Abstract
Random mutagenesis combined with phenotypic screening using carefully crafted functional tests has successfully led to the discovery of genes that are essential for a number of functions. This approach does not require prior knowledge of the identity of the genes that are involved and is a way to ascribe function to the nearly 6000 genes for which knowledge of the DNA sequence has been inadequate to determine the function of the gene product. In an effort to identify genes involved in the visual system via this approach, we have tested over 9000 first and third generation offspring of mice treated with the mutagen N-ethyl-N-nitrosourea (ENU) for visual defects, as evidenced by abnormalities in the electroretinogram and appearance of the fundus. We identified 61 putative mutations with this procedure and outline the steps needed to identify the affected genes.
Keywords: Mutagenesis, Forward genetics, Screening for visual function, Electroretinogram, Visual function in mouse, Mouse, Ethynitrosourea, ENU, a-wave, b-wave, c-wave, STR, Functional test for vision
1. Introduction
Mice have been recognized to be a valuable resource for the study of the visual system because they serve as a model organism for targeted mutations. However, mice are also valuable model organisms for vision because of the availability of a large genetically diverse set of isogenic strains, their short generation time, the availability of genomic sequence information and tools for mapping genes, and their ability to be mutagenized. One reason that the genetic advantages of mice have not been greatly exploited to study the visual system is that the mouse is not generally considered to be a “visual animal,” perhaps because the retinal degeneration (rd, now Pde6rd1) mutation occurs in many common laboratory strains and renders all of the mice in these strains incapable of normal responses to light (Chang et al., 2002). The fact that these laboratory strains are blind does not, of course, mean that the majority of strains of mice with the wild-type allele of the Pde6 are also blind. Mice, in fact, use their sense of vision under conditions of dim illumination. It is likely that this very fact is yet another reason why mice might not be considered “visual animals”: their circadian behavior is such that they are most active at night under dim illumination, not during the day under the bright illumination of a laboratory when they are usually observed. Another reason that the vision of mice is discounted is probably that their most obvious behaviors seen in the laboratory are sniffing and touching objects with whiskers. However, when observed by ethologists in more natural settings it has long been known that mice engage in exploratory, feeding and aggressive behaviors that clearly depend on vision (Crowcroft, 1966). Moreover, the overall architecture and biochemistry of the mouse retina is similar to those of human eyes, and a number of genes with disease causing mutations in mouse have orthologs responsible for retinal diseases in humans. Thus, mice are well suited to the study of the genetics of the visual system.
The use of mutant phenotypes to identify essential genes has facilitated rapid progress in diverse areas (Takahashi, Pinto, & Vitaterna, 1994). The demonstration that the Shaker gene encoded a potassium channel (Wu, Ganetzky, Haugland, & Liu, 1983) and the subsequent cloning of this gene (Papazian, Schwarz, Tempel, Jan, & Jan, 1987; Tempel, Papazian, Schwarz, Jan, & Jan, 1987) facilitated the identification of several other families of ion channels and led to a detailed knowledge of their structure– function relationship (Doyle et al., 1998). Likewise, the field of circadian biology was advanced from descriptive to mechanistic analyses with the identification of the per gene (Konopka & Benzer, 1971). The “forward genetic” approach of working from phenotype to gene has also been of great utility in the elucidation of developmental processes. The identification of mutants with aberrant pattern (body plan) formation in the fly (Nusslein-Volhard & Wieschaus, 1980) and zebrafish (Brand et al., 1996) has permitted an exponential increase in our understanding of the processes involved. The forward genetic approach differs from that of “reverse genetics”, in which a known gene is modified (e.g. with a “knockout”; see Keverne, 1997) in that prior knowledge of the gene's identity, sequence, map position or even gene family are not needed for the forward genetic approach. Thus, we have applied the forward genetics approach in order to discover novel genes and novel alleles of known genes involved in vision.
Two principal requirements need to be satisfied in order to use mutants to identify genes involved in vision. First, the population of animals used for the study should be genetically homogeneous, or isogenic, thereby permitting an induced mutation in the DNA sequence to be identified readily. Isogenicity is also helpful in reducing the variability of the phenotype under study, thus allowing animals with an abnormal phenotype to be identified reliably. Second, a reliable quantitative assay for vision needs to be established. Ideally, this assay should be neither labor intensive nor time consuming, and thus amenable to high throughput.
The mouse satisfies both of the above requirements. First, inbred strains are available for the mouse. Inbred strains are produced by 20 or more generations of crosses either between siblings or between offspring and the older parent. Thus, each member of a strain is considered to be homozygous at each locus across the genome. This also reduces variation among mice under investigation. However, it should be kept in mind that the reproducibility from animal to animal within a given inbred strain varies among the strains. For example, in our experience the circadian period of animals of the C57BL/6J strain is much more consistent than that of either the 129/Sv strain commonly used for gene targeting or the BALB/ cJ strain. The second requirement, for a reliable assay, is satisfied by relying on the electroretinogram (Dalke et al., 2004) and fundus photography. The electroretinogram is an important and relevant measure of retinal function (Peachey & Ball, 2003; Pinto & Enroth-Cugell, 2000). By adjusting the stimulation and adaptation conditions the component “waves” of the electroretinogram can be used to assay the activity of restricted sets of neurons in the retina. These “waves” are the a-, b-, and c-waves and the scotopic threshold response (STR).
The most sensitive wave of the mouse electroretinogram is the scotopic threshold response (STR), which originates with cells of the inner retinal layers (Saszik, Robson, & Frishman, 2002). The STR consists of a positive-going component followed by a negative-going component to dim stimuli that can be recorded only from fully dark-adapted animals.
