Abstract
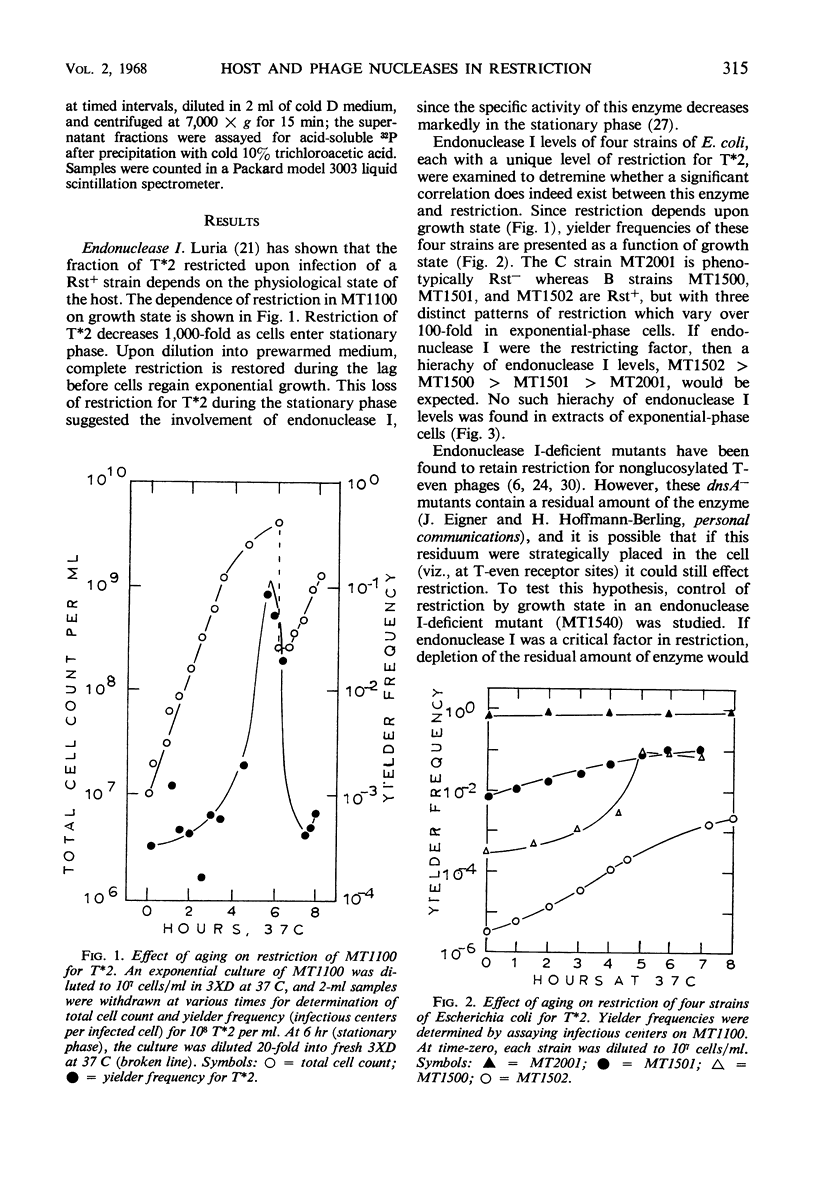
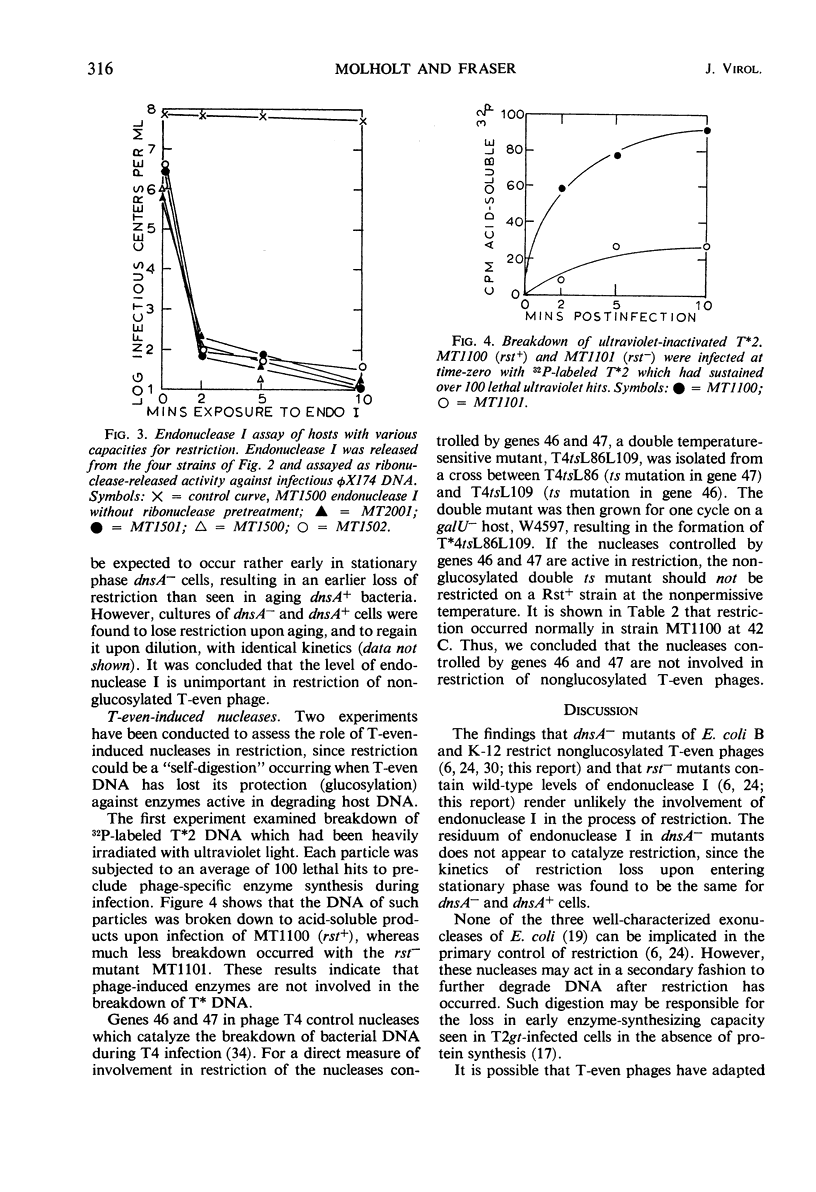
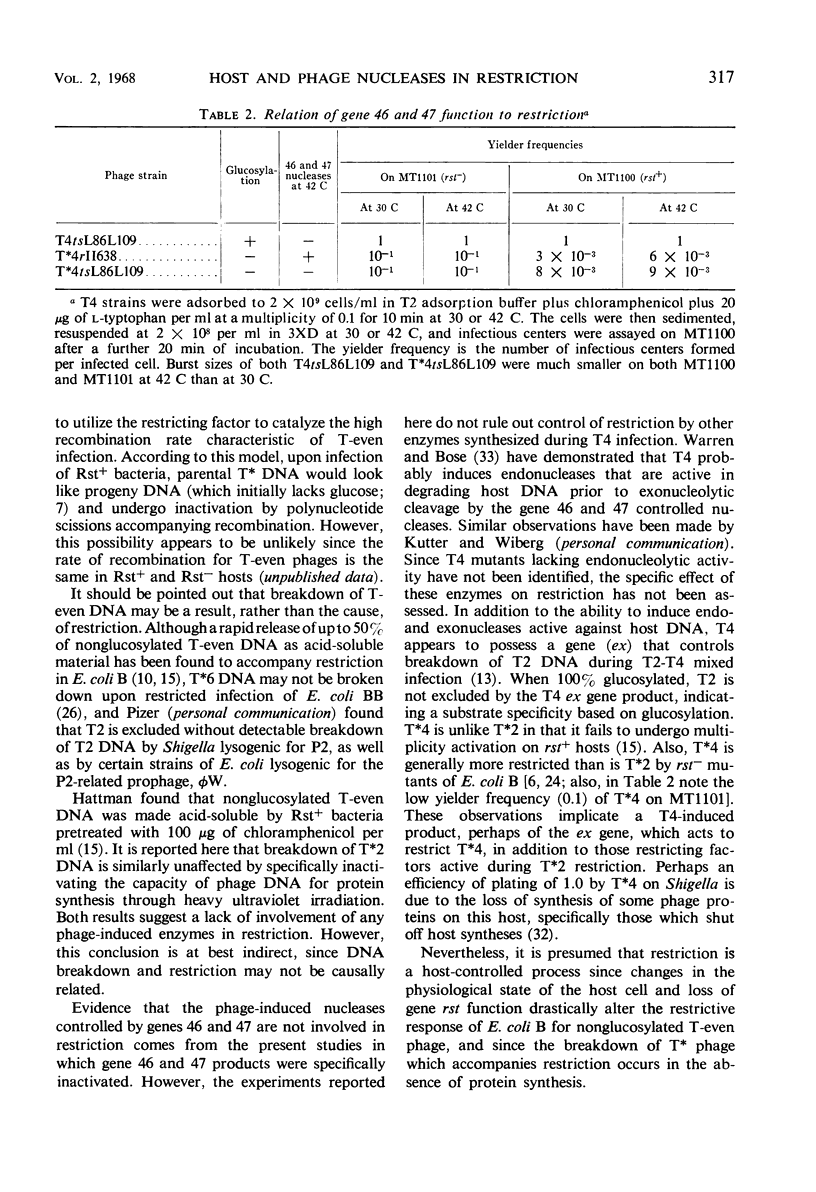
Restriction of nonglucosylated T2 phage (T*2) as a function of bacterial growth state was the same for endonuclease I-containing and endonuclease I-deficient strains of Escherichia coli B. Furthermore, E. coli strains with various levels of restriction for T2 had comparable endonuclease I activities. It was also found that a T4 mutant temperature-sensitive for gene 46 and 47 functions was fully restricted at 42 C. It therefore appears that neither endonuclease I nor the phage-induced nucleases whose activities are blocked by mutations in genes 46 and 47 catalyze the initial event in restriction of nonglucosylated T-even phages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., LATASTE-DOROLLE C. [Enlargement of the host area of bacteriophage lambda for Escherichia coli B]. Pathol Microbiol (Basel) 1961;24:1012–1018. [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. Conjugation in Escherichia coli. J Bacteriol. 1966 May;91(5):1767–1772. doi: 10.1128/jb.91.5.1767-1772.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C., Bernardi G. Localization of E. coli endonuclease I. Biochem Biophys Res Commun. 1965 Sep 8;20(5):555–559. doi: 10.1016/0006-291x(65)90434-1. [DOI] [PubMed] [Google Scholar]
