Abstract
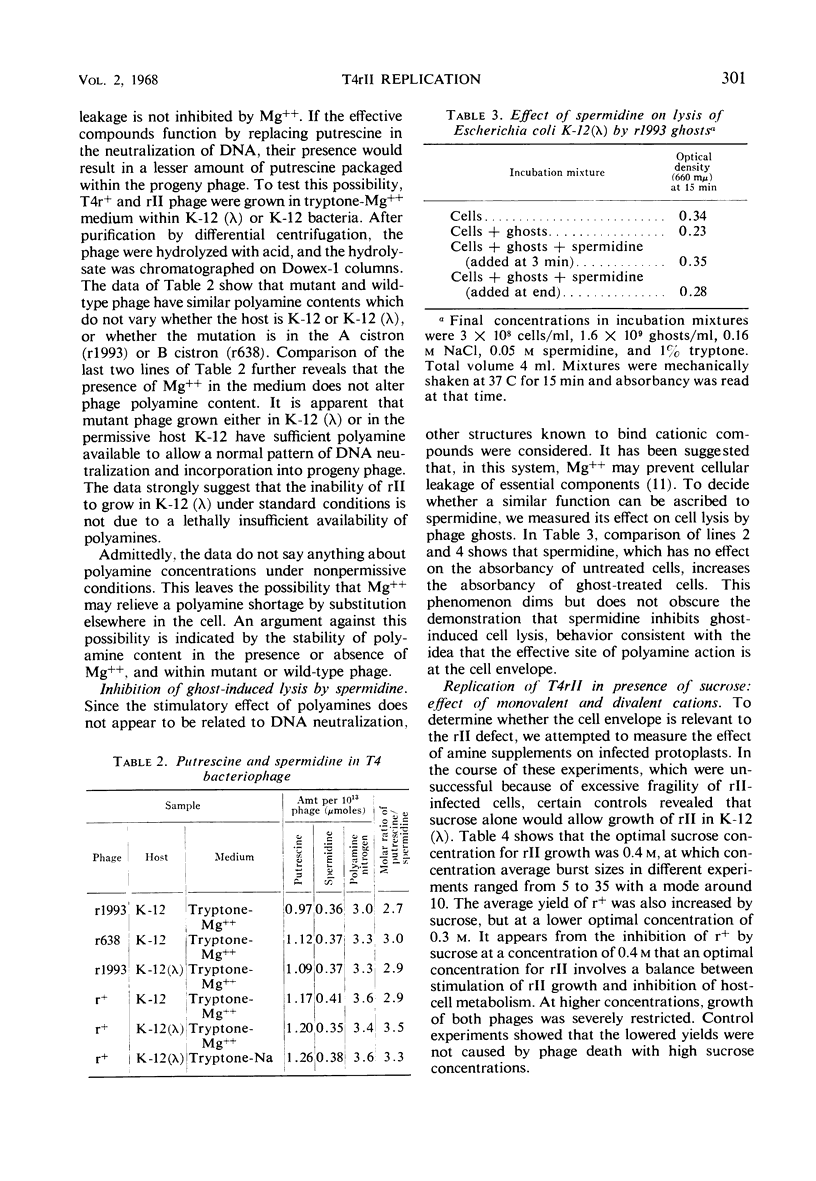
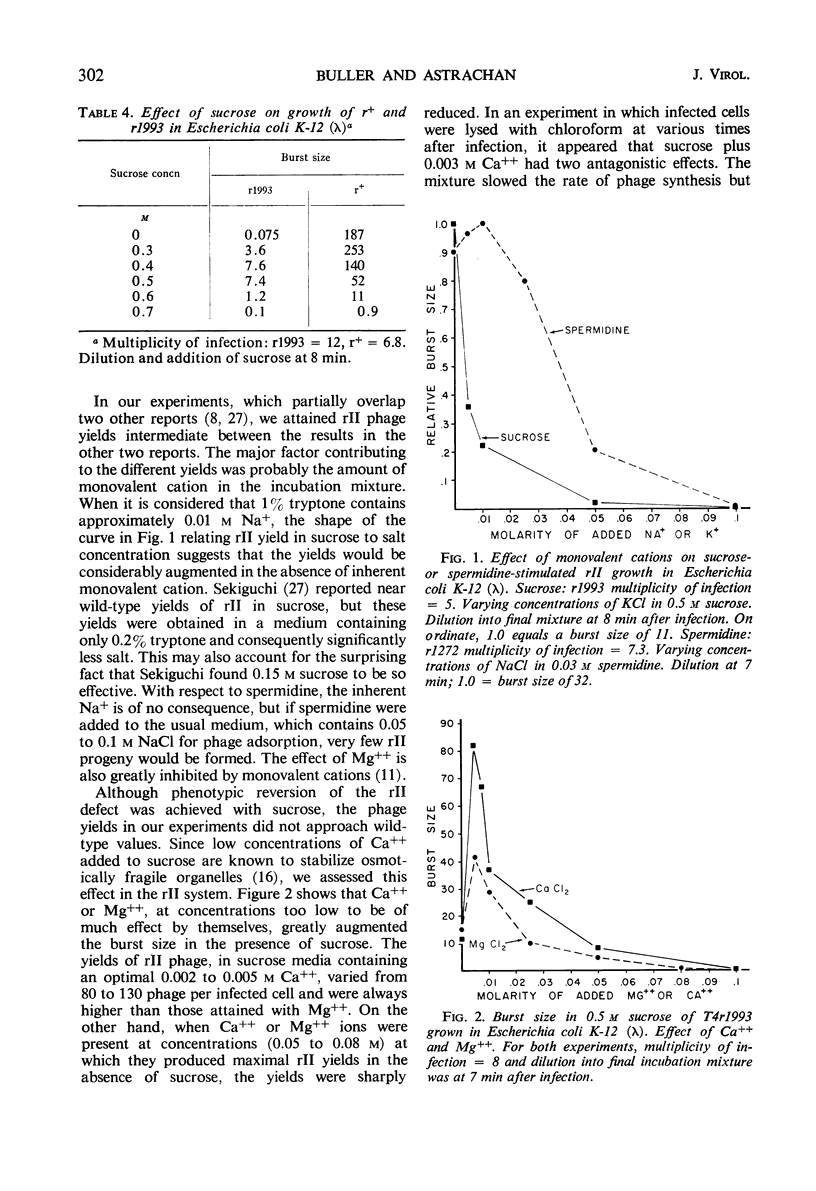
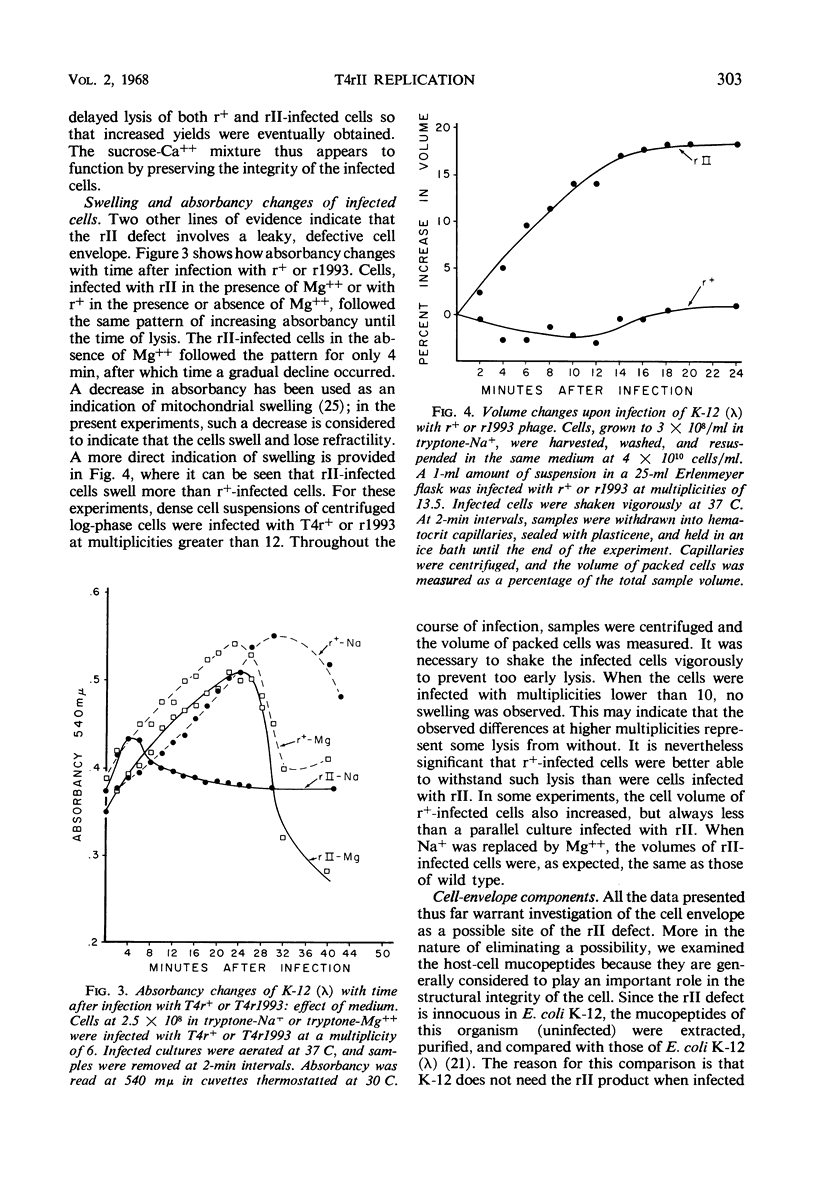
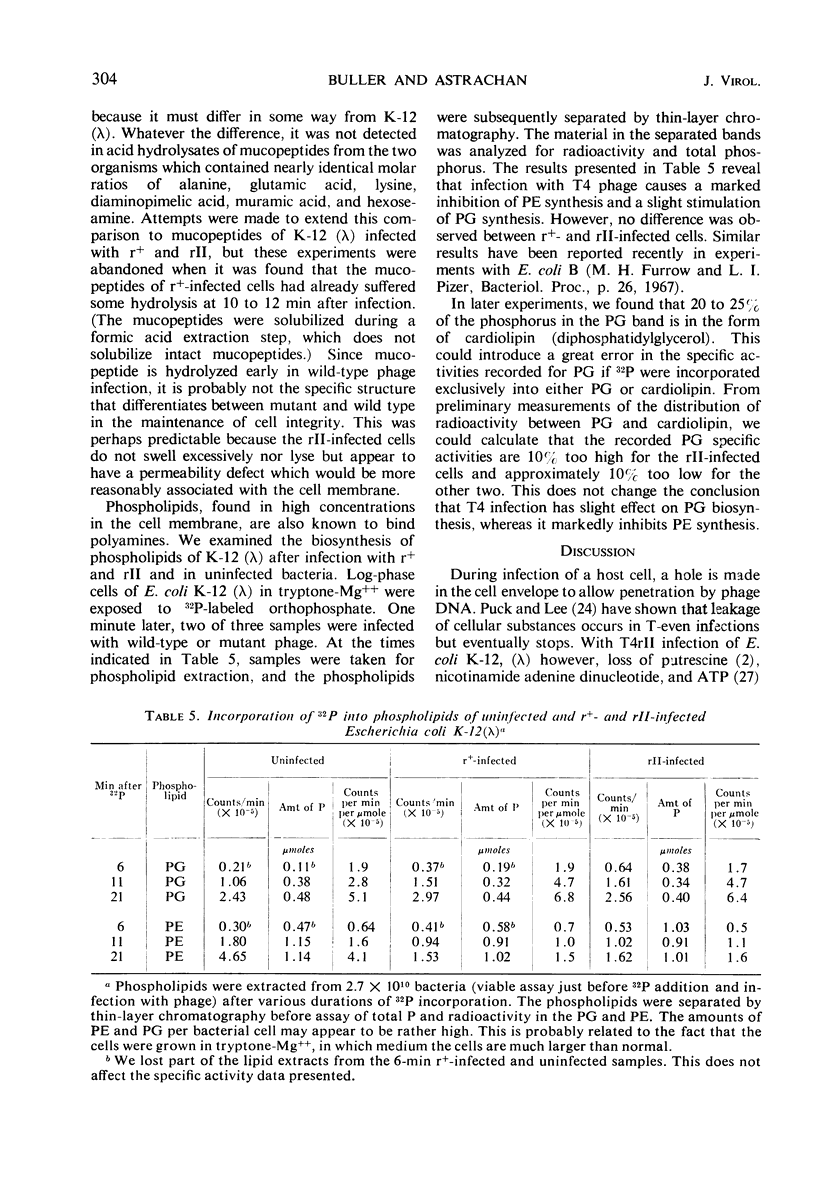
The defect of T4rII replication in Escherichia coli K-12 (λ) can be phenotypically reversed by various supplements to the growth medium. Arginine, lysine, spermidine, and a number of diamines allowed varying levels of rII replication. The best reversion was obtained with 0.4 m sucrose in 0.002 to 0.005 m Ca++. Monovalent cations severely inhibited reversion. A cell surface site of polyamine action is consistent with the fact that spermidine inhibits phage ghost-induced cell lysis and with the finding that sufficient polyamine is available within the cells to allow normal patterns of neutralization of phage deoxyribonucleic acid, as detected by the polyamine content of progeny phage. In the absence of effective supplements, rII-infected cells swelled and lost refractility. The data indicate that a leaky cell envelop is involved. No difference in mucopeptides of uninfected K-12 (λ) and K-12 was detected and, because the mucopeptide in r+ infected cells was found to be at least