The a-wave can be used as a highly quantitative monitor of the electrical activity of the rod and cone photoreceptors (Goto et al., 1996; Lyubarsky & Pugh, 1996; Peachey, Goto, Quiambao, & al-Ubaidi, 1995) in the mouse, which have high sensitivity to ultraviolet light (Calderone & Jacobs, 1995; Jacobs, Neitz, & Deegan, 1991). In the mouse, only about 2% of the receptors are cones (Carter-Dawson & LaVail, 1979), and under dark-adapted conditions the a-wave results from the activity of mostly rods (Lyubarsky, Falsini, Pennesi, Valentini, & Pugh, 1999; Pugh, Falsini, & Lyubarsky, 1998). The cellular basis for the a-wave is well understood in terms of the physiology of photoreceptors. In the dark, steady state condition, these cells are maintained in a depolarized state by a steady influx of cations which flow from the inner to the outer segments of the photoreceptors in the extracellular space, entering the outer segments through cGMP-activated channels. The inward current in the outer segment is balanced by an outward current in the inner segment carried primarily by K+ channels, and the net effect is that a “circulating current” flows in the extracellular space around the photoreceptors, creating a vitreal-positive electric field potential in the dark. Illumination quantitatively reduces the circulating current, and the consequent change in field potential generates a negative-going potential, the a-wave, that can be recorded in the vitreous or at the cornea. In some other species the activity of the second-order OFF-bipolar cells is thought to make an additional, prominent contribution to the a-wave (Bush & Sieving, 1994; Hood & Birch, 1996; Naarendorp & Williams, 1999; Robson & Frishman, 1996).
The cellular basis for the b-wave is not as well-defined as those of the a-and c-waves. It was once believed that this positive wave resulted from accumulation of K+ at the end-feet of the Mueller glial cells (Miller & Dowling, 1970), but this conclusion was contradicted by the findings that Ba2+ inhibits the responses of these glial cells but does not eliminate the b-wave (Frishman & Steinberg, 1989; Xu & Karwoski, 1994) and that disruption of the gene encoding a K+ channel in Mueller cells does not abolish the b-wave (Kofuji et al., 2000). Subsequent work in other species has led to the conclusion that the b-wave arises largely from the activity of bipolar cells (Gurevich & Slaughter, 1993; Hood & Birch, 1996; Robson & Frishman, 1995; Robson & Frishman, 1996; Tian & Slaughter, 1995). Even though the b-wave originates from neurons that are postsynaptic to the photoreceptors, it can be used as a useful indirect measure of certain aspects of photoreceptor and presumably ON-bipolar cell function. It may be particularly useful in the investigation of murine cone function (Lyubarsky et al., 1999), since the cone a-wave is very small and difficult to measure. Furthermore, the scotopic b-wave can be used to identify mutations that affect transmission from photoreceptors to second-order neurons (Pardue, McCall, LaVail, Gregg, & Peachey, 1998).
The c-wave originates in the retinal pigment epithelium (RPE). The positive voltage of this wave is observed after the a-and b-waves and results from hyperpolarization of the apical membrane of the RPE, which apposes the rod and cone photoreceptors. Photoreceptors hyperpolanze in response to light, resulting in decreased efflux of K+, and a consequent lowering of [K+] in the interphotoreceptor space (Oakley & Green, 1976; Steinberg, 1985), which hyperpolarizes the apical RPE membrane. Later components of the c-wave of the rodent are thought to arise from other retinal cells (Hanitzsch & Lichtenberger, 1997).
Mice are nocturnal animals and it is thus generally believed that their cone function is minimal. However this is not the case, as mice have ca. 2% (S-and L-tvpe) cones, distributed along a dorsal–ventral gradient (Applebury et al., 2000), and are not heavily concentrated in the area centralis (Carter-Dawson & LaVail, 1979). Cone function can be followed in the mouse with three types of stimuli: flashes that are applied against a dim background light that adapts the rods, with sinusoidally-modulated stimuli applied around a mean luminance sufficient to adapt the rods, and with pairs of flashes, the first of which adapts the rods (Ekesten, Gouras, & Moschos, 1998; Ekesten, Gouras, & Yamamoto, 2000). The electroretinogram recorded under these conditions reflects the contributions of several types of retinal cells, including opposing contributions from depolarizing and hypolarizing bipolar cells (Kondo & Sieving, 2001; Krishna, Alexander, & Peachey, 2002) and shows a dependence upon temporal frequency that differs from that of other species. Thus, the function of cones and the retinal neural pathways that lead from cones can be assessed in the mouse using electroretinography.
In addition to electroretinography, fundus examination is another, complementary technique for identifying vision phenotypes in mutant mice. Changes in the retinal vasculature, appearance of the optic nerve head, and ocular pigmentation can be compared with well-described fundus characteristics from inherited and acquired diseases in man. Putantive mutants may exhibit alterations in the ERG, fundus appearance, or both, and the use of both techniques in conjunction provides a robust screen for functional and structural changes with various genetic lesions. Indeed, our studies have shown that these tests can be used to identify offspring of mutagenized mice that are visually defective.
2. Methods
2.1. Mutagenesis
Male C57BL/6J mice were mutagenized with three weekly doses of N-nitroso-N-ethylurea (90–100mg/kg body weight). These ENU treated males were then bred with wild-type females to produce mutant G1 (generation 1) offspring. Each G1 mouse represents one mutagenized gamete from the G0 founder. G1 mice were either phenotyped to conduct a dominant screen for mutants or bred with wild-type mice to found mutant pedigrees to produce third generation (G3) mice to conduct a recessive screen. G3 mice were produced using two different breeding schemes—the intercross scheme and the backcross scheme. In the intercross scheme G1 males and G1 females from different G0 founders were intercrossed with one another to produce G2 mice. These G2 sibling mice were then intercrossed to generate G3 offspring for testing. In the backcross breeding scheme G1 male mice were mated with wild-type female mice to produce G2 daughters. The G2 females were then backcrossed to their G1 fathers to produce G3 mice for testing. All G3 mice were screened for a number of neurobiological traits starting at 6 weeks of age and were tested for vision starting at 12 weeks of age.
2.2. Electroretinogram
In order to detect visually defective mice a careful, quantitative study was made of the electroretinogram of each of the over 9000 mice that were screened. This was made possible by automated presentation of the stimuli and analysis of the responses of each mouse. The distribution of each measure was calculated, and the mean and variance of these measures were tracked for the population of G3 mice tested and for a smaller cohort of wild-type mice.
