Abstract
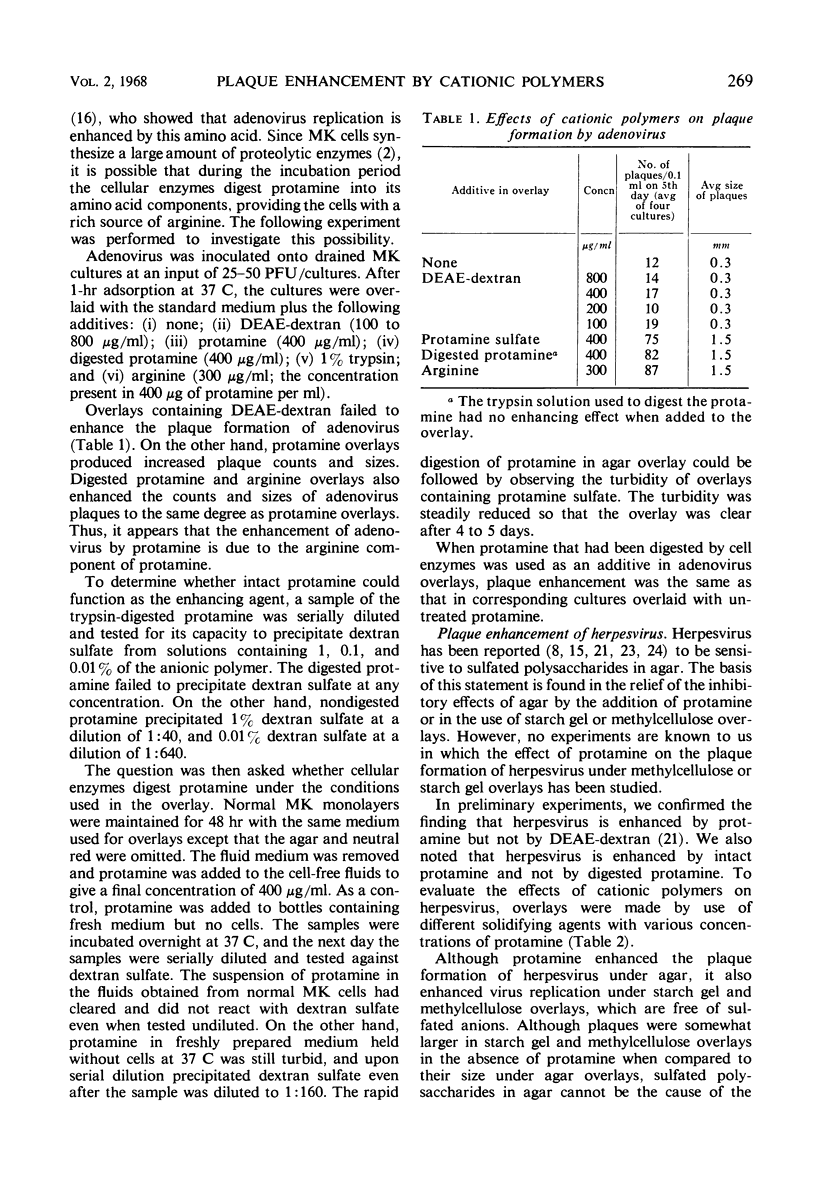
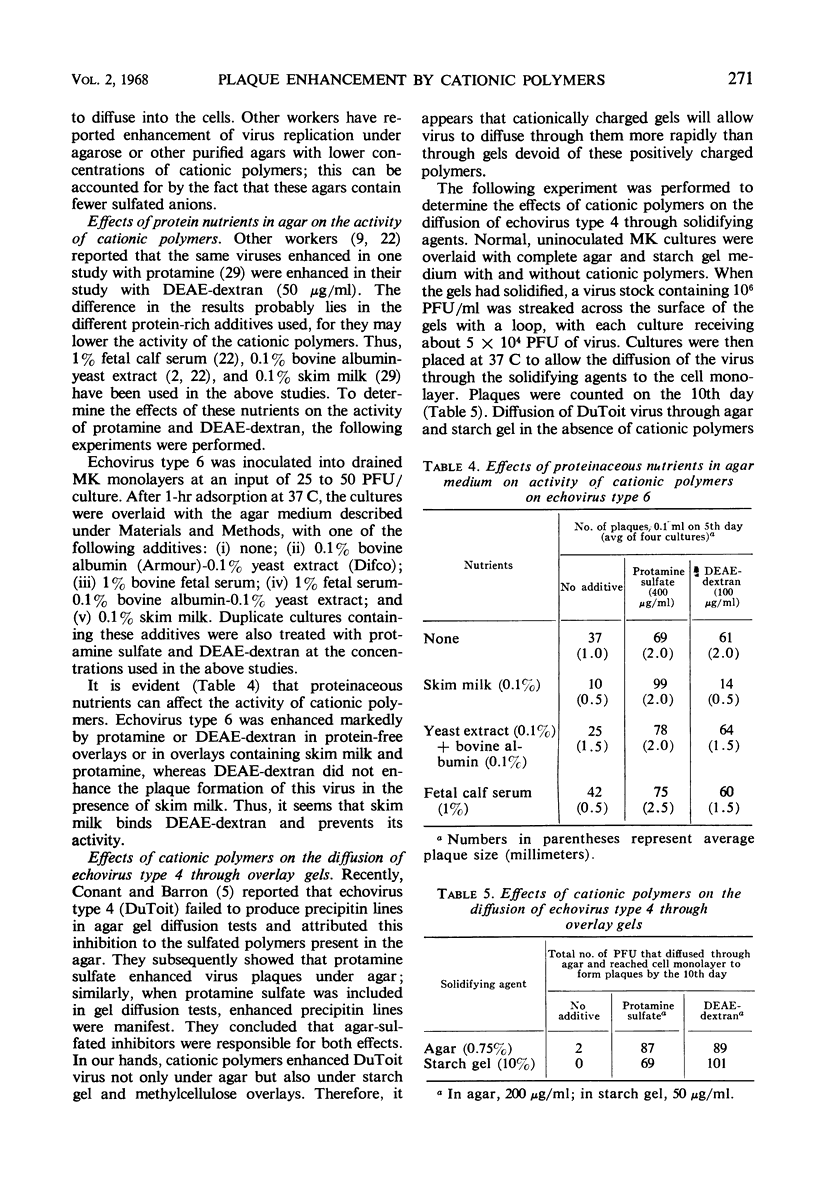
It has been assumed that plaque enhancement by cationic polymers is due to their binding of sulfated polysaccharides in agar. However, viruses that are enhanced by cationic polymers, diethylaminoethyl-dextran, and protamine were found not to be inhibited by polyanions in agar under the usual overlay conditions. In the case of adenovirus, enhancement by protamine seems to be due to the protamine serving as a source of arginine; enzymes released from the cultured cells digest the protamine and provide a reservoir of arginine for the cells. Other viruses (herpes and echovirus types 3, 4, 5, and 6) known to be susceptible to agar inhibitors were found to be enhanced by cationic polymers even under starch gel and methylcellulose overlays, which are free of polyanions. Since cationic polymers enhance the diffusion of virus through agar or starch gel, plaque enhancement seems to be the result of the gel becoming positively charged so that viruses can move effectively through them. The observation that starch gel and methylcellulose enhance plaque formation with viruses known to be inhibited under agar was also reinvestigated. When the consistency of the agar gel was reduced to the same viscosity of starch gel and methylcellulose overlays, the same plaque counts and sizes were observed under all three overlays.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- BARON S., LOW R. J. New maintenance medium for cell culture. Science. 1958 Jul 11;128(3315):89–90. doi: 10.1126/science.128.3315.89. [DOI] [PubMed] [Google Scholar]
