SUMMARY
The role of metabolism in ovarian aging is poorly described, despite the fact that ovaries fail earlier than most other organs. Growing interest in ovarian function is being driven by recent evidence that mammalian females routinely generate new oocytes during adult life through the activity of germline stem cells. In this perspective, we overview the female reproductive system as a powerful and clinically relevant model to understand links between aging and metabolism, and we discuss new concepts for how oocytes and their precursor cells might be altered metabolically to sustain or increase ovarian function and fertility in women.
INTRODUCTION
Early in life’s history, a complex signaling network evolved to maximize the number of descendants a cell could produce in a particular environment. This ancient network, which still exists in cells today, promotes growth and reproduction when the environment is favorable and suppresses these activities during harsh times (Kirkwood, 1987). This system explains in large part why many species gain health benefits from dietary restriction (DR) and how the body adapts to changing supplies and demands for energy. As we learn more about this survival network, it is becoming increasingly plausible to stimulate it pharmacologically. Indeed, molecules that mimic DR are in development for treatment of many aging-related health issues, such as type II diabetes, inflammation and muscle degeneration (Blum et al., 2011; Chiba et al., 2010). Despite rapid progress in this area, one aspect of human health that has been largely neglected is reproductive potential.
THE OVARY AS A MODEL FOR AGING STUDIES
The main functional unit of mammalian ovaries is a multi-cellular structure referred to as the follicle (Gougeon, 1996). Each follicle is composed of an oocyte, which is a partially differentiated female germ cell arrested in prophase of the first meiotic cell division, enclosed by one or more layers of specialized somatic cells that support the oocyte during its growth. Starting with a resting (primordial) follicle that contains an oocyte and just a single layer of somatic granulosa cells, each follicle attempts to complete progressive developmental stages associated with extensive replication of the granulosa cell population and the acquirement of a second somatic cell type known as theca-interstitial cells. Through complex cell-to-cell interactions, the oocyte gains developmental competence so that it can initiate embryogenesis if fertilized after ovulation (Matzuk et al., 2002; Orisaka et al., 2009). Simultaneously, the follicular somatic cells become highly responsive to circulating factors and secrete a spectrum of hormones that exert effects both locally in the ovaries and in many other tissues including the brain, bones, skin and cardiovascular system (Buckler, 2005; Prior, 1998).
Because of the central importance of follicles to maintaining endocrine function of the female gonads as well as to fertility, ovarian lifespan is dictated by the number of follicles present in the tissue—an endpoint often referred to as the ‘ovarian reserve’. Since the 1950s, it was widely believed that females of most mammalian species are provided with a non-renewable ovarian reserve around the time of birth (Zuckerman, 1951). Following growth activation, each primordial follicle in this reserve either completes maturation for release of its enclosed oocyte at ovulation, or undergoes a degenerative process referred to as atresia. Historical studies of mouse, rat and human ovaries have shown that atresia actually claims the vast majority of follicles present in the gonads, ultimately leading to complete exhaustion of the ovarian reserve long before death due to advanced chronological age (Faddy et al., 1992; Gosden et al., 1983; Richardson et al., 1987). More contemporary work has revealed that this massive oocyte loss occurs largely through apoptosis, involving an array of genes and signaling pathways that share many similarities to the regulation of apoptosis in other organ systems (Tilly, 2001). Follicle loss can be dramatically accelerated by external insults, including chemotherapy, radiation and environmental toxicants (Tilly, 2001), leading to the premature onset of many health problems associated with natural menopause.
The concept of irreversible exhaustion of the ovarian reserve in mammals is based on the presumed absence of replicative germ cells in postnatal ovarian tissue that could give rise to new oocytes. This contrasts sharply with observations from females of non-mammalian species, including files and fish, which retain germline stem cells (GSCs) that actively support oocyte renewal during adult life (Kirilly and Xie, 2007; Nakamura et al., 2010). However, in 2004 a study was published offering multiple lines of evidence for the existence of female GSCs (or, more appropriately, oogonial stem cells or OSCs, to be consistent with the nomenclature applied to spermatogonial stem cells—their male counterparts in the adult testis) in postnatal ovaries of mice that generate oocytes to form new follicles (Johnson et al., 2004). These findings countered dogma and thus were met with skepticism by many scientists (Powell, 2007).
Nonetheless, the possibility that the ovarian reserve could be replenished became a focal point of investigation for many laboratories (Tilly et al., 2009). These efforts ultimately led to the isolation of OSCs from neonatal and adult mouse ovaries by at least three groups using different strategies (Pacchiarotti et al., 2010; White et al., 2012; Zou et al., 2009), and the purification of a similar population of oocyte-producing progenitor germ cells from adult human ovaries (White et al., 2012). In addition, studies in mice have shown that when OSCs are reintroduced into adult ovaries, the cells differentiate to form follicle-enclosed oocytes that mature, ovulate and fertilize to produce viable embryos and offspring (White et al., 2012; Zou et al., 2009). In lower organisms, function of these types of germ cells has been tied to nutrient availability (McLeod et al., 2010; Jasper and Jones, 2010) and can even govern the pace of aging (Hsin and Kenyon, 1999; Flatt et al., 2008). These paradigm-shifting studies therefore provide a framework for introduction of OSCs into discussions of how female fertility and ovarian lifespan might be modulated in mammals.
DIETARY RESTRICTION, LONGEVITY GENES AND FEMALE FERTILITY
Mice and rats maintained on DR have reduced fertility or are completely infertile (Selesniemi et al., 2008; Visscher et al., 1952). Similarly, women below ideal body weight due to self-imposed DR have reduced fertility (Bates, 1985) and exhibit marked changes in gonadotropic hormones to levels that resemble those in women with ovarian insufficiency. Although the common wisdom is that DR negatively impacts fertility, it is less well known that DR can also have a positive impact. Almost a century ago, studies of rats noted that the “menopause has been postponed [by DR] long beyond the age at which it naturally appears” (Osborne et al., 1917). While rodents do not undergo a true menopause, this work, and several rodent studies that followed, clearly established that moderate DR extends functional ovarian lifespan in mammals.
At first glance, this finding appears at odds with the ‘Disposable Soma Theory’, in which the longevity of a species is a direct result of how it divides its resources between reproduction and protecting the soma (Kirkwood and Holliday, 1979). But it is not. Through analysis of physiological and ecological data on mouse survival and fertility, as well as life-history modeling, the temporary cessation of active fertility exhibited during DR in mice is believed to free up energy that can be used to enhance maintenance, thereby preserving viability and fertility for when the period of famine has passed (Shanley and Kirkwood, 2000).
Consistent with this idea, longevity can be achieved without sacrificing fertile potential in many species, including Podospora anserina (van Diepeningen et al., 2010), Saccharomyces cerevisiae (Jiang et al., 2000), Caenorhabditis elegans (Wood et al., 2004) and Drosophila melanogaster (Grandison et al., 2009). In nematodes, starvation shuts down reproduction through enforced quiescence of GSCs, which resume active gametogenesis for offspring production upon re-feeding (Angelo and Van Gilst, 2009). In flies, altering the balance of specific amino acids can increase longevity without reducing fertility (Grandison et al., 2009). The DR mimetic and SIRT1 activator resveratrol, which boosts mitochondrial function and extends lifespan in C. elegans and Drosophila, increases the number of eggs laid per organism (Wood et al., 2004). Together these data indicate that during adversity, many organisms down-regulate fertility but simultaneously up-regulate defense systems to preserve the germline. When conditions improve, fertility rebounds.