- Eigner J., Block S. Host-controlled restriction of T-even bacteriophages: relation of four bacterial deoxyribonucleases to restriction. J Virol. 1968 Apr;2(4):320–326. doi: 10.1128/jvi.2.4.320-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIERS W., SINSHEIMER R. L. The structure of the DNA of bacteriophage phi-X174. III. Ultracentrifugal evidence for a ring structure. J Mol Biol. 1962 Oct;5:424–434. doi: 10.1016/s0022-2836(62)80031-x. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- FUKASAWA T., JOKURA K., KURAHASHI K. A new enzymic defect of galactose metabolism in Escherichia coli K-12 mutants. Biochem Biophys Res Commun. 1962 Apr 3;7:121–125. doi: 10.1016/0006-291x(62)90158-4. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T. THE COURSE OF INFECTION WITH ABNORMAL BACTERIOPHAGE T4 CONTAINING NON-GLUCOSYLATED DNA ON ESCHERICHIA COLI STRAINS. J Mol Biol. 1964 Aug;9:525–536. doi: 10.1016/s0022-2836(64)80224-2. [DOI] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Georgopoulos C. P. Isolation and preliminary characterization of T4 mutants with nonglucosylated DNA. Biochem Biophys Res Commun. 1967 Jul 21;28(2):179–184. doi: 10.1016/0006-291x(67)90426-3. [DOI] [PubMed] [Google Scholar]
- HATTMAN S., FUKASAWA T. HOST-INDUCED MODIFICATION OF T-EVEN PHAGES DUE TO DEFECTIVE GLUCOSYLATION OF THEIR DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:297–300. doi: 10.1073/pnas.50.