- CAMPBELL J. B., COLTER J. S. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. 3. EFFECT OF OVERLAY AND POLYANIONS OF PLAQUE SIZE. Virology. 1965 Apr;25:608–619. doi: 10.1016/0042-6822(65)90089-9. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. II. INHIBITION OF INTERACTION WITH L CELLS BY AN AGAR INHIBITOR AND BY PROTAMINE. Virology. 1964 Dec;24:578–585. doi: 10.1016/0042-6822(64)90210-7. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. The plaque assay of animal viruses. Adv Virus Res. 1961;8:319–378. doi: 10.1016/s0065-3527(08)60689-2. [DOI] [PubMed] [Google Scholar]
- Conant R. M., Barron A. L. Enhanced diffusion of enterovirus antigens in agar gel in the presence of protamine. Virology. 1967 Nov;33(3):547–549. doi: 10.1016/0042-6822(67)90133-x. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Effect of polycations on growth and dissemination of the encephalomyocarditis virus in mice. J Virol. 1967 Oct;1(5):988–995. doi: 10.1128/jvi.1.5.988-995.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAEYER E., SCHONNE E. STARCH GEL AS AN OVERLAY FOR THE PLAQUE ASSAY OF ANIMAL VIRUSES. Virology. 1964 Sep;24:13–18. doi: 10.1016/0042-6822(64)90142-4. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Hannon W. H. Use of DEAE dextran in agar overlays to enhance size of ECHO virus plaques. Public Health Rep. 1966 Nov;81(11):1015–1018. [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S. A study of the herpes simplex virus-rabbit kidney cell system by the plaque technique. Virology. 1957 Dec;4(3):435–457. doi: 10.1016/0042-6822(57)90078-8. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. Alteration plaque morphology of EMC virus with polycations. Virology. 1961 Aug;14:502–504. doi: 10.1016/0042-6822(61)90349-x. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- NAHMIAS A. J., KIBRICK S., BERNFELD P. EFFECT OF SYNTHETIC AND BIOLOGICAL POLYANIONS ON HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:993–996. doi: 10.3181/00379727-115-29098. [DOI] [PubMed] [Google Scholar]