A similar situation may hold true for mammals. A stepwise 40% reduction in caloric intake in mice instituted after sexual maturation showed that while reproductive capacity is impaired during DR, these animals remain fertile much longer than continuously ad-libitum (AL)-fed controls once the DR females are allowed to resume AL feeding (Selesniemi et al., 2008). Furthermore, at advanced ages when offspring number per litter delivered by AL-fed females is close to zero, the number of pups born per litter by female mice maintained on DR for several months and then returned to an AL diet remains remarkably high (Selesniemi et al., 2008). The conclusion from these rodent studies is that if the DR regimen is mild, or if normal food intake is restored after a period of moderate DR, fertility is not negatively impacted and can actually be maintained for longer. In accordance with this, infertile women on DR rapidly regain their fertility if they increase their food consumption (Bates, 1985). An intriguing possibility is that DR mimetics provided to women on a normal diet could provide a means to activate germ cell defense networks, thereby maintaining or restoring oocyte quality and extending fertile lifespan.
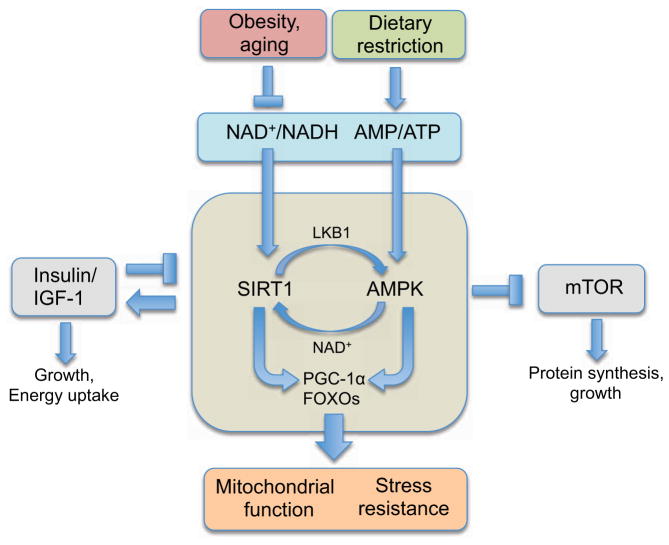
Such findings underscore the need to better define molecular mechanisms underlying organismal responses to DR or DR mimetics, and to then test these findings in the context of female fertility. The original idea that DR works passively by simply reducing metabolic rate or the generation of reactive oxygen species (ROS) has been largely discarded. In its place is a fundamentally different model in which DR works by triggering an active response that evolved to promote organismal survival during harsh conditions (Guarente, 2008; Kirkwood, 2005). At the center of this response are so-called ‘longevity regulatory pathways’ (Figure 1).
Figure 1. Longevity pathways that promote health and survival.
Current data indicate that environmental signals alter the pace of aging by modulating key metabolic sensors, such as SIRT1 and AMPK. These pathways interact with both mTOR and insulin/IGF-1 to control cell growth and energy intake. Obesity and aging reduce the ratios of NAD+/NADH and AMP/ATP, whereas DR has the opposite effect. Downstream, the actions of two transcriptional regulators, PGC-1α and FOXO, induce mitochondrial function and stress resistance, among other protective mechanisms. Together, this network coordinates cellular responses to stress, nutrient availability and metabolic demands, with mitochondria as key nexus points.
Although there are hundreds of longevity genes in dozens of species, four signaling pathways stand out as particularly important mediators of the DR response. These are insulin/insulin-like growth factor-1 (IGF-1) signaling, the mammalian target of rapamycin (mTOR) pathway, AMP-activated kinase (AMPK) and sirtuins. With the exception of sirtuins, which were first identified as mediators of gene silencing (Klar et al., 1979), these pathways were discovered for their roles in nutrient sensing decades before any role in aging was suspected. In the past few years, however, the fields of metabolism and aging have been united by the realization that all four of these metabolic sensors in eukaryotes form a complex regulatory network that responds to changes in a cell’s internal and external environment by storing or utilizing energy for processes that boost cellular defenses against tissue damage, deterioration and disease (Canto and Auwerx, 2009; Katewa and Kapahi, 2011).
As one might expect, at least some of these pathways appear operational in female germ cells, although much of this work has focused on oocyte growth activation and not egg quality (Reddy et al., 2008; Li et al., 2010). Exceptions include reports linking mTOR and the phosphatidylinositol 3-kinase (PI3K)/phosphatase and tensin homolog (PTEN) pathway, which ties into mTOR signaling, to meiotic progression in oocytes and embryonic genome activation in fertilized eggs, respectively (Lee et al., 2012; Zheng et al., 2010). In addition, several sirtuin family members are expressed in mouse oocytes. Of particular interest, loss of sirtuin-3 function in mouse eggs increases mitochondrial ROS production, leading to impaired pre-implantation embryonic development after fertilization (Kawamura et al., 2010).
EFFECTS OF DIET ON EGG QUALITY
Although early reports indicated that reduced dietary food intake can slow follicle depletion from ovaries in rats and mice (Lintern-Moore and Everitt, 1978; Nelson et al., 1985), the ovarian reserve in aged females subjected to DR followed by AL feeding is, like their age-matched AL-fed counterparts, severely diminished when compared to that of young adult females (Selesniemi et al., 2008). Thus, the beneficial effects of DR on female fertility, fecundity and offspring survival are apparently not due to maintenance of a larger follicle reserve with age. While it is conceivable that the benefits of DR in this model are partly a result of improved capacity of the uterus of aged females to establish and support a pregnancy, oocyte donation studies in humans have demonstrated that aging-related infertility can be effectively overcome by use of oocytes from young adult donors (Klein and Sauer, 2002; Sauer et al., 1992). In fact, given that women in their sixties have successfully carried pregnancies to term as surrogates (Paulson et al., 2002; Sauer et al., 1995), the single most important factor for determining pregnancy success rates in women of advanced maternal age seems to be oocyte quality and not uterine dysfunction (Navot et al., 1991).
Production of a developmentally competent egg requires the successful completion of meiosis, ultimately reducing its chromosome number to one-half once penetrated by the sperm at fertilization. The joining of the male and female pronuclei in the fertilized egg then restores a normal chromosome complement in the newly formed embryo. Unfortunately, the meiotic cell cycle is highly prone to errors with increasing age, leading to aneuploid oocytes (Hassold and Chiu, 1985; Hassold and Hunt, 2009; Hunt, 1998). Even with ovulation continuing in women into their early forties, the quality of oocytes ovulated by women as they grow older becomes compromised, elevating the risk for fertilization or embryonic failure, miscarriage and birth defects. The most widely known example of this maternal aging effect is the dramatic rise in risk for conception of offspring with trisomy 21 or Down syndrome, which increases from around 2% of clinical pregnancies for women in their twenties to 30% or more of clinical pregnancies for women in their forties (Hassold and Chiu, 1985).
