In recent years, our understanding of the genetic alterations underlying thyroid oncogenesis has greatly expanded. This review summarizes the current literature surrounding RAS and discusses its potential as a diagnostic and prognostic indicator in the management of thyroid cancer.
Keywords: RAS, Thyroid cancer, Thyroid nodules, Molecular markers
Learning Objectives
Explain the role of RAS mutations in thyroid carcinogenesis.
Describe the histologic spectrum and prognostic implications of RAS-associated thyroid neoplasms.
Explain the role of RAS mutation testing in patient care management algorithms.
Abstract
In recent years, our understanding of the genetic alterations underlying thyroid oncogenesis has greatly expanded. The use of molecular markers, including RAS, in the management of thyroid carcinoma is also increasing. This review summarizes the current literature surrounding RAS and discusses its potential as a diagnostic and prognostic indicator in the management of thyroid cancer.
Implications for Practice:
In recent years, our understanding of the molecular mechanisms underlying thyroid oncogenesis has greatly expanded. Further, the use of some molecular markers in the clinical management of thyroid cancer is increasing. Mutations in RAS represent the second most common genetic event in thyroid neoplasia. However, the significance of RAS-positive mutation status and the biological behavior of thyroid carcinomas that harbor RAS are not completely understood. The purposes of this review are to clarify the current literature surrounding RAS mutations in thyroid cancer and to examine the potential utility of RAS as a diagnostic tool to predict the presence of malignancy, thus altering subsequent clinical management. In addition, the prognostic value of RAS positivity in predicting the risk for tumor aggressiveness, recurrence, and mortality is discussed.
Introduction
Thyroid cancer (TC) is the most common endocrine malignancy, and its incidence is on the rise [1]. Tumors of follicular epithelial cell origin account for the vast majority of these cancers, and of these, well-differentiated papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) account for 95%; whereas, poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC) are observed far less frequently [2]. In recent years, our understanding of the molecular mechanisms underlying thyroid oncogenesis has greatly expanded, thus enabling differentiation of thyroid tumors based on characteristic genetic alterations in addition to traditional histologic criteria [3]. The most clinically relevant markers to date include point mutations in BRAF and RAS and RET/PTC and PAX8/PPARγ rearrangements. PTC is known to harbor BRAF most commonly, followed by RAS and RET/PTC, whereas FTC is characterized by the presence of either RAS or PAX8/PPARγ [3–6].
The use of these molecular markers in the management of thyroid carcinoma is increasing. BRAF is perhaps the most studied of the markers and has emerged as an important diagnostic and prognostic tool. For example, the finding of BRAF in a thyroid nodule with indeterminate cytology is associated with a PTC risk of nearly 100%, and further, patients with BRAF-positive PTC are more likely to have aggressive and recurrent disease [7–11]. Likewise, RAS represents the second most common genetic mutation in TC and was first implicated in thyroid neoplasia more than two decades ago [12]. Despite this, the significance of RAS-positive mutation status and the biological behavior of thyroid carcinomas that harbor RAS are still not completely understood. In part, this uncertainty is because RAS mutations have been reported in the full spectrum of thyroid neoplasms ranging from benign follicular adenomas to anaplastic carcinomas, thus obscuring its true clinical relevance [4, 5].
Therefore, the purpose of this review is to clarify the current literature surrounding RAS mutations in TC. Specifically, we will discuss the prevalence and isoform pattern of RAS, the pathogenesis of RAS-mediated oncogenesis, and the potential utility of RAS as a diagnostic and prognostic tool in the management of TC.
Prevalence and Isoform Pattern of RAS Mutations
The RAS gene encodes a family of three highly homologous isoforms: NRAS, HRAS, and KRAS. These 21-kDa membrane-associated proteins play a central role in the transduction of signals from tyrosine kinase and G protein-coupled receptors to effectors of the MAPK and PI3K-AKT signaling pathways, which mediate cell differentiation, proliferation, and survival [13, 14]. Under normal conditions, RAS activity is tightly regulated by GTP-mediated hydrolysis of activated GTP-bound RAS to inactivated GDP-bound RAS. Point mutations produce oncogenic alleles of RAS that exhibit either increased affinity for GTP (codons 12 and 13) or inhibition of autocatalytic GTP-ase function (codon 61). Both mechanisms result in constitutive, aberrant activation of the downstream MAPK and PI3/AKT signaling pathways, a critical event in thyroid tumorigenesis [4, 9, 12, 15, 16].
