerophthalmia by using light and electron microscopy. Much of the proteolytic damage in ulcerating xerophthalmia occurred extracellularly within the stromal matrix. In the ulcerating corneas, the stroma was heavily infiltrated with inflammatory cells and an extensive stromal degradation was observed in the central necrotic region of the lesion [17].
In a more recent study, Masuda et al. reported the role of oral nonsteroidal anti-inflammatory drugs (NSAIDs) in corneal perforation. In their report corneal perforation occurred in a 62-year-old woman and a 79-year-old woman, after 7 days and 5 months of oral NSAIDs administration, respectively [18]. After NSAIDs were discontinued, the cornea epithelialized and the anterior chamber formed within 14 and 10 days, respectively. It is well known that topical NSAIDs cause corneal perforation. However, observations in the present cases suggest that the oral administration of NSAIDs may also cause corneal damage, and hence, medical professionals should consider the risk of damage to the cornea when administering these drugs orally [18].
Moreover, it is suggested that the occurrence of an unbalance in collagenase levels, especially between metalloproteinases (MMP) and its tissue inhibitor (TIMP-1), may lead to a keratolysis process [19]. Smith et al. suggested that the progression of peripheral ulceration is correlated to abnormal MMP-2 production in the corneal stroma and the presence of MMP-9 in lacrimal gland secretion [20,21]. Galor and Thorne suggested that there is an abnormal activation of T cells, leading to the production of antibodies and formation of immune complexes that precipitate in the peripheral cornea [22].
As far as treatment is concerned, several therapeutic approaches are reported. Successful use of tissue adhesive has been reported in impending or actual corneal perforations [23]. However, its use is restricted to small perforations. Gottsch et al. report that treatment with topical cyclosporin alone may be considered in patients with sterile corneal ulcers associated with rheumatoid disease in the absence of systemic activation [24]. Clear guidelines regarding the institution of systemic chemotherapy in rheumatoid corneal perforations do not exist, although systemic immunosuppression, most commonly cyclophosphamide [25], and now cyclosporin [26], has been used for treating rheumatoid ulcerative keratitis.
Messmer and Foster report that the onset of necrotizing scleritis (NS) and peripheral ulcerative keratitis (PUK) in the clinical course of RA may reflect the presence of systemic, potentially lethal vasculitis [27]. Cytotoxic immunosuppressive therapy was instituted in all patients in their study with NS and/or PUK. Cyclophosphamide and methotrexate were the most successful agents used. It was observed that cytotoxic immunosuppressive drugs in conjunction with early aggressive surgical treatment halted the relentlessly progressive inflammation and preserved the integrity of the globe in 92% of eyes. Visual acuity could be stabilized or improved in 83% of patients with NS and in 68% with PUK.
A series of 40 eyes with rheumatoid keratolysis was reported by Malik et al. [28]. The mean duration of RA at presentation was 15 years. Most (55%) ulcers were peripheral, while 11 patients were immunosuppressed. They concluded that although the visual prognosis is often poor, surgical preservation of the eye can be achieved by penetrating keratoplasty and systemic immunosuppression, which appears to be safely tolerated with careful observation and regular monitoring.
Moreover, Thomas and Pflugfelder treated 3 patients with infliximab. These patients demonstrated progressive RA-associated peripheral ulcerative keratitis and were initially treated with conventional immunosuppressant therapy. It was found anti-TNF therapy (Infliximab) was effective in arresting progressive RA-associated peripheral ulcerative keratitis that was refractory to conventional immunomodulatory therapy [29].
Conclusions
Peripheral corneal ulceration is a disease of long-standing, sero-positive RA, which may have serious complications for the eye. It should therefore be regarded as a serious complication of RA and these patients should be kept under close observation. Close collaboration between ophthalmologists and rheumatologists is recommended to avoid poor visual outcome and associated mortality from other complications of systemic RA. The effective control of the underlying disease and early diagnosis of xerophthalmia in RA patients may prevent serious corneal complications, such as corneal ulceration, which are difficult to treat. Although the patient was already on immunosuppression, treatment was enhanced with steroids and methotrexate, halting the progression of keratolysis and assisting re-epithelization. The importance of the initiation of prednisone and methotrexate in the treatment of such complications is shown in the present case.
The patient had high levels of both RF and anti-CCP, which have been associated with worse clinical outcome and with extraarticular manifestations. Our patient presented with keratolysis and responded well to immunosuppressants. To further evaluate the prognostic factors, an immunogenetic investigation revealed the B*08, DRB1*0301 “super-haplotype”, which is strongly related to autoimmune predisposition in Caucasian patients.