The clinical importance of overcoming this aging-related decline in oocyte quality has become much more relevant as increasing numbers of women bear children in the second half of their fertile period (Matthews and Hamilton, 2009; Ventura, 1989). Compounding this problem is the inherent difficulty in circumventing fertility issues even with assisted reproductive technologies such as in-vitro fertilization (IVF). Since several factors contribute to the decline in oocyte quality with advancing age, a widely held assumption in the field of human reproduction is that any single or simple pharmacological intervention will be insufficient to overcome this problem. However, very recent studies with mice indicate that this fundamental belief, like that of a fixed ovarian reserve at birth, may be invalid. For example, recent studies show that female mice maintained on DR for 7 months and then allowed to AL feed for one month do not exhibit any of the hallmark features of deteriorating egg quality with age observed in AL-fed control females (Selesniemi et al., 2011). Notably, the increased incidence of aneuploidy, meiotic spindle abnormalities, chromosomal misalignment, mitochondrial aggregation and declining ATP levels detected in oocytes of aged AL-fed females are all absent in oocytes of aged females previously maintained on DR. Although additional studies are needed to address the mechanisms underlying these striking beneficial effects, aging-related aneuploidy and spindle defects in eggs at least no longer appear to be unreachable targets for therapeutic manipulation.
ENERGY, AGING AND THE ROLE OF MITOCHONDRIA
Whether one is working on metabolism or aging, it is hard to ignore mitochondria, the structures at the center of cellular energy production and utilization. These organelles are essential for generating most of the ATP in the body, which in humans amounts to about 65 kg per day to meet basic metabolic demands (Tornroth-Horsefield and Neutze, 2008). Other key functions of mitochondria include calcium buffering, reduction-oxidation (redox) homeostasis and programmed cell death (apoptosis). Mitochondria are continuously moving throughout the cell—undergoing fusion, fission, and degradation—to eliminate and replace damaged organelles, and to meet fluctuating energy needs (Palmer et al., 2011).
Data from flies, rats, mice, monkeys and humans show that as tissues age, both the number and activity of mitochondria decline, compensated by an increase in their overall size (Cho et al., 2011; Ferguson et al., 2005; Short et al., 2005; Wallace, 2001). Mitochondrial dysfunction is associated with, and potentially contributes to, common aging-related diseases such as atherosclerosis, obesity-induced type II diabetes, sarcopenia and neurodegenerative disorders (Di Lisa et al., 2009; Lin and Beal, 2006; Wallace, 2001). Underlying processes include a decline in mitochondrial membrane potential and ATP output, increased activation of the mitochondrial permeability transition pore (mPTP), mitochondrial membrane depolarization and leakage of mitochondrial matrix solutes into the cytoplasm (Di Lisa et al., 2001; Hafner et al., 2010; Liu et al., 2011; Wallace, 2001).
In addition to aging and aging-related diseases, considerable evidence supports a role for mitochondria as mediators of the benefits of DR in rodents and humans (Cerqueira et al., 2011; Civitarese et al., 2007; Lopez-Lluch et al., 2006; Lopez-Lluch et al., 2008). In a variety of species (Guarente, 2008; Johannsen and Ravussin, 2009), DR increases mitochondrial number and function. A recent rat study, however, did not observe this change (Hancock et al., 2011). The sirtuin-1 (SIRT1)-AMPK network, considered a mediator of DR physiology, acts to raise both the number and activity of mitochondria (Gerhart-Hines et al., 2007), as do DR mimetics that stimulate SIRT1 or AMPK activity, such as resveratrol (Baur et al., 2006; Feige et al., 2008; Funk et al., 2010; Lagouge et al., 2006), SRT1720 (Minor et al., 2011) and metformin (Canto et al., 2009; Suwa et al., 2006). In flies and nematodes, changes in mitochondrial metabolism are known to be necessary (Bahadorani et al., 2010; Bishop and Guarente, 2007; Zid et al., 2009) and sufficient (Bahadorani et al., 2010; Durieux et al., 2011; Rera et al., 2011) for DR to extend lifespan.
MITOCHONDRIA AND OOCYTE COMPETENCY
Of the many potential mechanisms by which DR benefits oocytes, one of the more plausible is prevention of abnormal mitochondrial aggregation and decreased ATP levels that occur in oocytes of aged females (Selesniemi et al., 2011). Many studies have proposed a link between insufficient ATP availability in eggs and defective chromosomal segregation—an outcome that probably ties to meiotic spindle abnormalities (Eichenlaub-Ritter et al., 2004; Schon et al., 2000; Zheng et al., 2007). Defective spindle formation would result in a reduced capacity for successful fertilization and the failure of zygotes produced from energetically compromised eggs to form viable blastocysts (Bentov et al., 2010). Consistent with this idea, disruption of mitochondrial oxidative phosphorylation in mouse oocytes results in reduced potential for meiotic maturation and fertilization, as well as decreased pre-implantation embryonic developmental potential (Van Blerkom et al., 1995).
Other studies with mice have demonstrated that failure of oocytes to adequately readjust ATP levels after sperm penetration disrupts intracellular calcium oscillations (Igarashi et al., 1997; Igarashi et al., 2005), which are critical for immediate post-fertilization events that ensure developmental competency of the embryo (Dumollard et al., 2004; Vitullo and Ozil, 1992). In human eggs, higher ATP levels have been correlated with a greater potential for successful embryonic development and implantation (Van Blerkom et al., 1995). These findings, along with observations that mitochondria in oocytes of women in their forties frequently exhibit swelling and abnormal cristae (Muller-Hocker et al., 1996), collectively support the idea that impaired bioenergetic capacity in oocytes is a primary contributor to declining egg and embryo quality with advancing maternal age.
Additional aspects of mitochondrial physiology must be considered when evaluating a central role for these organelles in oocyte development, meiotic maturation, fertilization and pre-implantation embryonic competency. Obviously, the generation of ROS during the oxidative phosphorylation steps associated with ATP production is closely linked to mitochondrial bioenergetics. In turn, it may be of little surprise that studies of mice have reported chronic antioxidant treatment throughout adult life can sustain egg quality in aging females (Tarin et al., 2002a). Unfortunately, the function of other cells and tissues in the reproductive tract is simultaneously impaired by this approach, with excessive fetal loss and diminished offspring numbers noted (Tarin et al., 2002b). Accordingly, long-term systemic administration of antioxidants has little, if any, clinical application for improving human female fertility.
On a more basic level, a striking aspect of mitochondrial physiology that warrants consideration is the amplification of mitochondrial numbers as human oocytes develop from their most immature state containing between 5–10 X 103 mitochondria, to mature metaphase II eggs containing 1–5 X 105 or more mitochondria (Jansen and Burton, 2004; Piko and Matsumoto, 1976). The reasons for this tremendous expansion of mitochondrial numbers during oocyte maturation are not fully understood. One belief is that the oocyte is actively preparing itself for the increased energy demands of successful fertilization and early cleavage divisions associated with embryonic development. This seems reasonable, especially if one considers that the machinery required for mitochondrial replication is shut off at the metaphase II egg stage and is not reactivated in the embryo until after the blastocyst implants in the uterine wall (Larrson et al., 1998). Accordingly, one would expect a steep decline in mitochondrial numbers as an embryo expands from the one-cell zygote to a blastocyst containing a hundred or more cells, a prediction supported by assessment of mitochondrial DNA (mtDNA) content during pre-implantation embryogenesis (Spikings et al., 2007; Wai et al., 2008) and mitochondrial numbers per cell in blastocyst-stage embryos (Van Blerkom, 2008).