Thyroid neoplasms are unique in that they have been associated with all three mutant isoforms of the RAS gene, although most series demonstrate predominance of NRAS61 [6, 17–23]. Further, the literature cites overall frequencies of RAS mutations in up to 48% of benign follicular adenomas (FA), 57% of FTC, and 21% of PTC (Table 1) [6, 18–21, 24–28]. However, the overall prevalence and pattern of specific isoform frequency varies significantly among reports. In part, this is because existing data are comprised mostly of small studies that often differ with respect to methodological criteria. For example, RAS prevalence is generally lower when analysis is limited to studies that use direct sequencing—the gold standard—either exclusively or for confirmation of mutation identification. This was illustrated by Vasko et al., who noted a significantly higher overall rate of mutation detection (17% vs. 12%, p < .01), particularly with respect to overestimation of HRAS61, when direct sequencing was not used [19].
Table 1.
Reported overall frequency of RAS mutations in thyroid neoplasms
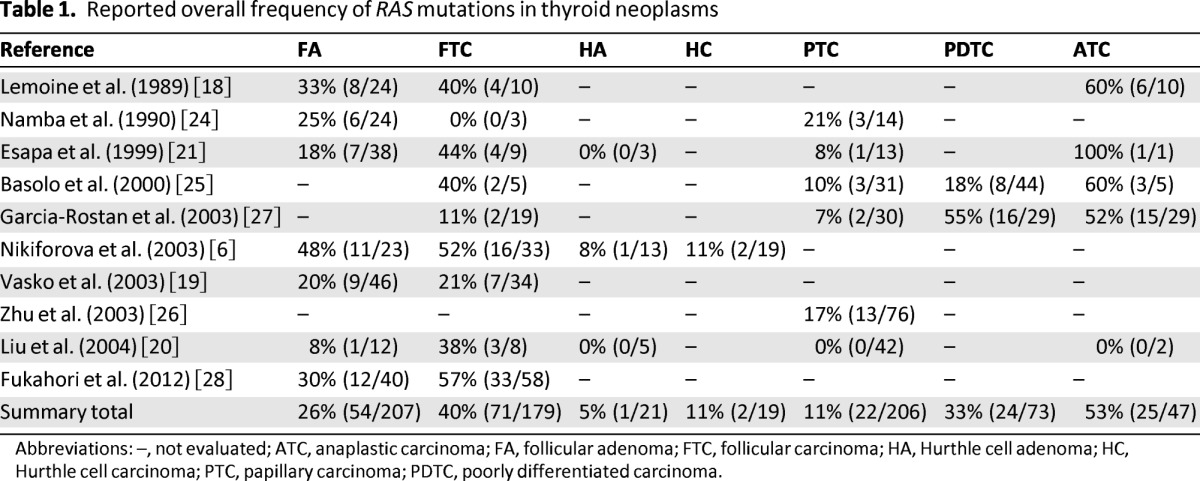
Abbreviations: –, not evaluated; ATC, anaplastic carcinoma; FA, follicular adenoma; FTC, follicular carcinoma; HA, Hurthle cell adenoma; HC, Hurthle cell carcinoma; PTC, papillary carcinoma; PDTC, poorly differentiated carcinoma.
To address these methodological limitations, Liu et al. performed a meta-analysis of 86 RAS tumors described in a restricted group of publications. In all the selected studies, tumors underwent direct sequencing for mutation identification and were routinely screened for all three mutant isoforms (H-, K-, and NRAS) in the same tumor. The study reported that mutations involving NRAS at codon 61 were by far the most numerous, accounting for 67% of all RAS mutations in the series [20]. This finding was corroborated by another pooled analysis of 22 studies with similar inclusion criteria, in which NRAS61 accounted for 88% of RAS mutations [19]. Both pooled analyses further concluded that RAS mutations were more prevalent in FTC than in benign FA, and were relatively uncommon in PTC. Liu et al. reported frequencies of 27%, 15%, and 6% for FTC, FA, and PTC, respectively, and Vasko et al. reported a frequency of 25%, 14%, and 5% for FTC, FA, and PTC, respectively [19, 20].
The almost exclusive occurrence of RAS mutations in follicular tumors and their rare appearance in PTC has also been suggested in other studies; however, there is increasing evidence that RAS-positive PTC may be restricted to the follicular variant subtype (FVPTC), which possesses the follicular growth pattern and architecture typical of follicular tumors [21, 26, 29]. For example, a recent study conducted to examine the prevalence of RAS mutations in 30 cases of FVPTC and 46 cases of non-FVPTC demonstrated that none of the non-FVPTC were RAS-mutation positive, but 43% of FVPTC were RAS-mutation positive [26]. This suggests that the occurrence of RAS mutations in PTC may be underestimated in study samples that do not include the follicular variant subtype.