2.2.1. Experimental details of electroretinogram recording
All procedures were performed in total darkness with the aid of infra-red image converters. Mice were dark-adapted for at least 2h prior to recording. Anesthesia was administered in the dark and the animals were placed in the recording chamber upon a temperature-controlled heating pad. Rectal temperature was measured and controlled to achieve a rectal temperature between 36.5 and 37 °C. We have found that the most important single experimental condition responsible for variability in the amplitude and sensitivity of the electroretinogram is the loss of body temperature of the animal after administration of anesthetic prior to reaching an anesthetic plane that allows the mouse to be placed on the heating pad where the electroretinogram is recorded. We attempted to minimize this variable by working in a warm room, placing warm objects in the mouse’s home cage while it was falling asleep, and remaining vigilant about the mouse’s body temperature. Mice were anesthetized with a mixture of ketamine (about 70mg/kg and xylazine (about 7mg/kg). We have had to adjust the dose of anesthetic very carefully to be sure that the breathing of the mouse remains normal and that the temperature of the mouse does not fall below 36.5–37 °C. We found it useful to be mindful of the relative amplitudes of the a-and b-waves because low body temperature is accompanied by greater decrease in the amplitude of the b-wave than in the amplitude of the a-wave (Kong & Gouras, 2003).
Mice were fitted with DTL fiber electrodes on each eye (Dawson, Trick, & Litzkow, 1979) and the electroretinogram recorded differentially between the eyes to reduce artifacts from the electrocardiogram and respiratory muscles. The corneas were wetted with a solution of 1.2% cellulose in 0.9% NaCl. One eye was covered with a clear contact lens and the other with an opaque contact lens. Diffuse stimuli were presented from LEDs to the eye covered with the clear lens. Nine full-field flash stimuli (luminance 7X 10−4 to 300cds/m2) were presented against a dark background and a dim adapting light (0.5cd/m2) was presented steadily in order to light-adapt the retina while a flash stimulus (luminance 0.2cds/m2) was presented in order to evoke the cone electroretinogram. It was necessary for the mice to breed after we found them to be visually defective and, thus, to ensure their health, we allowed them to remain anesthetized for only about 20min. Unfortunately, this short time did not permit us to measure the recovery of sensitivity after offset of an adapting light (Kang Derwent, Qtaishat, & Pepperberg, 2002; Silva, Hetling, & Pepperberg, 2001) or to use a two-flash protocol to isolate cone responses (Ekesten et al., 1998; Pugh et al., 1998). The electroretinogram was recorded at 1kHz with a resolution of 0.5lV. Response averaging was employed for the responses to dim stimuli to increase signal-to-noise ratio. All stimulus presentation and data recording were controlled by computer using software we have made available to the community (www.neuromice.org; look under “assays” or http://www.genome.northwestern.edu/neuro/vision_protocol.cfm). Analysis of the electroretinograms was done automatically using MATLAB analysis software. For the analysis, the dependence of the peak amplitude of each wave upon stimulus luminance and the amplitude of the responses recorded under conditions to isolate cone inputs were measured.
2.3. Fundus photography
The fundi of over 15 inbred strains of mice have been characterized with fundus photography (Hawes et al., 1999), and we used the methods that were developed by these workers for our study. After collection, the digital images were transmitted electronically from Northwestern University to the University of Iowa, where the images were evaluated and the results of the evaluation returned for follow-up where necessary.
2.3.1. Experimantal details of fundus photography
These observations were performed on anesthetized mice one to three days after the electroretinogram was measured. In our experience this time interval is adequate to allow the mice to recover from the anesthesia for electroretinography. The iris was dilated with 1% Mydriacil and the corneas kept moist with saline solution during photography. We have also found it to be essential to maintain the body temperature of the mouse during fundus photography and thus the mouse was also positioned on a heating pad during photography. Photographs were made with a Kowa small animal fundus camera (2.5 megapixels) equipped with a 66 diopter supplemental lens. Fundus photographs were evaluated by a certified ophthalmic photographer (EH) for retinal phenotypes characteristic of human disease conditions, including irregularities in the optic nerve head, caliber of retinal vessels, presence of dot-like lesions, and evidence of pigmentary abnormalities. Attenuation of arterioles is an ophthalmoscopic feature of photoreceptor degenerations such as retinitis pigmentosa, and was assessed in fundus photographs. Phenotypic changes were confirmed by a retina specialist (EMS), and breeding recommendations are made.
2.4. Testing heritability
Once a putative mutant is identified, it is important to verify that the abnormal phenotype is heritable, i.e., that the abnormality has a genetic basis. While there are multiple methods to accomplish this, our preference is to breed the affected individual and confirm heritability by “vertical transmission” to that individual's descendents. This has the advantage of providing a direct and clear demonstration that the trait is heritable. In cases in which the animal has a phenotype that might impair fertility, or in which invasive testing methods are used, breeding an affected individual may not be possible. In these cases, first degree relatives (parents or siblings) are bred and recurrence of the trait in the resulting progeny provide indirect evidence of a mutation. This is less preferable, since the relatives may fail to carry or transmit the mutation. In addition, recurrence of the phenotype in siblings of the original animal could be attributable to a common environmental cause (e.g., injury from maternal care) and not due to shared genetic alteration.
Because we screened G3 mice, both dominant and recessive mutations may have been recovered. We thus have selected a test cross scheme that will allow us to distinguish between these classes of mutations. G3 putative mutants are first backcrossed to wild-type C57BL/6J mice to produce G4 backcross progeny. We phenotypically tested these G4 mice, since if the G3 founder was a heterozygote for a dominant mutation, half of the G4 mice would be expected to harbor the mutation. If none of the G4 progeny display the phenotype, then it is more likely that the G3 founder was a homozygote for a recessive mutation, and thus the G4 progeny would all be heterozygotes and not exhibit the recessive phenotype. We intercrossed G4 mice with one another to produce G5 mice. One-fourth of these would be expected to be homozygous for the mutation, and we thus one would expect to find affected individuals in this generation if a recessive mutation is present.