It bears mentioning that mtDNA content rather than absolute numbers of mitochondria, which contain 1–10 copies of mtDNA per organelle, may actually be a more reliable indicator of the competence of a given oocyte. Past studies have shown that mtDNA copy numbers in oocytes and early-stage embryos positively correlate with fertilization and developmental potential, respectively (Almeida-Santos et al., 2006; Spikings et al., 2006). In fact, successful embryogenesis has been tied to threshold levels of mtDNA content per egg at the time of fertilization, with those oocytes at the low range of this threshold more prone to failed maturation, reduced fertilization rates and embryonic developmental arrest (El Shourbagy et al., 2006; Piko and Taylor, 1987; Reynier et al., 2001; Santos et al., 2006; Wai et al., 2010). In addition, studies of porcine oocytes have revealed that suppression of mtDNA replication during in vitro maturation to the metaphase II egg stage results in reduced fertilization competence as well as pre-implantation embryonic developmental arrest, and that the severity of these outcomes is tightly linked to a minimal threshold copy number of mtDNA (Spikings et al., 2007).
Another intriguing feature of the oocyte is that its mitochondria tend to be very small (≤1 μm in diameter), with electron-dense matrices and few cristae. Despite these structural features, oocyte mitochondria are highly active and produce the majority of energy needed by the egg and early embryo (Dumollard et al., 2007; Motta et al., 2000; Van Blerkom et al., 1995). After fertilization, mitochondria in developing embryos undergo striking ultrastructural changes. By the time of blastocyst formation, these organelles have acquired an elongated appearance with complex cristae and less electron-dense matrices, more typical of mitochondria in somatic cells (Sathananthan and Trounson, 2000; Van Blerkom, 1989a, 1989b, 1993; Van Blerkom and Motta, 1979; Van Blerkom et al., 1973). The significance of this mitochondrial remodeling is unclear; however, human embryos that arrest in vitro often contain mitochondria that have failed to transform into a more orthodox morphology, a step that is presumably necessary to meet the energy demands of the developing embryo (Van Blerkom, 1989a).
Finally, mitochondria in oocytes play another critical role in reproduction: they serve as the source for uniparental inheritance of the mitochondrial genome from one generation to the next. It is fairly well established that paternal (sperm-derived) mitochondria are degraded in newly formed embryos within the first few cleavage divisions, leaving maternally derived mitochondria as the sole pool for replication of these organelles in the embryo and resultant offspring (Cummins, 1998; Giles et al., 1980; Hutchinson et al., 1974; Kaneda et al., 1995; Sutovsky et al., 2000). The mechanisms underlying this sex-specific selection against the transmission of paternal mtDNA are not completely understood. In many species, however, the selection appears to result from ubiquitination of sperm-derived mitochondria, which allows for their subsequent removal from the embryo (Sutovsky et al., 2004). Equally unclear is why paternal mtDNA inheritance is actively selected against. One idea is that it minimizes the transmission of mtDNA mutations that arise in sperm exposed to ROS during spermatogenesis (Aitken, 1995). Consistent with this idea, sperm mitochondria often harbor mtDNA mutations and deletions (Reynier et al., 1998), which in turn are linked to poor sperm motility and malefactor infertility (Kao et al., 1995; Ruiz-Pesini et al., 2000; St. John et al., 2001).
Irrespective of the mechanisms that drive uniparental mtDNA inheritance or the advantages it provides, this process necessitates strict maintenance of mtDNA integrity in the female germline. Otherwise, a cumulative mutational disaster could occur, as mitochondria from the oocyte are used to seed the new embryo in each successive generation (Jansen and De Boer, 1998). To ensure asexual maintenance of mitochondrial genome integrity, a multistep process has been proposed that involves an initial quantitative restriction on mtDNA genotypes that will potentially be passed (the so-called ‘mitochondrial bottleneck’), followed by a period of tremendous amplification. Then, under pressure to achieve improved fitness in subsequent generations, a competitive mass selection occurs (Jansen, 2000). The quantitative restriction event takes place in the developing embryo as mtDNA content in the embryo, and mitochondrial numbers per cell, decline exponentially from peak levels at the fertilized egg stage to extremely low levels in the implanting blastocyst. In fact, embryonic primordial germ cells contain 10 or fewer mitochondria per cell, in stark contrast to the hundreds of thousands of mitochondria present in each egg (Jansen and Burton, 2004; Piko and Matsumato 1976). This latter point exemplifies the importance of the second step in the process of mtDNA selection, which entails a period of tremendous amplification with minimal selection that presumably allows for some degree of genetic drift (Brown et al., 2001). The third and final stage of the process—competitive mass selection—is the least established in terms of actual mechanism.
One theory is that constant culling of oocyte-containing follicles by atresia serves to identify a given follicle that will release an egg at ovulation with the highest degree of mtDNA integrity (Jansen and Burton, 2004; Jansen and De Boer, 1998). Although this is an attractive proposal, direct evidence for it is currently lacking. However, the existence of a tightly controlled surveillance system for ensuring maternal mtDNA integrity is supported by observations that mtDNA deletions are usually not transmitted to the offspring of clinically symptomatic women. In addition, the common ΔmtDNA4977 deletion is greater in unfertilized human eggs than in early cleavage-stage embryos (Brenner et al., 1998; Perez et al., 2000), and two-thirds of degenerated or arrested oocytes carry the ΔmtDNA4977 deletion (Duran et al., 2011).
ENERGETICS, AGING AND FEMALE FERTILITY: CONNECTING THE DOTS
Direct causative relationships between impaired mitochondrial function, suboptimal bioenergetic capacity and reduced developmental competency of eggs in aging females have not yet been unequivocally established. Nevertheless, evidence for such relationships continues to solidify, as does the link between longevity pathways and fertility. Studies of hamsters and mice have reported that maternal aging is associated with significant decreases in ATP content and mitochondrial numbers in oocytes. In addition, the demonstration that DR instituted during adulthood not only benefits multiple parameters of egg quality in aging female mice (Selesniemi et al., 2011), but also extends the natural reproductive period (Selesniemi et al., 2008), argues strongly that the same signaling pathways mediating responses of somatic cells to DR are also at work in the female germline. Further supporting this conclusion are recent data showing that sirtuins are expressed in rat and mouse oocytes (Kawamura et al., 2010; Luo et al., 2012), that DR increases the expression of sirtuins in the rat ovary (Luo et al., 2012), and that the development of embryos arising from oocytes lacking mitochondrial-associated sirtuin-3 is significantly impaired (Kawamura et al., 2010).
Although progress has been made in connecting the dots, we are still far from understanding or manipulating the bioenergetic and longevity pathways that impact female fertility. Thus, the experimental and clinical data currently in hand can be viewed as opening chapters in a saga that may one day offer unprecedented opportunities for the clinical management of egg quality, fertilization and pre-implantation embryogenesis in human assisted reproduction. Looking ahead, we will conclude with two examples of potential future chapters that integrate many of the concepts discussed herein.