Reported prevalence may also be influenced by the inclusion of, or failure to distinguish, oncocytic variants of follicular tumors. Hurthle cell carcinoma (HC) and its benign counterpart, Hurthle cell adenoma (HA), account for one third of all follicular lesions and are far less likely to harbor RAS mutations than conventional FTC and FA [6, 20, 30]. A recent study examining a series of 33 FTC, 23 FA, 19 HC, and 13 HA detected RAS mutations in 52% of FTC and 48% of FA, but in only 11% of HC and 8% of HA [20]. Furthermore, the isoform pattern between conventional FTC and HC also differed with mutations of NRAS at codon 61 common in the former but absent in the latter. Hurthle cell tumors exhibited mutations only in HRAS at codon 61 and KRAS at codon 12, once again underscoring the importance of the subtype histologic variant when interpreting overall and individual isoform prevalence data [20].
Pathogenesis of RAS-Positive Thyroid Carcinoma
Although the ability of activated mutant RAS to induce thyroid neoplasia in both in vivo and in vitro experimental studies has been known for a number of years [31, 32], the clinical applicability of detecting this mutation has only recently been described. The current understanding is that RAS mutations that occur in well differentiated thyroid cancer (WDTC) represent distinct molecular events that are mutually exclusive with other genetic alterations that occur in FTC and PTC. It is postulated that up to 85% of conventional FTC develop along one of two described molecular pathways involving either RAS mutation or PAX8-PPARγ gene rearrangement [6]. A recent investigation reported that 49% and 36% of conventional FTC were positive for RAS and PAX8-PPARγ, respectively [6]. Furthermore, only 1 out of 33 FTC in this series had both alterations, supporting the concept of two discrete, essentially non-overlapping molecular mechanisms [6]. With respect to papillary cancer, RAS mutations are generally restricted to the follicular variant subtype, as previously mentioned [21, 23, 26]. Furthermore, the most common alterations found in non-FVPTC, BRAF V600E and RET/PTC, are infrequent in FVPTC, again suggesting a distinct RAS-mediated mechanism for tumorigenesis in PTC [26, 33].
The precursor lesion for RAS-mediated development of FTC and FVPTC is currently hypothesized to be RAS-positive FA [3, 4, 6]. This theory stems from the consistent demonstration of RAS mutations in FA, a feature that is not typical of other well-described genetic events in TC, such as PAX8-PPARγ, BRAF V600E, and RET/PTC mutations [6, 18]. A recent series of RAS-positive tumors demonstrated distribution along the full histologic spectrum from benign FA, encapsulated and indolent FVPTC, encapsulated FVPTC with capsular invasion, non-encapsulated infiltrative FVPTC, FTC, and ATC [23]. Similarly, in a group of follicular tumors, RAS mutations were found in both benign FA, with no morphologic indicators of malignancy, and in FTC that ranged from minimally, overtly, and widely invasive [6].
The above-mentioned studies seem to suggest that RAS mutation occurs as an early event in FA and may increase the potential for malignant transformation. Further, there are also data to suggest that RAS may predispose WDTC to subsequent de-differentiation into poorly differentiated thyroid cancer (PDTC) and ATC [34]. RAS is present at all stages of tumor differentiation, which is not a characteristic feature of other molecular alterations [18, 25]. For example, in one of the first studies to examine the prevalence of RAS in a full spectrum of thyroid tumors, RAS mutations were found in 33% of FA, 53% of WDTC, and 60% of ATC [18]. In addition, PDTC and ATC seem to maintain RAS activation while also gaining mutations in p53, beta-catenin, PTEN, and/or PI3KCA genes [35–38]. The acquisition of these “late” genetic events in RAS-positive tumors further supports the notion of stepwise RAS-mediated oncogenesis, in which an early transformative mutation in RAS predisposes to future molecular alterations that promote development of cancer and subsequent de-differentiation. The natural history and progression of RAS-positive neoplasms is unfortunately difficult to confirm definitively, and these theories remain speculative.