3. Results
3.1. Properties of the electroretinograms of the population studied
We recorded the electroretinograms of over 100 wild-type C57BL/6J mice and over 9000 G1 and G3 offspring of ENU-mutagenized mice. We did not attempt to record the electroretinograms of the 1–2% of these mice that were microphthalmic. Two types of electroretinograms were recorded. First, the animals were completely dark-adapted and handled in the dark with infra-red image converters and infra-red illumination and the dark-adapted electroretinogram was recorded in response to a series of 9 full-field flash stimuli (luminance 7 · 10 −4 to 300cds/m2). Second, a dim adapting light (0.5 cd/m2) was presented steadily in order to light-adapt the retina and one of the flash stimuli (luminance 0.2cds/m2) was presented in order to evoke the cone electroretinogram.
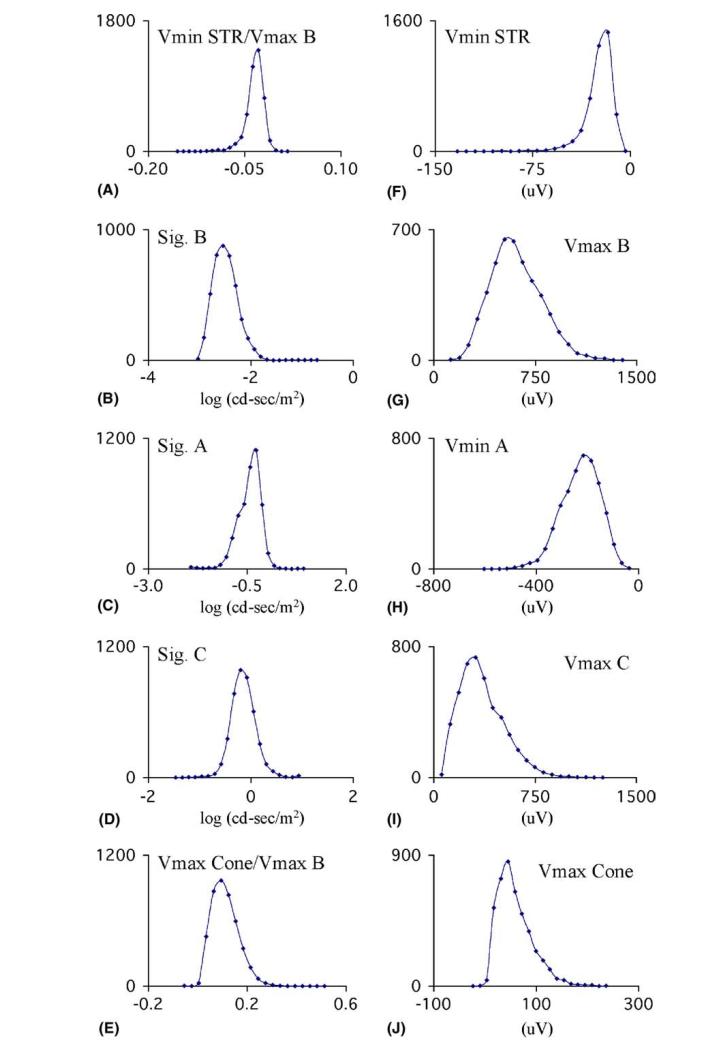
Dim stimuli normally evoked the scotopic threshold response (STR), which has a positive and negative component (Fig. 1). We quantified the amplitude of the negative component of the STR as a fraction of the maximal amplitude of the b-wave (STR/VmaxB in Fig. 2) and found that this ratio had a sufficiently small variation (Fig. 2) that mice with very small or very large values would be detectable. The response that is detectable with slightly more luminous stimuli is the b-wave (Fig. 1), attributable mostly to the bipolar cells. We plotted the amplitude of the b-wave against the stimulus luminance, fitted a saturation curve to these data and interpolated to find the luminance required to evoke a response of half-maximal amplitude (SigB) and plotted the distribution of SigB (Fig. 2). Likewise, the amplitudes of the a-and c-waves, resulting from photoreceptor and retinal pigment epithelial cell activity respectively, were measured, plotted against stimulus luminance and the distribution of the respective SigA and SigB values were calculated (Fig. 2).
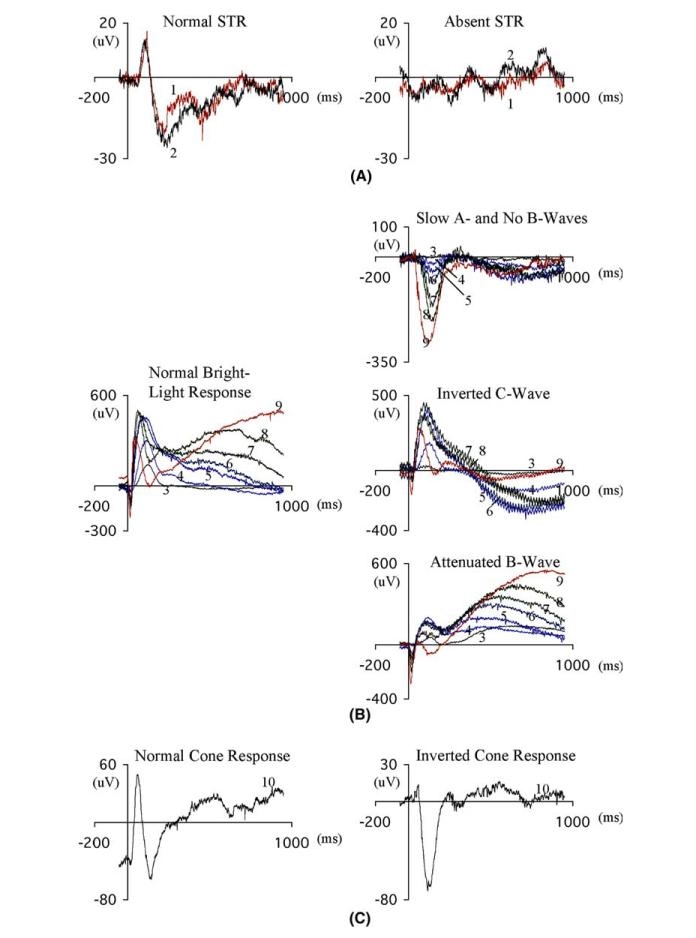
Figure 1.