partially hydrolyzed midway through the lytic cycle, it did not appear that the rII defect concerned mucopeptide synthesis. The pattern of cell phospholipid synthesis changes after phage infection, but no difference was detected between r+ and rII with regard to biosynthesis of phosphatidylethanolamine and phosphatidylglycerol.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BACHRACH U., COHEN I. Spermidine in the bacterial cell. J Gen Microbiol. 1961 Sep;26:1–9. doi: 10.1099/00221287-26-1-1. [DOI] [PubMed] [Google Scholar]
- BROCK M. L. THE EFFECTS OF POLYAMINES ON THE REPLICATION OF T4RII MUTANTS IN ESCHERICHIA COLI K-12 (LAMBDA). Virology. 1965 Jun;26:221–227. doi: 10.1016/0042-6822(65)90049-8. [DOI] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Freeman A. R. Plasma membrane: substructural changes correlated with electrical resistance and pinocytosis. Science. 1967 Feb 3;155(3762):582–585. doi: 10.1126/science.155.3762.582. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. Preparation, purification, and properties of E. coli virus T2. J Gen Physiol. 1952 May;36(1):17–28. doi: 10.1085/jgp.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON F. B., SETTERFIELD G., STERN H. The isolation of nucleoli from ungerminated pea embryos. J Biophys Biochem Cytol. 1959 Aug;6(1):53–56. doi: 10.1083/jcb.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- MAGER J. Influence of osmotic pressure on the polyamine requirement of Neisseria perflava and Pasteurella tularensis for growth in defined media. Nature. 1955 Nov 12;176(4489):933–934. doi: 10.1038/176933a0. [DOI] [PubMed] [Google Scholar]
- MAGER J. Spermine as a protective agent against osmotic lysis. Nature. 1959 Jun 27;183:1827–1828. doi: 10.1038/1831827a0. [DOI] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Preparation and properties of the mucopeptides of cell walls of gram-negative bacteria. Biochem J. 1962 Aug;84:294–299. doi: 10.1042/bj0840294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. I. An increase in host cell permeability induced by bacteriophage infection. J Exp Med. 1954 May 1;99(5):481–494. doi: 10.1084/jem.99.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECKNAGEL R. O., MALAMED S. The osmotic nature of mitochondrial swelling produced by carbon tetrachloride and inorganic phosphate. J Biol Chem. 1958 Jun;232(2):705–713. [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Barclay M., Reichman E. S., Good J. J. Separation of acidic phospholipids by one-dimensional thin-layer chromatography. Biochim Biophys Acta. 1967 Feb 14;137(1):80–89. doi: 10.1016/0005-2760(67)90010-0. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- Wells M. A., Dittmer J. C. A microanalytical technique for the quantitative determination of twenty-four classes of brain lipids. Biochemistry. 1966 Nov;5(11):3405–3418. doi: 10.1021/bi00875a004. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Crawford I. P. THE EFFECTS OF DELETIONS, POINT MUTATIONS, REVERSIONS AND SUPPRESSOR MUTATIONS ON THE TWO COMPONENTS OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Jul;45(7):1016–1026. doi: 10.1073/pnas.45.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- ZILLIG W., KRONE W., ALBERS M. [Investigations on the biosynthesis of proteins. III. Contribution to the knowledge of the composition and structure of ribonucleoprotein particles]. Hoppe Seylers Z Physiol Chem. 1959;317:131–143. doi: 10.1515/bchm2.1959.317.1.131. [DOI] [PubMed] [Google Scholar]