Diagnostic Utility of RAS Mutation
Thyroid nodules are common, and the clinical challenge is to identify the 5%–15% that harbor or that are at increased risk for developing malignancy [39]. Fine-needle aspiration biopsy (FNAB) is the recommended diagnostic procedure of choice but can yield an indeterminate cytology result up to 30% of the time [40, 41]. Patients with indeterminate cytology may ultimately require diagnostic surgery to exclude malignancy; however, this carries with it the potential for operative complications and undue health care costs [42]. As a result, much recent attention has focused on improving the diagnostic accuracy of FNAB through the use of adjunctive molecular testing for those with indeterminate cytology, a practice that is supported by current American Thyroid Association guidelines [39, 43].
Molecular testing should occur as part of a panel which is an effective strategy because the most commonly described alterations, BRAF, RAS, RET/PTC, and PAX8-PPARγ, are not only mutually exclusive but can be found in up to 70%–80% of TC [4, 5]. Three recent studies that examined the utility of concurrent testing of FNAB specimens for the above-mentioned alterations found that the presence of any mutation was a strong predictor of cancer, with histologic confirmation of malignancy in 89%–97% of specimens [7, 8, 44]. Yet, unlike BRAF and RET/PTC, which nearly always confer a malignant diagnosis, the predictive value of a detected RAS mutation is less definitive [7, 8, 44, 45]. This is because RAS mutations are consistently found in benign FA, leading some to even suggest that RAS-positive FA should be classified as “false-positive” molecular results [44]. However, this contrasts with the prevailing notion that RAS-positive FA is likely a precursor to RAS-positive follicular-patterned cancer [6, 22, 23, 46].
In tumors of follicular cell origin, RAS mutations are essentially restricted to FA, FTC, and FVPTC which are difficult to differentiate as benign or malignant based on cytology alone, and are therefore often indeterminate by FNAB. It is precisely in this group of FNAB results that RAS mutation testing may be most clinically useful. In fact, in a recent series of 67 prospectively identified RAS-positive thyroid nodules, cytology was malignant in 3%, benign in 3%, but indeterminate in 94% [23]. Similarly, Nikiforov et al. evaluated a large series of FNAB specimens with indeterminate cytology and found that RAS was the most common mutation detected (72%), followed by BRAF (21%), PAX8-PPARγ (6%), and RET/PTC (1%) [7]. Further, in this study, the probability of cancer associated with RAS-positive mutation status was 85%, which is consistent with other reports in the literature that range from 74% to 88% [7, 8, 43, 45].
The markedly elevated increased risk for cancer (∼85%) when RAS mutation is present potentially alters initial surgical management for the group of patients with otherwise indeterminate cytology.
Although identification of RAS mutation in the FNAB specimen is not 100% predictive of cancer, it is certainly highly suggestive of either FTC or FVPTC and thus has significant diagnostic value. The markedly elevated increased risk for cancer (∼85%) when RAS mutation is present potentially alters initial surgical management for the group of patients with otherwise indeterminate cytology [7]. Even if an RAS-positive indeterminate nodule is histologically confirmed as FA, some would argue that FA has an increased risk for malignant degeneration and may be best treated by surgical resection.
Although it is not the focus of this discussion, it should also be mentioned that RAS mutation has been implicated in the pathogenesis of non-familial, RET-negative medullary thyroid cancer (MTC), with a reported prevalence ranging from 17% to 81% [47–49]. In the context of sporadic MTC, RAS and RET are thought to represent alternative genetic events, and thus knowledge of RAS mutation status has potential diagnostic value and can aid in the selection of targeted therapies [50].
Prognostic Utility of RAS
Most patients with WDTC have an excellent prognosis, with an average 20-year survival following tumor resection above 90% [51]. The clinical challenge remains in identifying the subset of tumors that have aggressive biological behavior and therefore have a negative impact on patient morbidity and mortality. To date, few publications have addressed the prognostic significance of RAS-positive TC, and no universal conclusions can be drawn at this time. There is, however, some evidence to suggest that a select group of patients with RAS-positive WDTC may be at risk for RAS-mediated tumor de-differentiation, distant metastases, and shortened survival [25, 27, 28, 52, 53].
Poorly differentiated and anaplastic subtypes of TC typically arise from stepwise de-differentiation of PTC and FTC, a process that may be facilitated by mutation in the RAS oncogene [2, 3]. Evidence in favor of this theory stems in part from the in vitro finding that mutant RAS can promote chromosomal instability [54]. Further, it has been noted that well differentiated FTC and PTC with focal areas of poorly differentiated histology are often RAS mutation positive. Zhu et al. reported that all identified cases of PTC with focal areas of poorly differentiated carcinoma were of the follicular variant subtype, and that 67% of these cases harbored mutations in RAS [26]. Similarly, Nikiforova et al. found that FTC with focal areas of poorly differentiated histology contained mutated RAS [6].