Electroretinograms from normal (left column) and putative mutant (right column) mice in response to flash stimuli against a dark background (A and B) or against a steady adapting background (3cd/m2, C). Stimuli are indicated by numbers near each response. (A) The STR (scotopic threshold response) is found in every normal, fully dark-adapted mouse but was not found in 4 putative mutants. (B) Putative mutants were found with either inverted b-wave or slowed a-wave and absent b-wave (upper traces), inverted c-wave (middle traces) or attenuated b-wave (lower traces). (C) Cone responses are evoked in the presence of a steady adapting light (3cd/m2) but in one putative mutant mouse the cone response was inverted in sign. Illumination of the various stimuli were: 1, 0.00017cds/m2; 2, 0.00028cds/m2; 3, 0.00066cds/m2; 4, 0.0083cds/m2; 5, 0.158cds/m2;6, 0.331cds/m2; 7, 0.637cds/m2; 8, 1.19cds/m2; 9, 27.88cds/m2.
Figure 2.
Distribution of values found for variables associated with the electroretinogram of 4375 mice over a one-year period. (A) The amplitude of the negative component of the STR, normalized to the maximal amplitude of the b-wave. (B–D). log illumination required to evoke a response of half maximal amplitude for the b-wave, the a-wave and the c-wave. (E) Amplitude of the negative component of the cone response normalized to the maximal amplitude of the b-wave. (F) Negative component of the STR. (G–J) Maximal amplitudes of the b-wave, a-wave, c-wave and negative component of the cone erg.
In order to assess cone activity a flash stimulus of luminance 0.6cd/m2 was presented against a steady background of luminance3cds/m2. The cone signal consisted of a positive-going wave that peaked about 75ms after the stimulus flash, followed by a negative-going wave at about 150ms. The amplitude of the positive-going wave, evoked in the presence of the steady dim light, was normalized to the amplitude of the b-wave evoked by the same stimulus against a dark background (“Cone B/VmaxB” in Fig. 2).
3.2. Fundus appearance for the population studied
The normal fundus of the C57BL/6J mouse is darkly pigmented. This pigmentation is due to RPE melanosomes and the dense bed of choroidal melanocytes which are continuous through the posterior pole except at the optic nerve head, from which the main array of retinal vessels radiates. Arterioles and venules can be distinguished as the latter are of larger diameter. A series of hypopigmented fibers corresponding to the nerve fiber layer also radiate from the optic nerve head in many cases and are not considered atypical (Fig. 3A). In later stages of the screen fundus examination was performed with un-anesthetized animals. An advantage of using non-anesthetized animals is that the lack of corneal drying results in better clarity and contrast, probably owing to better transmission through the more moist surface during handling and photography.
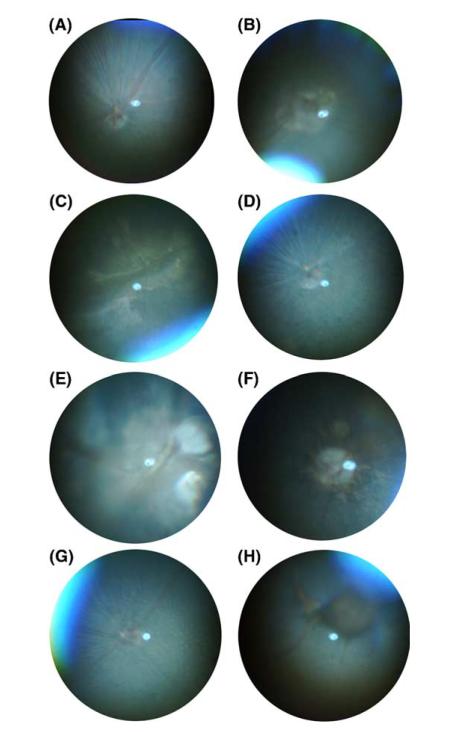
Figure 3.
Examples of fundus photographs from a normal and various putative mutant mice. (A) Normal fundus, (B) fundus lesion around optic disk, (C) heavy lesions, (D) grainy fundus texture, (E) “Morning Glory disk,” (F) fundus lesion around optic disk and flecks, (G) hypopigmented flecks throughout the fundus, (H) large areas of hyperpigmentation.
3.3. Animals with abnormal electroretinograms
Over 500 of the 9000 mice tested were found to have abnormalities of the electroretinogram when first tested. Each of these mice was retested, and 43 of them were found to have the same electroretinographic abnormality in the second test. These mice were considered putative mutants, and five types of abnormalities were most notable. (1) No detectable electroretinogram (7 putative mutants), (2) undetectable STR (4 putative mutants); (3) slow a-wave or inverted/small b-wave (16 putative mutants); (4) attenuated or inverted c-wave (12 putative mutants); and (5) inverted cone response (one putative mutant). Examples of these five categories are shown in the right column of Fig. 1. In a few cases, both the a-and c-waves or both the b-and c-waves were affected. In addition to these categories, we also found 11 putative mutants for which the amplitudes of each of the waves of the electroretinogram was decreased to about 10% of its normal value and 4 putative mutants in which the amplitude of only the a-wave was reduced or was slowed in its time course. The electroretinographic abnormality of one of these, Noerg-1, has been shown to be heritable, demonstrating that the founder was indeed a mutant. A total of 43 putative mutants with electroretinographic defects were found, and 20 of these were found to have abnormal fundus appearance. Most of these putative mutants are in the process of heritability testing at the present time, but for one of them with reduced b-wave amplitude, heritability was not confirmed after testing 30 G5 animals.