AUTOLOGOUS GERMLINE MITOCHONDRIAL ENERGY TRANSFER AND EGG QUALITY
In the mid-to-late 1990s, 27 female subjects who had repeatedly failed to become pregnant following assisted reproduction due to poor embryo quality and implantation failure participated in a trial of a new fertility protocol termed ooplasmic transfer (Barritt et al., 2001; Brenner et al., 2000; Cohen et al., 1997; Cohen et al., 1998; Harvey et al., 2007). Under the assumption that the recurrent failure of these women to achieve pregnancies was due, at least in part, to an age-related impairment in the quality of their eggs, their next cycle of IVF included transfer of a small amount of cytoplasm extracted from young donor oocytes (viz., obtained from different women) into their oocytes. Thirty attempts of ooplasmic transfer were performed by the first clinic, resulting in 13 live births (17 babies total, comprised of 11 singletons, 1 set of twins, and 1 set of quadruplets) and 1 first-trimester miscarriage (45, XO karyotype). The twin pregnancy resulted in birth of a female with a normal karyotype (46, XX) and a chromosomally abnormal sibling (45, XO). Of the babies born, the rate of chromosomal abnormalities (1 of 17, or 5.9%) was within the normal range of IVF outcomes for women at the ages tested in that region of the United States (Harvey et al., 2007). The high pregnancy success rates achieved in this relatively small cohort of patients, who had repeatedly failed all prior IVF attempts, raised hopes that human assisted reproduction would finally have a new tool in its arsenal to combat infertility associated with poor egg and embryo quality.
Other clinical sites were quick to join in (Lanzendorf et al., 1999); however, enthusiasm for widespread adoption of ooplasmic transfer as a clinical protocol was short-lived, in part because of concerns about mitochondrial heteroplasmy (Barritt et al., 2001; Brenner et al., 2000). Although the procedure involved transfer of cytoplasm from donor eggs, and not purified mitochondria, it is widely believed that the benefit to recipient eggs came from the transfer of active donor mitochondria (Bentov et al., 2011; Van Blerkom et al. 1998). This conclusion has been substantiated by animal studies (El Shourbagy et al., 2006; Yi et al., 2007) and, to a degree, by follow-up studies of the children born through ooplasmic transfer who carry mitochondria from both the biological mother and the egg donor (Brenner et al., 2000; Barritt et al., 2001). At present, the negative health impact, if any, of heteroplasmy in these children is unknown, but animal models indicate there may be at least some cause for concern. For example, studies in mice show that mitochondrial heteroplasmy can produce an adult-onset phenotype consistent with metabolic syndrome (Acton et al., 2007). Other work has shown that heteroplasmy can also negatively impact cognitive function (Sharpley et al., 2012).
Additionally, oocyte mitochondria contain genetic material that is distinct from nuclear genes contributed by the biological mother and father. Accordingly, the children conceived following this procedure possess genetic material derived from not two but three distinct sources: the biological mother, the biological father and the egg donor. Aside from an array of potential ethical and legal issues associated with heterologous ooplasmic transfer, the U.S. Food and Drug Administration (FDA) viewed this procedure as genetic manipulation of human germ cells for the purpose of generating embryos. Thus, in 2001 the FDA ruled that heterologous ooplasmic transfer could no longer be used for human assisted reproduction unless the procedure was submitted for review and testing under Investigational New Drug (IND) guidelines (Zoon, 2001). While use of autologous mitochondria from a woman’s own somatic cells would avoid mitochondrial heteroplasmy, somatic mitochondria are prone to aging-related mtDNA damage resulting in heritable mutations. Introduction of these mitochondria into oocytes at fertilization could lead to propagation of mutant mitochondria in newly formed embryos and resultant offspring, a risk too great to consider for clinical protocol development.
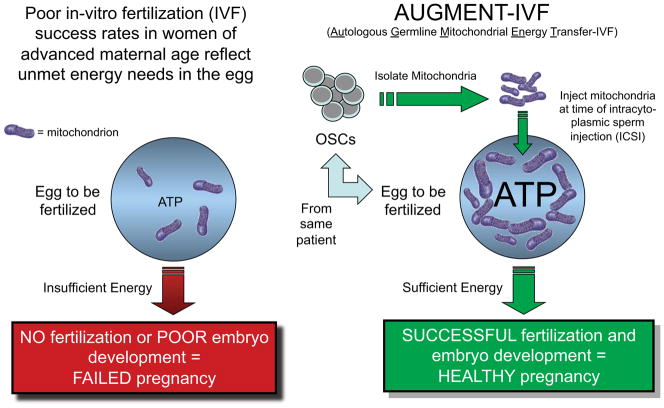
However, the discovery that OSCs are present in ovaries of not just adult mice but also of reproductive age women (Johnson et al., 2004; White et al., 2012; Zou et al., 2009) has opened prospects for bringing a modified version of ooplasmic transfer into clinical practice. Termed AUGMENT (for autologous germline mitochondrial energy transfer; Woods et al., 2013), this procedure would provide an autologous germline-derived source of cytoplasmic extract or purified mitochondria for the bioenergetic reinvigoration of eggs whose capacity for fertilization and embryogenesis has been compromised by aging (Figure 2).
Figure 2. Proposed method of improving human IVF outcomes with AUGMENT.
Reduced mitochondrial activity and bioenergetic potential contribute to aging-related impairments in female fertility, even with the use of assisted reproductive technologies such as IVF. Delivery of mitochondria derived from a patient’s own natural egg precursor cells (OSCs) into that same patient’s eggs during intracytoplasmic sperm injection raises the threshold level of mitochondria in that egg, providing sufficient energy to support successful fertilization and embryogenesis.
The use of OSCs as a source of mitochondria for enhancing egg and embryo quality is attractive for reasons other than simply being a patient-matched source of these important energy-boosting organelles. First, since OSCs function as natural precursor cells for the generation of oocytes (White et al., 2012; Zou et al., 2009). If mitochondria in the female germline are indeed managed quite differently from those in somatic cells, the use of OSCs as a source of mitochondria for rejuvenation of eggs would be compatible with the natural processes of surveillance and selection that govern maternal mitochondrial transfer from one generation to the next. Second, OSC mitochondria, being derived from slowly dividing stem/progenitor cells, are more likely to be free of cumulative damage to their genomes than mitochondria in patient-matched somatic cells. Preliminary observations from studies of human OSCs support this contention (Woods and Tilly, 2013). Perhaps equally important are observations that the bioenergetic potential of mitochondria in human OSCs, as measured by ATP generation over time, far exceeds that of equivalent numbers of mitochondria isolated from several other human cell lineages, including embryonic and adult somatic stem cells (Woods and Tilly, 2013). Finally, the cell lineage-specific transfer of key nuclear-encoded proteins into mitochondria would be better preserved through the use of OSCs versus non-germline cells. Given all of these considerations, along with the preclinical and clinical proof-of-concept data available from prior studies discussed above, the use of AUGMENT for safely improving human assisted reproduction without the ethical, legal and biological issues surrounding heterologous ooplasmic transfer is an exciting prospect to consider.