Given the high probability of cancer, an RAS-positive lesion with indeterminate cytology could be considered for initial upfront total thyroidectomy to eliminate the need for, risks of, and costs of re-operative completion thyroidectomy.
In addition to the correlation between RAS and loss of histologic features characteristic of WDTC, a high frequency of RAS mutations in PDTC and ATC has been reported in many observational studies (Table 1) [18, 21, 25, 27]. An early study by Lemoine et al. found RAS mutations in 60% of undifferentiated ATC, and a more recent investigation described a similar prevalence of RAS in 55% of PDTC and 52% of ATC [18, 27]. Another study by Volante et al. sought to characterize the individual frequency of molecular alterations in a group of 65 PDTC diagnosed using strict Turin criteria and reported a predominance of RAS mutations in 23% of cases (n = 15) [53]. In contrast, only one mutation was identified in BRAF V600E and no mutations were detected in KRAS, RET/PTC, or PAX8-PPARγ, again suggesting the exclusive potential ability of RAS to predispose to de-differentiation and/or anaplastic transformation [53].
It has also been suggested that RAS may confer a more aggressive phenotype in some cases, increasing a patient's risk for tumor recurrence, distant metastases, and death (Table 2) [25, 27, 28, 52, 53, 55, 56]. In 91 cases of PTC, Hara et al. found NRAS61 mutation in 14% (n = 13) of patients overall, with a significantly higher incidence of RAS in patients with distant metastases than in those without (28% vs. 8%, p = .01), and in patients who died versus those who were still alive (33% vs. 10%, p = .02) [52]. Kaplan-Meier survival analysis further revealed that patients in the RAS-positive group experienced significantly greater mortality and recurrence than those in the RAS-negative group [52].
Table 2.
RAS mutation and poor outcome
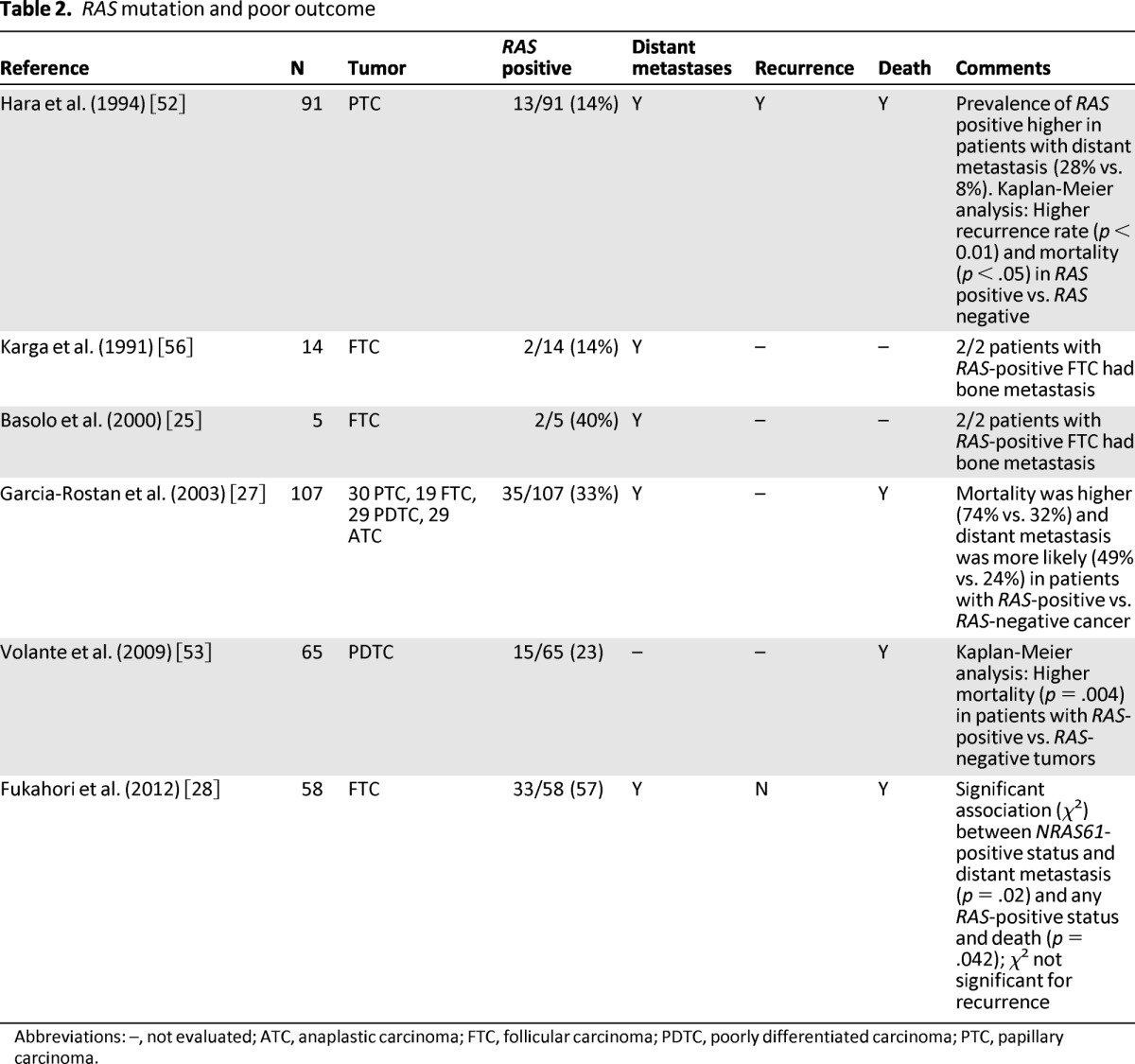
Abbreviations: –, not evaluated; ATC, anaplastic carcinoma; FTC, follicular carcinoma; PDTC, poorly differentiated carcinoma; PTC, papillary carcinoma.
Several additional studies drew similar conclusions regarding RAS as a marker for poor prognosis, although the sample size in each study was small and mutation testing was performed only on selected patients. Manenti et al. and Karga et al. reported an association between RAS mutations and hematogenous bone metastases [55, 56]. Garcia-Rostan et al. examined a heterogeneous group of TC, including WDTC, PDTC, and ATC, and found that RAS was an independent predictor of poor survival even when restricting the analysis to differentiated cancers [27]. Finally, Fukahori et al. reported that RAS was significantly associated with both distant metastases and death in their series of patients with FTC [28].