3.4. Animals with abnormal fundus appearance
A number of atypical fundus phenotypes have been identified in the offsprings of ENU treated mice (Fig. 3). These may be divided into several categories and include animals with bilateral, regularly spaced and evenly distributed hypopigmented lesions; fundus lesions around the optic disk; large areas of geographic hypopigmentation; and solitary hyperpigmented lesions of variable size; a grainy fundus texture, which likely results from a photoreceptor degeneration, similar to the appearance of eyes with retinitis pigmentosa; and a coloboma resembling the “Morning Glory” condition in human eyes. A total of 40 putative mutants displayed abnormalities of the fundus of one kind or another; for 20 of these a defect of the electroretinogram was also detected. We did not consider variations of the branching patterns of the retinal vessels to represent abnormalities, as there was considerable variation of the branching pattern from animal to animal.
The range of funduscopic lesions in the offspring of mutagenized mice is impressive, with numerous phenotypes resembling human diseases or syndromes. For example, morning glory disc anomaly in humans is a congenital defect of the optic nerve head that may be unilateral or bilateral, and may be associated with a number of other central nervous system abnormalities. In some patients, the morning glory disk is associated with mutations in the pax6 gene (Azuma et al., 2003).
Histologically, we have examined a few of these phenotypes. A grainy fundus and/or areas of pigment mottling are often associated with photoreceptor dropout. Other pigmentation abnormalities may be due to persistent vessels or neovessels in the vitreous surrounded by pigment. Some flecks may be due to the presence of pigment laden cells in the subretinal space, which may be migrating RPE cells or melanophages, as described in the rd6 mouse (Hawes et al., 2000). We are in the process of determining the heritability of these fundus phenotypes with the goal of identifying additional animal models for retinal diseases.
3.5. Practical difficulties encountered
The greatest difficulty we have faced is in breeding the putative mutant mice. In a number of cases the mice have simply failed to produce viable offspring. There are two possible reasons for this. First, the G3 mice are tested for vision when they are 12–14 weeks of age, because the vision tests require anesthesia and other behavioral tests thus need to be performed first. At this age the females have passed their reproductive peak. As C57BL/6J females often cannibalize their first litter, this short window of opportunity can cause serious problems for breeding female putative mutants. Secondly, ENU mutagenesis at the doses used in this study induces hundreds of mutations (Russell & Russell, 1996; Shedlovsky, Guenet, Johnson, & Dove, 1986), and it is likely that some mutations resulting in decreased fecundity or fertility are harbored in the genome of putative vision mutants. In fact, 19 of the putative mutants we identified so far have failed to produce any offspring. Examination of the pedigree of a G3 putative mutant may help to provide evidence for heritability of the trait for which the putative mutant was identified. When we first isolated the G3 Noerg-1 mutant founder animal, our finding that the animal had defective vision was reinforced by the simultaneous finding of other members of the pedigree that fell off the open arms of the elevated plus maze or performed abnormally in the open field maze. In fact, there have been 8 pedigrees (G1 families) for which we have identified two or more putative mutants with visual defects. If additional pedigree members are produced as a result of finding a putative mutant, the chances for identifying a fecund putative mutant with the desired trait will be increased.
We also encountered practical difficulties in recording the electroretinograms of this large number of mice. First was the difficulty in maintaining the temperature of each mouse within the normal range when many mice were being tested at one time. Second was the occasional inability to record the c-wave of the electroretinogram that resulted from failure of the DTL electrode. We discovered that this resulted from elevated electrode resistance, which made it impossible to drive the low frequency components of the c-wave into the AC-coupled recording amplifier. This could be minimized by replacing the DTL electrodes after two weeks of use.
4. Discussion
4.1. Comparison with previous studies
Recently, the amplitude and implicit times for the a-and b-waves of 8 strains of mice have been measured, among them the C57BL/6J strain (Dalke et al., 2004); the average values we measured for over 9000 G3 mice, the descendents of mutagenized C57BL/6J mice, are in agreement with these measurements. We know of no prior studies in which screening of mutagenized mice has been performed using electroretinography, but many forward genetic studies have been performed in Drosophila using electroretinography, resulting in the discovery of many gene products that control either phototransduction (Pearn, Randall, Shortridge, Burg, & Pak, 1996) or neuronal signal processing in the lamina or medulla (Poeck, Fischer, Gunning, Zipursky, & Salecker, 2001).
Another approach to identifying genes that are involved in traits which affect the visual system is to take advantage of the natural variation that occurs among inbred strains of the mouse. This approach employs QTL analysis and has been used to understand the genetic basis for differences in ganglion cell number among mouse strains. The number of retinal ganglion cells in the mouse retina depends on the strain background and can be attributed to commonly-occurring polymorphisms. In a study of 17 inbred laboratory strains and 8 wild strains of mice, it was found that the number of ganglion cells could be described with a bimodal distribution with modes at about 56,000 and about 64,000 cells (Williams, Strom, Rice, & Goldowitz, 1996). The distributions of ganglion cells for each strain had a low coefficient of variation and thus individuals from one strain could be distinguished from individuals from another strain. In order to identify the location of the genes that underlie the difference in ganglion cell number, recombinant inbred (RI) strains between a strain having a low number of ganglion cells (C57BL/6) and each of two strains having a high number of ganglion cells (DBA and C3H) were employed. These two sets of RI strains (BXD and BXH) were used to identify a locus on chromosome 11 that is responsible for the majority of the variation observed, and candidate genes in the region were identified (Williams, Strom, & Goldowitz, 1998). However, the gene(s) responsible for determining the number of ganglion cells have not been identified.
4.2. Interactions with other phenotypes
It is very important to note that mice found to be defective in other neurological tests may also be defective in vision. There are a number of mutations not thought to be “visual” that result in degenerations that affect the visual system. Among these are the Tubby mutation, the nervous mutation (see Chang et al., 2002 for review) and the fierce mutation (Young et al., 2002), none of which were originally identified by their visual phenotypes. Thus, it makes sense to examine 'other' neurological mutants for defective vision. Conversely, it is also possible that mutations found to cause visual defects act pleiotropically to produce defects in other neuronal systems.