MITOCHONDRIAL ACTIVATORS TO BOOST EGG QUALITY
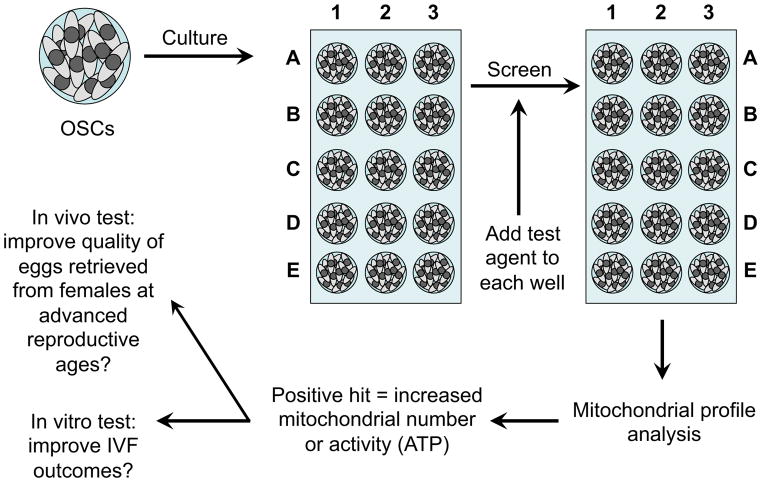
Another potential approach to overcoming energy deficits in eggs is to identify new biological and chemical entities that enhance mitochondrial numbers or the efficacy of mitochondrial ATP generation in oocytes. The development of such compounds that safely reproduce the striking benefits of DR on egg quality in aging females (Selesniemi et al., 2011) could represent a significant leap forward in human assisted reproduction. The main hurdle faced is the extreme rarity of oocytes for conducting large-scale mitochondrial screening assays, coupled with the cost and complexities of performing aging studies with mice. However, the availability of mouse and human OSCs, which can be maintained and expanded ex vivo to generate essentially unlimited numbers of cells for screening (White et al., 2012), may provide a solution (Figure 3). Because these cells function as natural oocyte progenitors, it is reasonable to predict that compounds identified as mitochondrial boosters in OSCs would exhibit similar properties in oocytes. In addition, use of OSCs as a screening platform offers an opportunity to map molecular events through which a given compound, or family of compounds, boosts mitochondrial numbers or activity in female germ cells. Such studies would further benefit by generating OSC lines that carry desired manipulations of key components of the longevity regulatory pathways discussed earlier (Figure 1). Establishment of these types of female germ cell lines could facilitate identification of control points that coordinate bioenergetic potential in oocytes. In turn, this may lead to a more directed approach for identifying potential lead compounds that could be tested in aging female mice for their ability to improve egg quality.
Figure 3. Identification and utility of female germ cell mitochondrial boosters.
Culture of human OSCs, which are natural precursor cells for human oocytes, allows high-throughput screening of biological and pharmacological entities for their ability to increase various aspects of mitochondrial dynamics, including mtDNA content, mitochondrial membrane potential, and ATP-generating capacity. Positive hits can be further tested using a combination of in vitro and in vivo assays to assess if aging-related impairments in egg quality, embryonic developmental competence and fertility can be minimized.
There is ample reason to believe that IVF outcomes for patients with energetically compromised eggs or embryos would benefit from mitochondrial activators (Bentov et al., 2010; Van Blerkom, 2011), similar to the energetic boost provided to eggs by direct mitochondrial transfer. However, mitochondrial activators may also prove valuable as in-vivo agents to ensure that oocytes ultimately released from the ovaries at ovulation or retrieved from IVF patients after ovarian stimulation are fully prepared, from a bioenergetic perspective, to undergo the final maturational steps needed for full developmental competency. One example of their potential utility would be to combat the increase in oocyte aneuploidy associated with maternal reproductive aging, a process that, at least in mice, has been tied to mitochondrial dysfunction and energy deficits in the ovulated eggs (Selesniemi et al., 2011).
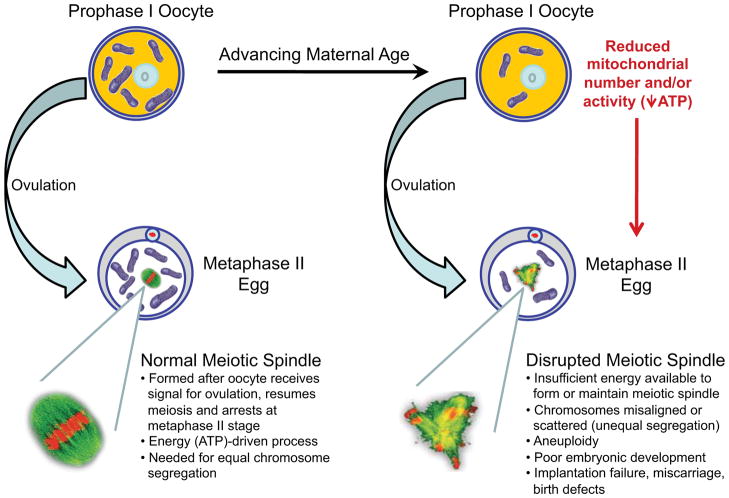
Mechanistically, the formation and maintenance of the meiotic spindle is an energy-driven process that is highly susceptible to failure in oocytes of aged females (Figure 4). The resultant chromosomal misalignment or unequal chromosomal segregation produces an egg with too few or too many chromosomes, leading to genetic errors that can be passed to resultant embryos after fertilization (Gaulden, 1992). Clinically, maternal aging-related increases in egg and embryo aneuploidy are tied directly to parallel increases in trisomic conceptions, implantation failures and miscarriages (Benadiva et al., 1996; Hassold and Chiu, 1985; Munné and Cohen, 1998; Munné et al., 1995). Identification of orally active compounds that boost the energetic capacity of oocytes prior to ovulation or retrieval for IVF may therefore provide a novel strategy to maximize the chances of obtaining eggs from females at advanced reproductive ages that are free from genetic errors and other problems that contribute to post-fertilization embryonic failure. In turn, such strategies might also mitigate the maternal aging-related increase in risk for miscarriage and birth defects, including Down syndrome.
Figure 4. Negative impact of maternal aging on ovulated egg quality and female fertility.
Schematic depiction of how maternal aging-associated deficits in bioenergetic potential in oocytes compromises their ability to form fertilization-competent eggs. Insufficient energy in the eggs of older females leads to impaired meiotic spindle formation and maintenance, unequal segregation of genetic material at the completion of meiosis during fertilization, and aneuploid conceptions, which result in pre-implantation embryonic growth arrest, implantation failure, miscarriage and birth defects.
Acknowledgments
Work conducted by the lab of J.L.T. was supported by a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279), the Glenn Foundation for Medical Research, and the Henry and Vivian Rosenberg Philanthropic Fund. Work conducted by the lab of D.A.S. was supported by NIH Grant R01-AG028730, the Ellison Medical Foundation, the Glenn Foundation for Medical Research, the United Mitochondrial Disease Foundation, and a philanthropic gift from R. Shulsky-David. The authors thank D.C. Woods for helpful discussions and citation of work from preliminary studies conducted with J.L.T.