Potential Management Algorithm Incorporating RAS Mutation Status
Molecular testing for RAS mutation of the FNAB specimen can provide useful clinical information that can be of diagnostic value and may alter patient management. As mentioned previously, several studies have demonstrated that the finding of RAS mutation in a thyroid nodule is highly suggestive of malignancy, with predictive values ranging between 74% and 88% [7, 8, 43, 44]. Because RAS mutations are found with high frequency in nodules with indeterminate cytology, this added information has a significant impact, particularly when diagnostic lobectomy is the next step in management. Given the high probability of cancer, an RAS-positive lesion with indeterminate cytology could be considered for initial upfront total thyroidectomy to eliminate the need for, risks of, and costs of re-operative completion thyroidectomy. There is also evidence that RAS-positive cancers are frequently bilateral, further supporting the choice of upfront total thyroidectomy for these patients [23]. A study by Gupta et al. in 46 patients with RAS-positive malignancy found bilateral cancer in 43% (n = 20): 45% of these contralateral cancers were positive for RAS mutation, 5% were positive for BRAFV600E, and 50% did not undergo molecular testing. Notably, the majority of patients with bilateral cancer underwent diagnostic lobectomy as their initial procedure, resulting in the need for a second operative procedure in most [23].
In some circumstances when the diagnosis of cancer is known preoperatively, total thyroidectomy is indicated. In the case of PTC, central neck dissection may also be considered because of the propensity of PTC to spread to central compartment lymph nodes and its association with recurrent disease [39, 57]. However, RAS-positive PTC is nearly always FVPTC, which has been characterized by a lack of central compartment lymph node metastases in several studies [23, 26, 33, 58]. Thus, central neck dissection in RAS-positive cancers can likely be deferred in the absence of clinically evident or suspicious nodal disease.