4.3. Advantages and disadvantages of the forward genetic approach using induced mutations
There are several advantages to adopting a forward genetic approach. (1) It requires no prior knowledge of the mechanism or components of the behavior to be studied and it makes no assumptions about the genes that are involved. (2) It is often possible to isolate a number of mutant alleles of one gene that alter the function of the gene product in different ways, i.e., an allelic series. From these various alterations it is often possible to understand the function of the gene product better than by studying only the wild-type gene product. (3) Once a single gene that is essential for a visual phenotype has been found, it is possible to use this gene to facilitate the search for other genes that are essential. (4) In some instances, point mutations may be more informative than targeted null mutations. (5) Gain-of-function alleles can be identified. Finally, it is possible to achieve certain economies in the process of screening for mutants. For example, one set of mutagenized animals can be screened for several behaviors.
There are three primary disadvantages to the forward genetic approach with induced mutations, two theoretical and one practical. Theoretically, should the behavior under study require the activity of a protein that is essential for life, then mutation of the gene encoding the essential protein could have a negative effect on the general state of health of the animal. Therefore, it may be difficult to tell if the reduced performance of the animal in the behavior under study is due to a specific effect in the behavioral mechanism or is due to general deterioration. An example of this is seen in the Wheels mutation, which was identified by screening of mutagenized animals. Heterozygous animals demonstrate a lengthened circadian period (Pickard, Sollars, Rinchik, Nolan, & Bucan, 1995). However, the Wheels mutation, which is homozygous lethal, also demonstrates abnormal development of the inner ear, leading to the conclusion that the circadian defects are secondary to the inner ear defects (Nolan, Kapfhamer, & Bucan, 1997). In brief, even for a behavior as basic as the circadian clock, a mutation that alters the behavior may not be fully informative about the mechanism for the behavior. One way to distinguish a primary from a secondary defect is to characterize several behaviors. If only one is altered, this finding strengthens the argument that the single defect is primary. A second theoretical shortcoming of the approach is that one function may be performed by two gene products, either of which is sufficient to sustain the function Thus, removal of either gene product will not alter the function subserved by both. In other words, a gene must be vital in order for it to be detected by this approach. The number of vital genes in a region can be estimated by studying the frequency of lethal mutations linked to markers in the region (Shedlovsky et al., 1986). The principal practical shortcoming is that, having identified a mutant phenotype, the mutated gene still needs to be cloned and its gene product identified.
5. Summary
We have shown that it is possible to generate mutant mice that have defective vision by performing mutagenesis, breeding and highly refined screening for visual structure and function. The mutations that have been generated occurred in random locations in the genome and thus will serve as a source to discover novel vision genes from among the ca. 30% of the mammalian genes for which the function is not known.
Acknowledgments
We thank Drs. Laura Frishman, Neal Peachey and John Robson for their help in establishing a method for high-throughput electroretinography, Dr. Ilan Lampl for writing the programs for automatic data collection and analysis and Dr. John Heckenlively and Mr. Norman Hawes for help in mastering fundus photography of mice. Supported by NIH Cooperative Research Agreement U01MH61915.
References
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Azuma N, Yamaguchi Y, Handa H, Tadokoro K, Asaka A, Kawase E, et al. Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. American Journal of Human Genetics. 2003;72(6):1565–1570. doi: 10.1086/375555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Bush RA, Sieving PA. A proximal retinal component in the primate photopic ERG a-wave. Investigative Ophthalmology & Visual Science. 1994;35(2):635–645. [PubMed] [Google Scholar]
- Calderone JB, Jacobs GH. Regional variations in the relative sensitivity to UV light in the mouse retina. Visual Neuroscience. 1995;12(3):463–468. doi: 10.1017/s0952523800008361. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. Journal of Comparative Neurology. 1979;188(2):245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Research. 2002;42(4):517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. Mice all over. Foulis; London: 1966. p. 121. [Google Scholar]
- Dalke C, Loster J, Fuchs H, Gailus-Durner V, Soewarto D, Favor J, et al. Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Investigative Ophthalmology & Visual Science. 2004;45(2):601–609. doi: 10.1167/iovs.03-0561. [DOI] [PubMed] [Google Scholar]
- Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Investigative Ophthalmology & Visual Science. 1979;18(9):988–991. [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Ekesten B, Gouras P, Moschos M. Cone properties of the light-adapted murine ERG. Documenta Ophthalmologica. 1998;97(1):23–31. doi: 10.1023/a:1001869212639. [DOI] [PubMed] [Google Scholar]
- Ekesten B, Gouras P, Yamamoto S. Cone inputs to murine retinal ganglion cells. Vision Research. 2000;40(19):2573–2577. doi: 10.1016/s0042-6989(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Steinberg RH. Light-evoked increases in [K+]o in proximal portion of the dark-adapted cat retina. Journal of Neurophysiology. 1989;61(6):1233–1243. doi: 10.1152/jn.1989.61.6.1233. [DOI] [PubMed] [Google Scholar]
- Goto Y, Peachey NS, Ziroli NE, Seiple WH, Gryczan C, Pepperberg DR, et al. Rod phototransduction in transgenic mice expressing a mutant opsin gene. Journal of the Optical Society of America A—Optics & Image Science. 1996;13(3):577–585. doi: 10.1364/josaa.13.000577. [DOI] [PubMed] [Google Scholar]
- Gurevich L, Slaughter MM. Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vision Research. 1993;33(17):2431–2435. doi: 10.1016/0042-6989(93)90122-d. [DOI] [PubMed] [Google Scholar]
- Hanitzsch R, Lichtenberger T. Two neuronal retinal components of the electroretinogram c-wave. Documenta Ophthalmologica. 1997;94(3):275–285. doi: 10.1007/BF02582985. [DOI] [PubMed] [Google Scholar]
- Hawes NL, Chang B, Hageman GS, Nusinowitz S, Nishina PM, Schneider BS, et al. Retinal degeneration 6 (rd6): a new mouse model for human retinitis punctata albescens. Investigative Ophthalmology & Visual Science. 2000;41(10):3149–3157. [PubMed] [Google Scholar]
- Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Molecular Vision. 1999;5:22. [PubMed] [Google Scholar]