Footnotes
DISCLOSURES
J.L.T. declares interest in intellectual property described in U.S. Patent 7,955,846, related to work discussed herein; J.L.T. and D.A.S. are co-founders of OvaScience, Inc. (Cambridge, MA); D.A.S. is a co-founder of and consultant to Cohbar (Washington, DC) and Sirtris (Cambridge, MA), a GlaxoSmithKline company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton BM, Lai I, Shang X, Jurisicova A, Casper RF. Neutral mitochondrial heteroplasmy alters physiological function in mice. Biol Reprod. 2007;77:569–576. doi: 10.1095/biolreprod.107.060806. [DOI] [PubMed] [Google Scholar]
- Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- Almeida-Santos T, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Bahadorani S, Cho J, Lo T, Contreras H, Lawal HO, Krantz DE, Bradley TJ, Walker DW. Neuronal expression of a single-subunit yeast NADH-ubiquinone oxidoreductase (Ndi1) extends Drosophila lifespan. Aging Cell. 2010;9:191–202. doi: 10.1111/j.1474-9726.2010.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- Bates GW. Body weight control practice as a cause of infertility. Clin Obstet Gynecol. 1985;28:632–644. doi: 10.1097/00003081-198528030-00018. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benadiva CA, Kligman I, Munné S. Aneuploidy 16 in human embryos increases significantly with maternal age. Fertil Steril. 1996;66:248–255. [PubMed] [Google Scholar]
- Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93:272–275. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Blum CA, Ellis JL, Loh C, Ng PY, Perni RB, Stein RL. SIRT1 modulation as a novel approach to the treatment of diseases of aging. J Med Chem. 2011;54:417–432. doi: 10.1021/jm100861p. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Barritt JA, Willadsen S, Cohen J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril. 2000;74:573–578. doi: 10.1016/s0015-0282(00)00681-6. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Wolny YM, Barritt JA, Matt DW, Munné S, Cohen J. Mitochondrial DNA deletion in human oocytes and embryos. Mol Hum Reprod. 1998;4:887–892. doi: 10.1093/molehr/4.9.887. [DOI] [PubMed] [Google Scholar]
- Brown DT, Samuels DC, Michael EM, Turnbull DM, Chinnery PF. Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet. 2001;68:533–536. doi: 10.1086/318190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler H. The menopause transition: endocrine changes and clinical symptoms. J Br Menopause Soc. 2005;11:61–65. doi: 10.1258/136218005775544525. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Laurindo FR, Kowaltowski AJ. Mild mitochondrial uncoupling and calorie restriction increase fasting eNOS, AKT and mitochondrial biogenesis. PLoS One. 2011;6:e18433. doi: 10.1371/journal.pone.0018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Tsuchiya T, Komatsu T, Mori R, Hayashi H, Shimokawa I. Development of calorie restriction mimetics as therapeutics for obesity, diabetes, inflammatory and neurodegenerative diseases. Curr Genom. 2010;11:562–567. doi: 10.2174/138920210793360934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Hur JH, Walker DW. The role of mitochondria in Drosophila aging. Exp Gerontol. 2011;46:331–334. doi: 10.1016/j.exger.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of an infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, Wu L, Brenner CA, Warner C, Willadsen S. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- Cummins J. Mitochondrial DNA in mammalian reproduction. Rev Reprod. 1998;3:172–182. doi: 10.1530/ror.0.0030172. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol Rep. 2009;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Topics Dev Biol. 2007;77:21–49. doi: 10.1016/S0070-2153(06)77002-8. [DOI] [PubMed] [Google Scholar]
- Duran HE, Simsek-Duran F, Oehninger SC, Jones HW, Jr, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96:384–388. doi: 10.1016/j.fertnstert.2011.05.056. [DOI] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulden M. The enigma of Down syndrome and other trisomic conditions. Mutat Res. 1992;269:69–88. doi: 10.1016/0165-1110(92)90033-6. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–260. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria – a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ, Gibson TC, Quebedeaux TM, Brenner CA. Impact of assisted reproductive technologies: a mitochondrial perspective of cytoplasmic transplantation. Curr Top Dev Biol. 2007;77:229–249. doi: 10.1016/S0070-2153(06)77009-0. [DOI] [PubMed] [Google Scholar]
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Hunt PA. The control of female meiosis: factors that influence chromosome segregation. J Assist Reprod Genet. 1998;15:246–252. doi: 10.1023/A:1022580024402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson CA, Newbold JE, Potter SS. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974;251:536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Takahashi E, Hiroi M, Doi K. Aging-related changes in calcium oscillations in fertilized mouse oocytes. Mol Reprod Dev. 1997;48:383–390. doi: 10.1002/(SICI)1098-2795(199711)48:3<383::AID-MRD12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72:1256–1261. doi: 10.1095/biolreprod.104.034926. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod. 2000;15(Supplement 2):112–128. doi: 10.1093/humrep/15.suppl_2.112. [DOI] [PubMed] [Google Scholar]
- Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion. 2004;4:577–600. doi: 10.1016/j.mito.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Jansen RP, de Boer K. The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998;145:81–88. doi: 10.1016/s0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 2010;12:561–565. doi: 10.1016/j.cmet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Kaneda H, Hayashi J, Takahama S, Taya C, Lindahl KF, Yonekawa H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc Natl Acad Sci USA. 1995;92:4542–4546. doi: 10.1073/pnas.92.10.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Chao HT, Wei YH. Mitochondrial deoxyribonucleic acid 4977-bp deletion is associated with diminished fertility and motility of human sperm. Biol Reprod. 1995;52:729–736. doi: 10.1095/biolreprod52.4.729. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest. 2010;120:2817–2828. doi: 10.1172/JCI42020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Holliday R. The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci. 1979;205:531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Immortality of the germ-line versus disposability of the soma. Basic Life Sci. 1987;42:209–218. doi: 10.1007/978-1-4613-1939-9_15. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Fogel S, Macleod K. MAR1 – a regulator of the HMa and HMα loci in Saccharomyces cerevisiae. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Sauer MV. Oocyte donation. Best Prac Res Clin Obstet Gynaecol. 2002;6:277–291. doi: 10.1053/beog.2002.0288. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Mayer JF, Toner J, Oehninger S, Saffan DS, Muasher S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil Steril. 1999;71:575–577. doi: 10.1016/s0015-0282(98)00504-4. [DOI] [PubMed] [Google Scholar]
- Larrson N, Wang J, Wilhemsson H, Oldfors A, Rustin P, Lewandoski M, Barsh G, Clayton D. Mitochondria transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Lee SE, Sun SC, Choi HY, Uhm SJ, Kim NH. mTOR is required for asymmetric division through small GTPases in mouse oocytes. Mol Reprod Dev. 2012;79:356–366. doi: 10.1002/mrd.22035. [DOI] [PubMed] [Google Scholar]
- Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lintern-Moore S, Everitt AV. The effect of restricted food intake on the size and composition of the ovarian follicle population in the Wistar rat. Biol Reprod. 1978;19:688–691. doi: 10.1095/biolreprod19.3.688. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu J, Brink PR, Glass PS, Rebecchi MJ. Age-associated differences in the inhibition of mitochondrial permeability transition pore opening by cyclosporine A. Acta Anaesthes Scand. 2011;55:622–630. doi: 10.1111/j.1399-6576.2011.02421.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo LL, Chen XC, Fu YC, Xu JJ, Li L, Lin XH, Xiang YF, Zhang XM. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin Exp Res. 2012;24:125–133. doi: 10.3275/7660. [DOI] [PubMed] [Google Scholar]
- Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1–8. [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken E, Abdelmohsen K, Shin Y, Canto C, Scheibye-Knudsen, et al. SRT1720 improves survival and healthspan of obese mice. Scientific Reports. 2011;1:1–10. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15(Supplement 2):129–147. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- Muller-Hocker J, Schafer S, Weis S, Munscher C, Strowitzki T. Morphological-cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–958. doi: 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–391. [PubMed] [Google Scholar]
- Munné S, Cohen J. Chromosome abnormalities in human embryos. Hum Reprod Update. 1998;4:842–855. doi: 10.1093/humupd/4.6.842. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB, Ferry EL. The effect of retardation of growth upon the breeding period and duration of life of rats. Science. 1917;45:294–295. doi: 10.1126/science.45.1160.294. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, Francis MM, Jain JK. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. J Am Med Assoc. 2002;288:2320–2323. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature. 2000;403:500–501. doi: 10.1038/35000651. [DOI] [PubMed] [Google Scholar]
- Piko L, Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976;49:1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Piko L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Powell K. Going against the grain. PloS Biol. 2007;5:e338. doi: 10.1371/journal.pbio.0050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy C, Shen Y, Du C, Tang W, Hämäiäinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr, Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynier P, Chrétien MF, Savagner F, Larcher G, Rohmer V, Barrière P, Malthièry Y. Long PDR analysis of human gamete mtDNA suggests defective mitochondrial maintenance in spermatozoa and supports the bottleneck theory for oocytes. Biochem Biophys Res Commun. 1998;252:373–377. doi: 10.1006/bbrc.1998.9651. [DOI] [PubMed] [Google Scholar]
- Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapeña AC, Díez C, Alvarez E, Enríquez JA, López-Pérez MJ. Seminal quality correlates with mitochondrial functionality. Clin Chim Acta. 2000;300:97–105. doi: 10.1016/s0009-8981(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod. 2000;15(Suppl 2):148–159. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- Sauer MV, Paulson RJ, Lobo RA. Reversing the natural decline in human fertility. An extended clinical trial of oocyte donation to women of advanced reproductive age. J Am Med Assoc. 1992;268:1275–1279. doi: 10.1001/jama.268.10.1275. [DOI] [PubMed] [Google Scholar]
- Sauer MV, Paulson RJ, Lobo RA. Pregnancy in women 50 or more years of age: outcomes of 22 consecutively established pregnancies from oocyte donation. Fertil Steril. 1995;64:111–115. [PubMed] [Google Scholar]
- Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ. Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod. 2000;15(Suppl 2):160–172. doi: 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, Lin CS, Masubuchi S, Friend N, Koike M, Chalkia D, Macgregor G, Sassone-Corsi P, Wallace DC. Heteroplasmy of mouse mtDNA Is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spikings EC, Alderson J, St John JC. Transmission of mitochondrial DNA following assisted reproduction and nuclear transfer. Hum Reprod Update. 2006;12:401–415. doi: 10.1093/humupd/dml011. [DOI] [PubMed] [Google Scholar]
- Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327–335. doi: 10.1095/biolreprod.106.054536. [DOI] [PubMed] [Google Scholar]
- St John JC, Jokhi RP, Barratt CL. Men with oligoasthenoteratozoospermia harbour higher numbers of multiple mitochondrial DNA deletions in their spermatozoa, but individual deletions are not indicative of overall aetiology. Mol Hum Reprod. 2001;7:103–111. doi: 10.1093/molehr/7.1.103. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod. 2000;63:582–590. doi: 10.1095/biolreprod63.2.582. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Van Leyen K, McCauley T, Day BN, Sutovsky M. Degradation of paternal mitochondria after fertilization: implications for heteroplasmy, assisted reproductive technologies and mtDNA inheritance. Reprod Biomed Online. 2004;8:24–33. doi: 10.1016/s1472-6483(10)60495-6. [DOI] [PubMed] [Google Scholar]
- Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1α protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Pérez-Albalá S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002a;61:385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Pérez-Albalá S, Pertusa JF, Cano A. Oral administration of pharmacological doses of vitamins C and E reduces reproductive fitness and impairs the ovarian and uterine functions of female mice. Theriogenology. 2002b;57:1539–1550. doi: 10.1016/s0093-691x(02)00636-2. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornroth-Horsefield S, Neutze R. Opening and closing the metabolite gate. Proc Natl Acad Sci USA. 2008;105:19565–19566. doi: 10.1073/pnas.0810654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J. Developmental failure in human reproduction associated with preovulatory oogenesis and preimplantation embryogenesis. In: Van Blerkom J, Motta P, editors. Ultrastructure of Human Gametogenesis and and Embryogenesis. Kluwer Academic Publishers; Boston: 1989a. pp. 125–180. [Google Scholar]
- Van Blerkom J. Morphodynamics of nuclear and cytoplasmic reorganization during the resumption of arrested meiosis in the mouse oocyte. Prog Clin Biol Res. 1989b;294:33–51. [PubMed] [Google Scholar]
- Van Blerkom J. Development of human embryos to the hatched blastocyst stage in the presence or absence of a monolayer of Vero cells. Hum Reprod. 1993;8:1525–1539. doi: 10.1093/oxfordjournals.humrep.a138293. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod Biomed Online. 2008;16:553–569. doi: 10.1016/s1472-6483(10)60463-4. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Manes C, Daniel JC., Jr Development of preimplantation rabbit embryos in vivo and in vitro. I An ultrastructural comparison. Dev Biol. 1973;35:262–282. doi: 10.1016/0012-1606(73)90023-7. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Motta P. The Cellular Basis of Mammalian Reproduction. Urban and Schwarzenberg; Munich: 1979. [Google Scholar]
- Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Sinclair J, Davis P. Mitochondrial transfer between oocytes: potential applications of mitochondrial donation and the issue of heteroplasmy. Hum Reprod. 1998;13:2857–2868. doi: 10.1093/humrep/13.10.2857. [DOI] [PubMed] [Google Scholar]
- van Diepeningen AD, Slakhorst SM, Koopmanschap AB, Ikink GJ, Debets AJ, Hoekstra RF. Calorie restriction in the filamentous fungus Podospora anserina. Exp Gerontol. 2010;45:516–524. doi: 10.1016/j.exger.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Ventura SJ. Trends and variations in first births to older women, United States, 1970–86. Vital Health Stat. 1989;47:1–27. [PubMed] [Google Scholar]
- Visscher MB, King JT, Lee CP. Further studies on influence of age and diet upon reproductive senescence in strain A female mice. Am J Physiol. 1952;170:72–76. doi: 10.1152/ajplegacy.1952.170.1.72. [DOI] [PubMed] [Google Scholar]
- Vitullo AD, Ozil JP. Repetitive calcium stimuli drive meiotic resumption and pronuclear development during mouse oocyte activation. Dev Biol. 1992;151:128–136. doi: 10.1016/0012-1606(92)90220-b. [DOI] [PubMed] [Google Scholar]
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. Oogonial stem cells: a source for autologous germline mitochondria. Reprod Sci. 2013;20(Supplement) in the press. [Google Scholar]
- Woods DC, White YAR, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci. 2013;20:7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi YC, Chen MJ, Ho JYP, Guu HF, Ho ES. Mitochondrial transfer can enhance the murine embryo development. J Assist Reprod Genet. 2007;24:445–449. doi: 10.1007/s10815-007-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Vassena R, Latham KE. Effects of in vitro oocyte maturation and embryo culture on the expression of glucose transporters, glucose metabolism and insulin signaling genes in rhesus monkey oocytes and preimplantation embryos. Mol Hum Reprod. 2007;13:361–371. doi: 10.1093/molehr/gam014. [DOI] [PubMed] [Google Scholar]
- Zheng W, Gorre N, Shen Y, Noda T, Ogawa W, Lundin E, Liu K. Maternal phosphatidylinositol 3-kinase signalling is crucial for embryonic genome activation and preimplantation embryogenesis. EMBO Rep. 2010;11:890–895. doi: 10.1038/embor.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon KC. Human cells used in therapy involving the transfer of genetic material by means other than the union of gamete nuclei. U.S. Food and Drug Administration; 2001. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm105852.htm. [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]