Conclusion
Mutations in RAS represent the second most commonly identified genetic alteration in TC. RAS mutations are primarily found in follicular-patterned tumors, including FA, follicular cancer, and the follicular variant of papillary cancer. There is increasing evidence that RAS mutation status has significant diagnostic utility when used concurrently with FNAB. This is particularly true for lesions with indeterminate cytology, for which the detection of RAS may have a potential impact on initial surgical management. Although some data suggest that RAS mutation positivity is associated with tumor progression to histologic subtypes associated with poor prognosis, future studies will be needed to determine further whether prospective RAS testing has equal prognostic significance.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Linwah Yip, Gina M. Howell, Steven P. Hodak
Collection and/or assembly of data: Linwah Yip, Gina M. Howell
Data analysis and interpretation: Linwah Yip, Gina M. Howell
Manuscript writing: Linwah Yip, Gina M. Howell
Final approval of manuscript: Linwah Yip, Gina M. Howell, Steven P. Hodak
Disclosures
The authors indicated no financial relationships.
Section editors: Herbert Chen: None; Stan Sidhu: None
Reviewer “A”: None
Reviewer “B”: None
Reference
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.DeLellis RA, Lloyd RV, Heitz PU, et al., editors. Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 3.Nikiforova MN, Nikiforov YE. Molecular Testing of Thyroid FNA Samples. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Diagnostic Pathology and Molecular Genetics of the Thyroid. Baltimore, MD: Lippincott Williams & Wilkins; 2009. pp. 94–102. [Google Scholar]
- 4.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 5.Nikiforov YE. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod Pathol. 2008;21:S37–43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: Evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 7.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 9.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocrine Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 10.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–471. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–4090. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 12.Suarez HG, du Villard JA, Caillou B, et al. Detection of activated ras oncogenes in human thyroid carcinomas. Oncogene. 1988;2:403–406. [PubMed] [Google Scholar]
- 13.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 14.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos JL. Ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 16.Pai EF, Kabsch W, Krengel U, et al. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989;341:209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- 17.Suarez HG, du Villard JA, Severino M, et al. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990;5:565–570. [PubMed] [Google Scholar]
- 18.Lemoine NR, Mayall ES, Wyllie FS, et al. High frequency of RAS oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989;4:149–164. [PubMed] [Google Scholar]
- 19.Vasko V, Ferrand M, Di Cristofaro J, et al. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003;88:2745–2752. doi: 10.1210/jc.2002-021186. [DOI] [PubMed] [Google Scholar]
- 20.Liu R-T, Hou C-Y, You H-L, et al. Selective occurrence of ras mutations in benign and malignant thyroid follicular neoplasms in Taiwan. Thyroid. 2004;14:616–621. doi: 10.1089/1050725041692882. [DOI] [PubMed] [Google Scholar]
- 21.Esapa CT, Johnson SJ, Kendall-Taylor P, et al. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol. 1999;50:529–535. doi: 10.1046/j.1365-2265.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 22.Banito A, Pinto AE, Espadinha C, et al. Aneuploidy and RAS mutations are mutually exclusive events in the development of well-differentiated thyroid follicular tumours. Clin Endocrinol. 2007;67:706–711. doi: 10.1111/j.1365-2265.2007.02949.x. [DOI] [PubMed] [Google Scholar]
- 23.Gupta N, Dasyam A, Carty SE, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–E922. doi: 10.1210/jc.2012-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990;4:1474–1479. doi: 10.1210/mend-4-10-1474. [DOI] [PubMed] [Google Scholar]
- 25.Basolo F, Pisaturo F, Pollina LE, et al. N-ras mutation in poorly differentiated thyroid carcinomas: Correlation with bone metastases and inverse correlation to thyroglobulin expression. Thyroid. 2000;10:19–23. doi: 10.1089/thy.2000.10.19. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z, Gandhi M, Nikiforova MN, et al. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2003;120:71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Rostan G, Zhao H, Camp RL, et al. ras Mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21:3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 28.Fukahori M, Yoshida A, Hayashi H, et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid. 2012;22:683–689. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]