- RAPP F. VARIANTS OF HERPES SIMPLEX VIRUS: ISOLATION, CHARACTERIZATION, AND FACTORS INFLUENCING PLAQUE FORMATION. J Bacteriol. 1963 Nov;86:985–991. doi: 10.1128/jb.86.5.985-991.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- STYK B., RADA B. INTERACTION OF MYXOVIRUSES WITH DEXTRAN SULFATES. II. SPECTRUM OF INHIBITORY ACTIVITY. CHARACTERISTICS OF ANTIVIRAL EFFECTS OF DEXTRAN SULFATES IN TISSUE CULTURE. Acta Virol. 1964 Jul;8:312–326. [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INFLUENCE OF ACID POLYSACCHARIDES ON PLAQUE FORMATION BY INFLUENZA A2 AND B VIRUSES. Proc Soc Exp Biol Med. 1963 Dec;114:811–814. doi: 10.3181/00379727-114-28806. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- TYTELL A. A., NEUMAN R. E. A medium free of agar, serum and peptone for plaque assay of herpes simplex virus. Proc Soc Exp Biol Med. 1963 Jun;113:343–346. doi: 10.3181/00379727-113-28362. [DOI] [PubMed] [Google Scholar]
- TYTELL A. A., TOROP H. A., McCARTHY F. J. Adenovirus plaque formation in grivet monkey kidney cells. Proc Soc Exp Biol Med. 1962 Apr;109:916–918. doi: 10.3181/00379727-109-27377. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- VAHERI A. HEPARIN AND RELATED POLYIONIC SUBSTANCES AS VIRUS INHIBITORS. Acta Pathol Microbiol Scand Suppl. 1964:SUPPL 171–17298. [PubMed] [Google Scholar]
- VOSS H., MUENDLER M., PLOETZE G. VIRUS UND POLYELEKTROLYTE. I. PLAQUEVERFAHREN, D-MARKER UND AGARPOLYSACCHARID. Zentralbl Bakteriol Orig. 1964 Mar;192:137–157. [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A. Growth of rubella virus in BHK21 cells. II. Enhancing effect of DEAE-dextran, semicarbazide and low doses of metabolic inhibitors. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1092–1098. doi: 10.3181/00379727-125-32284. [DOI] [PubMed] [Google Scholar]
- Wallis C., Morales F., Powell J., Melnick J. L. Plaque enhancement of enteroviruses by magnesium chloride, cysteine, and pancreatin. J Bacteriol. 1966 May;91(5):1932–1935. doi: 10.1128/jb.91.5.1932-1935.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