References:
- 1.Grassi W, De Angelis R, Lamanna G, Cervini C. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27:18–24. doi: 10.1016/s0720-048x(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 2.Scutellari PN, Orzincolo C. Rheumatoid arthritis: sequences. Eur J Radiol. 1998;27:31–38. doi: 10.1016/s0720-048x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 3.Baldassano VF., Jr Ocular manifestations of rheumatic diseases. Curr Opin Ophthalmol. 1998;9:85–88. doi: 10.1097/00055735-199812000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Polanska V, Sery O, Fojtik Z, Hlinomazova Z. The presence of dry eye syndrome and corneal complications in patients with rheumatoid arthritis and its association with -174 gene polymorphism for interleukin 6. Cesk Slov Oftalmol. 2008;64:77–80. [PubMed] [Google Scholar]
- 5.Zlatanovic G, Veselinovic D, Cekic S, et al. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn J Basic Med Sci. 2010;10:323–27. doi: 10.17305/bjbms.2010.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy SC, Rao UR. Ocular complications of adult rheumatoid arthritis. Rheumatol Int. 1996;16:49–52. doi: 10.1007/BF01816435. [DOI] [PubMed] [Google Scholar]
- 7.Fox RI, Stern M, Michelson P. Update in Sjogren syndrome. Curr Opin Rheumatol. 2000;12:391–98. doi: 10.1097/00002281-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–35. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 9.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with non-contact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 10.Gokhale NS. Rheumatoid corneal melting. Indian J Ophthalmol. 1997;45:238–39. [PubMed] [Google Scholar]
- 11.Vivino FB, Minerva P, Huang CH, Orlin SE. Corneal melt as the initial presentation of primary Sjogren’s syndrome. J Rheumatol. 2001;28:379–82. [PubMed] [Google Scholar]
- 12.Kervick GN, Pflugfelder SC, Haimovici R, et al. Paracentral rheumatoid corneal ulceration. Clinical features and cyclosporine therapy. Ophthalmology. 1992;99:80–88. doi: 10.1016/s0161-6420(92)32006-8. [DOI] [PubMed] [Google Scholar]
- 13.Villani E, Galimberti D, Viola F, et al. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2008;49:560–64. doi: 10.1167/iovs.07-0893. [DOI] [PubMed] [Google Scholar]
- 14.Villani E, Galimberti D, Viola F, et al. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48:2017–22. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 15.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–49. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 16.Riley GP, Harrall RL, Watson PG, et al. Collagenase (MMP-1) and TIMP-1 in destructive corneal disease associated with rheumatoid arthritis. Eye (Lond) 1995;9:703–18. doi: 10.1038/eye.1995.182. [DOI] [PubMed] [Google Scholar]
- 17.Twining SS, Hatchell DL, Hyndiuk RA, Nassif KF. Acid proteases and histologic correlations in experimental ulceration in vitamin A deficient rabbit corneas. Invest Ophthalmol Vis Sci. 1985;26:31–44. [PubMed] [Google Scholar]
- 18.Masuda I, Matsuo T, Okamoto K, et al. Two cases of corneal perforation after oral administration of nonsteroidal anti-inflammatory drugs: oral NSAID-induced corneal damage. Eur J Ophthalmol. 2010;20:454–56. doi: 10.1177/112067211002000230. [DOI] [PubMed] [Google Scholar]
- 19.Silva BL, Cardozo JB, Marback P, et al. Peripheral ulcerative keratitis: a serious complication of rheumatoid arthritis. Rheumatol Int. 2010;30:1267–68. doi: 10.1007/s00296-009-1161-7. [DOI] [PubMed] [Google Scholar]
- 20.Smith VA, Hoh HB, Easty DL. Role of ocular matrix metalloproteinases in peripheral ulcerative keratitis. Br J Ophthalmol. 1999;83:1376–83. doi: 10.1136/bjo.83.12.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85:147–53. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007;33:835–54. doi: 10.1016/j.rdc.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss JL, Williams P, Lindstrom RL, Doughman DJ. The use of tissue adhesive in corneal perforations. Ophthalmology. 1983;90:610–15. doi: 10.1016/s0161-6420(83)34508-5. [DOI] [PubMed] [Google Scholar]
- 24.Gottsch JD, Akpek EK. Topical cyclosporin stimulates neovascularization in resolving sterile rheumatoid central corneal ulcers. Trans Am Ophthalmol Soc. 2000;98:81–87. discussion 87–90. [PMC free article] [PubMed] [Google Scholar]
- 25.Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis. Effects of systemic immunosuppression. Ophthalmology. 1984;91:1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 26.Bernauer W, Ficker LA, Watson PG, Dart JK. The management of corneal perforations associated with rheumatoid arthritis. An analysis of 32 eyes. Ophthalmology. 1995;102:1325–37. doi: 10.1016/s0161-6420(95)30867-6. [DOI] [PubMed] [Google Scholar]
- 27.Messmer EM, Foster CS. Destructive corneal and scleral disease associated with rheumatoid arthritis. Medical and surgical management. Cornea. 1995;14:408–17. doi: 10.1097/00003226-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Malik R, Culinane AB, Tole DM, Cook SD. Rheumatoid keratolysis: a series of 40 eyes. Eur J Ophthalmol. 2006;16:791–97. doi: 10.1177/112067210601600602. [DOI] [PubMed] [Google Scholar]
- 29.Thomas JW, Pflugfelder SC. Therapy of progressive rheumatoid arthritis-associated corneal ulceration with infliximab. Cornea. 2005;24:742–44. doi: 10.1097/01.ico.0000154391.28254.1d. [DOI] [PubMed] [Google Scholar]