- 29.Rosai J, Zampi G, Carcangiu ML. Papillary carcinoma of the thyroid: A discussion of its several morphologic expressions, with particular emphasis on the follicular variant. Am J Surg Pathol. 1983;7:809–817. [PubMed] [Google Scholar]
- 30.Schark C, Fulton N, Jacoby RF, et al. N-RAS 61 oncogene mutations in Hurthle cell tumors. Surgery. 1990;108:994–999. [PubMed] [Google Scholar]
- 31.Bond JA, Wyllie FS, Rowson J, et al. In vitro reconstruction of tumour initiation in human epithelium. Oncogene. 1994;9:281–290. [PubMed] [Google Scholar]
- 32.Rochefort P, Caillou B, Michiels FM, et al. Thyroid pathologies in transgenic mice expressing a human activated Ras gene driven by a thyroglobulin promoter. Oncogene. 1996;12:111–118. [PubMed] [Google Scholar]
- 33.Di Cristofaro J, Marcy M, Vasko V, et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: Association of N-ras mutation in codon 61 with follicular variant. Hum Pathol. 2006;37:824–830. doi: 10.1016/j.humpath.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Motoi N, Sakamoto A, Yamochi T, et al. Role of ras mutation in the progression of thyroid carcinoma of follicular epithelial cell origin. Pathol Res Pract. 2000;196:1–7. doi: 10.1016/S0344-0338(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Rostan G, Tallini F, Herrero A, et al. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 36.Garcia-Rostan G, Costa AM, Pereira-Castro I, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 37.Fagin JA, Matsuo K, Karmakar A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianoukakis AG, Giannelli SM, Salameh WA, et al. Well differentiated follicular thyroid neoplasia: Impact of molecular and technological advances on detection, monitoring and treatment. Mol Cell Endocrinol. 2011;332:9–20. doi: 10.1016/j.mce.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 39.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 40.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 41.Baloch ZW, LiVoisi VA. Fine-needle aspiration of thyroid nodules: Past, present, and future. Endocr Pract. 2004;10:234–241. doi: 10.4158/EP.10.3.234. [DOI] [PubMed] [Google Scholar]
- 42.Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: A summation. Cytojournal. 2008;5:6. doi: 10.1186/1742-6413-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moses W, Weng J, Sansano I, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34:2589–2594. doi: 10.1007/s00268-010-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 45.Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.”. Cancer Cytopathol. 2010;118:17–23. doi: 10.1002/cncy.20063. [DOI] [PubMed] [Google Scholar]
- 46.Vasko VV, Gaudart J, Allasia C, et al. Thyroid follicular adenomas may display features of follicular carcinoma and follicular variant of papillary carcinoma. Eur J Endocrinol. 2004;151:779–786. doi: 10.1530/eje.0.1510779. [DOI] [PubMed] [Google Scholar]
- 47.Ciampi R, Mian C, Fugazzola L, et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid. 2013;23:50–57. doi: 10.1089/thy.2012.0207. [DOI] [PubMed] [Google Scholar]
- 48.Moura MM, Cavaco BM, Pinto AE, et al. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:E863–E868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- 49.Boichard A, Croux L, Al Ghuzlan A, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab. 2012;97:E2031–E2035. doi: 10.1210/jc.2012-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida MQ, Hoff AO. Recent advances in the molecular pathogenesis and targeted therapies of medullary thyroid carcinoma. Curr Opin Oncol. 2012;24:229–234. doi: 10.1097/CCO.0b013e328351c71a. [DOI] [PubMed] [Google Scholar]
- 51.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–99): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 52.Hara H, Fulton N, Yashiro T, et al. N-ras mutation: An independent prognostic factor for aggressiveness of papillary thyroid carcinoma. Surgery. 1994;116:1010–1016. [PubMed] [Google Scholar]
- 53.Volante M, Rapa I, Gandhi M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab. 2009;94:4735–4741. doi: 10.1210/jc.2009-1233. [DOI] [PubMed] [Google Scholar]
- 54.Saavedra HI, Knauf JA, Shirokawa JM, et al. The RAS oncogene induces genomic instability in thy-roid PCCL3 cells via the MAPK pathway. Oncogene. 2000;19:3948–3954. doi: 10.1038/sj.onc.1203723. [DOI] [PubMed] [Google Scholar]
- 55.Manenti G, Pilotti S, Re FC, et al. Selective activation of ras oncogenes in follicular and undifferentiated thyroid carcinomas. Eur J Cancer. 1994;30A:987–993. doi: 10.1016/0959-8049(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 56.Karga H, Lee JK, Vickery AL, et al. Ras oncogene mutations in benign and malignant thyroid neoplasms. J Clin Endocrinol Metab. 1991;73:832–836. doi: 10.1210/jcem-73-4-832. [DOI] [PubMed] [Google Scholar]
- 57.Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer. Am J Surg. 1978;136:107–112. doi: 10.1016/0002-9610(78)90209-x. [DOI] [PubMed] [Google Scholar]
- 58.Jain M, Khan A, Patwardhan N, et al. Follicular variant of papillary thyroid carcinoma: A comparative study of histopathologic features and cytology results in 141 patients. Endocr Pract. 2001;7:79–84. doi: 10.4158/EP.7.2.79. [DOI] [PubMed] [Google Scholar]