- Hood DC, Birch DG. Beta wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. Journal of the Optical Society of America A. 1996;13(3):623–633. doi: 10.1364/josaa.13.000623. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Deegan JFD. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353(6345):655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Kang Derwent JJ, Qtaishat NM, Pepperberg DR. Excitation and desensitization of mouse rod photoreceptors in vivo following bright adapting light. Journal of Physiology. 2002;541(Pt 1):201–218. doi: 10.1113/jphysiol.2001.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. An evaluation of what the mouse knockout experiments are telling us about mammalian behaviour. Bioessays. 1997;19(12):1091–1098. doi: 10.1002/bies.950191208. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. Journal of Neuroscience. 2000;20(15):5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Sieving PA. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Investigative Ophthalmology & Visual Science. 2001;42(1):305–312. [PubMed] [Google Scholar]
- Kong J, Gouras P. The effect of body temperature on the murine electroretinogram. Documenta Ophthalmologica. 2003;106(3):239–242. doi: 10.1023/a:1022988332578. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the USA. 1971;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna VR, Alexander KR, Peachey NS. Temporal properties of the mouse cone electroretinogram. Journal of Neurophysiology. 2002;87(1):42–48. doi: 10.1152/jn.00489.2001. [DOI] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN., Jr. UV-and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. Journal of Neuroscience. 1999;19(1):442–455. doi: 10.1523/JNEUROSCI.19-01-00442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Pugh EN., Jr. Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. Journal of Neuroscience. 1996;16(2):563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Dowling JE. Intracellular responses of the Mullercells of mudpuppy retina: their relation to b-wave of the electroretinogram. Journal of Neurophysiology. 1970;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- Naarendorp F, Williams GE. The d-wave of the rod electroretinogram of rat originates in the cone pathway. Visual Neuroscience. 1999;16(1):91–105. doi: 10.1017/s0952523899161054. [DOI] [PubMed] [Google Scholar]
- Nolan PM, Kapfhamer D, Bucan M. Random mutagenesis screen for dominant behavioral mutations in mice. Methods. 1997;13(4):379–395. doi: 10.1006/meth.1997.0545. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Oakley BD, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. Journal of Neurophysiology. 1976;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Investigative Ophthalmology & Visual Science. 1998;39(12):2443–2449. [PubMed] [Google Scholar]
- Peachey NS, Ball SL. Electrophysiological analysis of visual function in mutant mice. Documenta Ophthalmologica. 2003;107(1):13–36. doi: 10.1023/a:1024448314608. [DOI] [PubMed] [Google Scholar]
- Peachey NS, Goto Y, Quiambao AB, al-Ubaidi MR. Functional consequences of oncogene-induced photoreceptor degeneration in transgenic mice. Visual Neuroscience. 1995;12(3):513–522. doi: 10.1017/s0952523800008427. [DOI] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. Journal of Biological Chemistry. 1996;271(9):4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Sollars PJ, Rinchik EM, Nolan PM, Bucan M. Mutagenesis and behavioral screening for altered circadian activity identifies the mouse mutant, Wheels. Brain Research. 1995;705(1-2):255–266. doi: 10.1016/0006-8993(95)01171-4. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Enroth-Cugell C. Tests of the mouse visual system. Mammalian Genome. 2000;11(7):531–536. doi: 10.1007/s003350010102. [DOI] [PubMed] [Google Scholar]
- Poeck B, Fischer S, Gunning D, Zipursky SL, Salecker I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001;29(1):99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Falsini B, Lyubarsky AL. The origin of the major rod-and cone-driven components of the rodent electroretinogram and the effect of age and light-rearing history on the magnitude of these components. In: Thistle WA, editor. Photostasis and related phenomena. Plenum; New York: 1998. [Google Scholar]
- Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Visual Neuroscience. 1995;12(5):837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Photoreceptor and bipolar cell contributions to the cat electroretinogram: a kinetic model for the early part of the flash response. Journal of the Optical Society of America A. 1996;13(3):613–622. doi: 10.1364/josaa.13.000613. [DOI] [PubMed] [Google Scholar]
- Russell LB, Russell WL. Spontaneous mutations recovered as mosaics in the mouse specific-locus test [published erratum appears in Proceedings of the National Academy of Sciences USA. Proceedings of the National Academy of Sciences of the USA. 1996 1997 Apr 15;9493(8)(23):4233, 13072–13077. doi: 10.1073/pnas.93.23.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. Journal of Physiology. 2002;543(Pt 3):899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlovsky A, Guenet JL, Johnson LL, Dove WF. Induction of recessive lethal mutations in the T/t-H-2 region of the mouse genome by a point mutagen. Genetical Research. 1986;47(2):135–142. doi: 10.1017/s0016672300022977. [DOI] [PubMed] [Google Scholar]
- Silva GA, Hetling JR, Pepperberg DR. Dynamic and steady-state light adaptation of mouse rod photoreceptors in vivo. Journal of Physiology. 2001;534(Pt 1):203–216. doi: 10.1111/j.1469-7793.2001.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Documenta Ophthalmologica. 1985;60(4):327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Pinto LH, Vitaterna MH. Forward and reverse genetic approaches to behavior in the mousesee [see comments] Science. 1994;264(5166):1724–1733. doi: 10.1126/science.8209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Tian N, Slaughter MM. Correlation of dynamic responses in the ON bipolar neuron and the b-wave of the electroretinogram. Vision Research. 1995;35(10):1359–1364. doi: 10.1016/0042-6989(95)98715-l. [DOI] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Goldowitz D. Natural variation in neuron number in mice is linked to a major quantitative trait locus on Chr 11. Journal of Neuroscience. 1998;18(1):138–146. doi: 10.1523/JNEUROSCI.18-01-00138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. Journal of Neuroscience. 1996;16(22):7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-F, Ganetzky B, Haugland F, Liu A-X. Potassium currents in drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Xu X, Karwoski CJ. Current source density analysis of retinal field potentials. II. Pharmacological analysis of the b-wave and m-wave. Journal of Neurophysiology. 1994;72(1):96–105. doi: 10.1152/jn.1994.72.1.96. [DOI] [PubMed] [Google Scholar]
- Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, et al. Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behavioural Brain Research. 2002;132(2):145–158. doi: 10.1016/s0166-4328(01)00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]