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S. THE FUNCTIONING OF T-EVEN PHAGES WITH UNGLUCOSYLATED DNA IN RESTRICTING ESCHERICHIA COLI HOST CELLS. Virology. 1964 Nov;24:333–348. doi: 10.1016/0042-6822(64)90171-0. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. Some minor components of bacteriophage T2 particles. Virology. 1957 Oct;4(2):237–264. doi: 10.1016/0042-6822(57)90061-2. [DOI] [PubMed] [Google Scholar]
- Hattman S., Revel H. R., Luria S. E. Enzyme synthesis directed by nonglucosylated T-even bacteriophages in restrictive hosts. Virology. 1966 Nov;30(3):427–438. doi: 10.1016/0042-6822(66)90120-6. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., ROUSSOS G. G., PRATT E. A. The deoxyribo-nucleases of Escherichia coli. III. Studies on the nature of the inhibition of endonuclease by ribonucleic acid. J Biol Chem. 1962 Mar;237:829–833. [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Molholt B., Fraser D. Reversal of restriction for host modified T2 and T4 DNA upon conversion of non-permissive Escherichia coli to spheroplasts. Biochem Biophys Res Commun. 1965 May 18;19(5):571–575. doi: 10.1016/0006-291x(65)90376-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- REVEL H. R., HATTMAN S., LURIA S. E. MUTANTS OF BACTERIOPHAGES T2 AND T6 DEFECTIVE IN ALPHA-GLUCOSYL TRANSFERASE. Biochem Biophys Res Commun. 1965 Feb 17;18:545–550. doi: 10.1016/0006-291x(65)90788-6. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORTMAN K., LEHMAN I. R. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. VI. CHANGES IN ENZYME LEVELS IN RESPONSE TO ALTERATIONS IN PHYSIOLOGICAL STATE. J Biol Chem. 1964 Sep;239:2964–2974. [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYMONDS N., STACEY K. A., GLOVER S. W., SCHELL J., SILVER S. The chemical basis for a case of host-induced modification in phage T2. Biochem Biophys Res Commun. 1963 Jul 26;12:220–222. doi: 10.1016/0006-291x(63)90193-1. [DOI] [PubMed] [Google Scholar]
- Takano T., Watanabe T., Fukasawa T. Specific inactivation of infectious lambda DNA by sonicates of restrictive bacteria with R factors. Biochem Biophys Res Commun. 1966 Oct 20;25(2):192–198. doi: 10.1016/0006-291x(66)90579-1. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi M. Studies on the mechanism of bacteriophage T4 interference with host metabolism. J Mol Biol. 1967 Aug 28;28(1):37–44. doi: 10.1016/s0022-2836(67)80075-5. [DOI] [PubMed] [Google Scholar]
- Warren R. J., Bose S. K. Bacteriophage-induced inhibition of host functions. I. Degradation of Escherichia coli deoxyribonucleic acid after T4 infection. J Virol. 1968 Apr;2(4):327–334. doi: 10.1128/jvi.2.4.327-334.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot B. The bar-properties, in particular glucsylation of deoxyribonucleic acid, in crosses of bacteriophages T2 and T4. Genet Res. 1967 Apr;9(2):149–158. doi: 10.1017/s0016672300010454. [DOI] [PubMed] [Google Scholar]


