Abstract
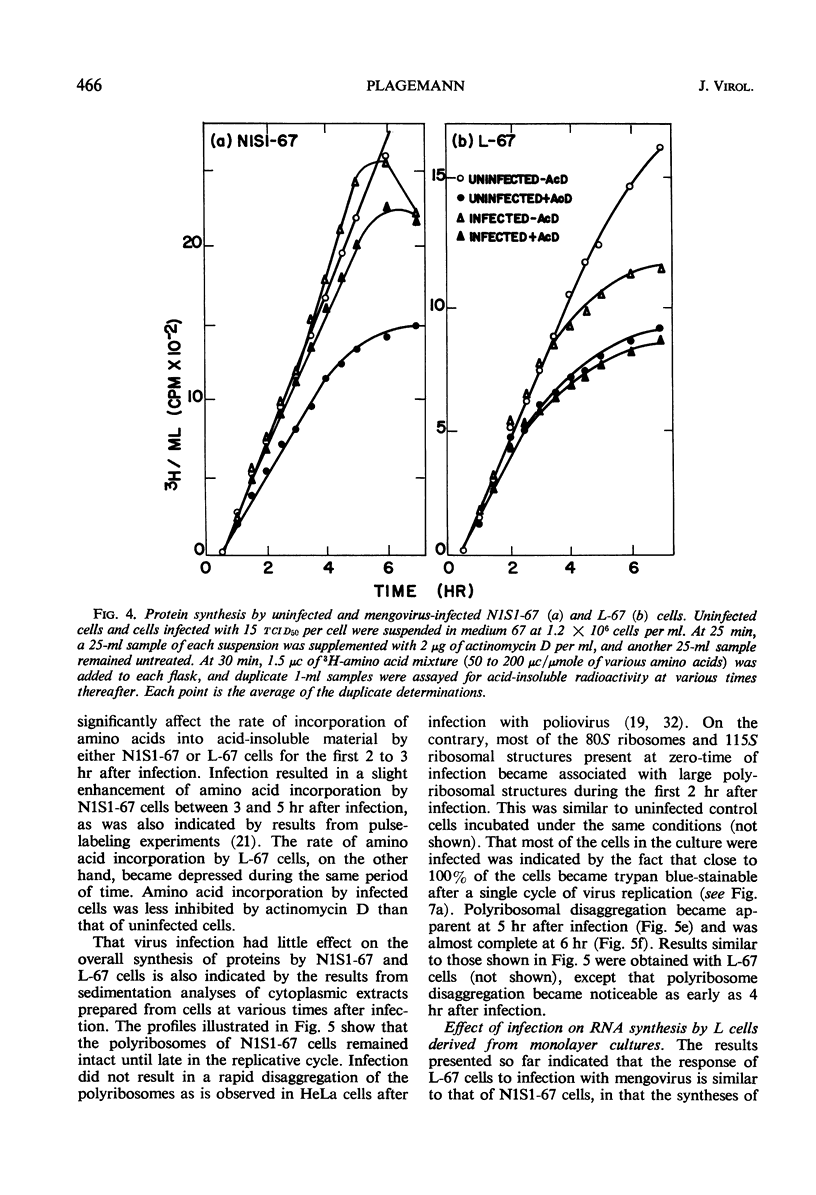
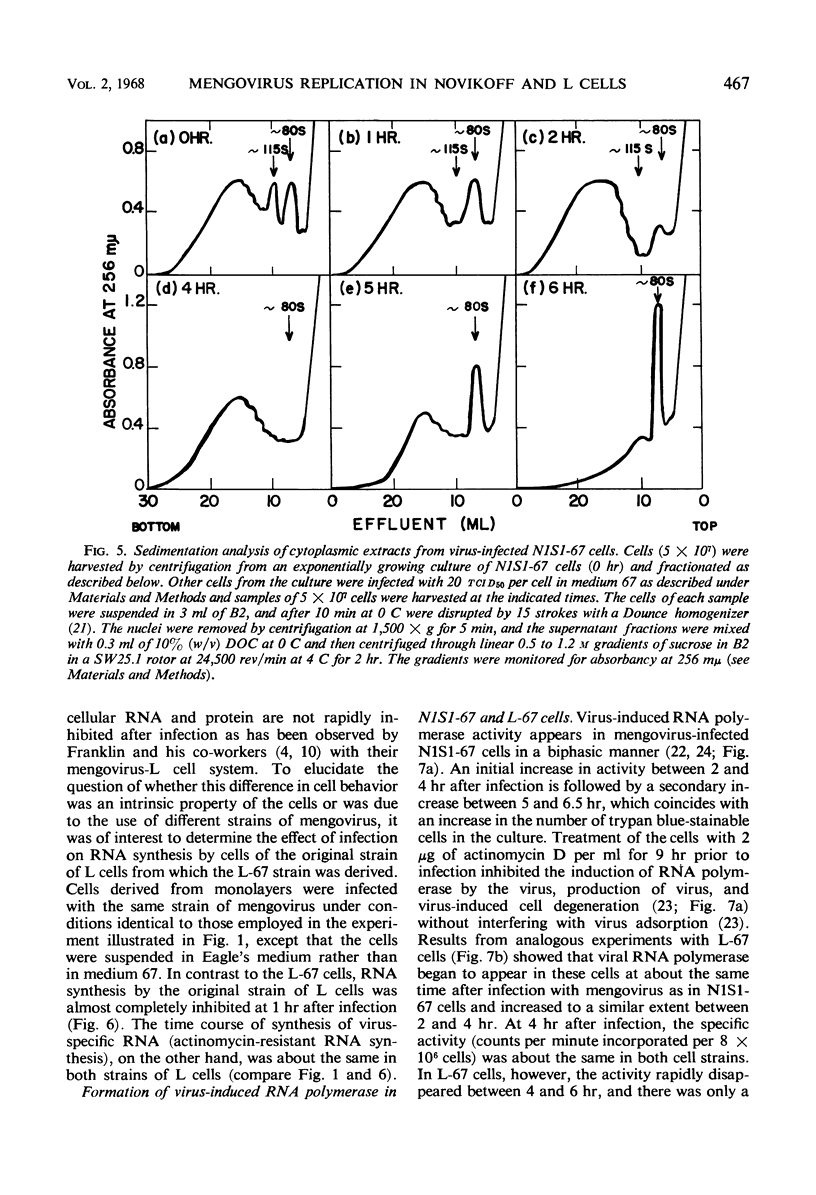
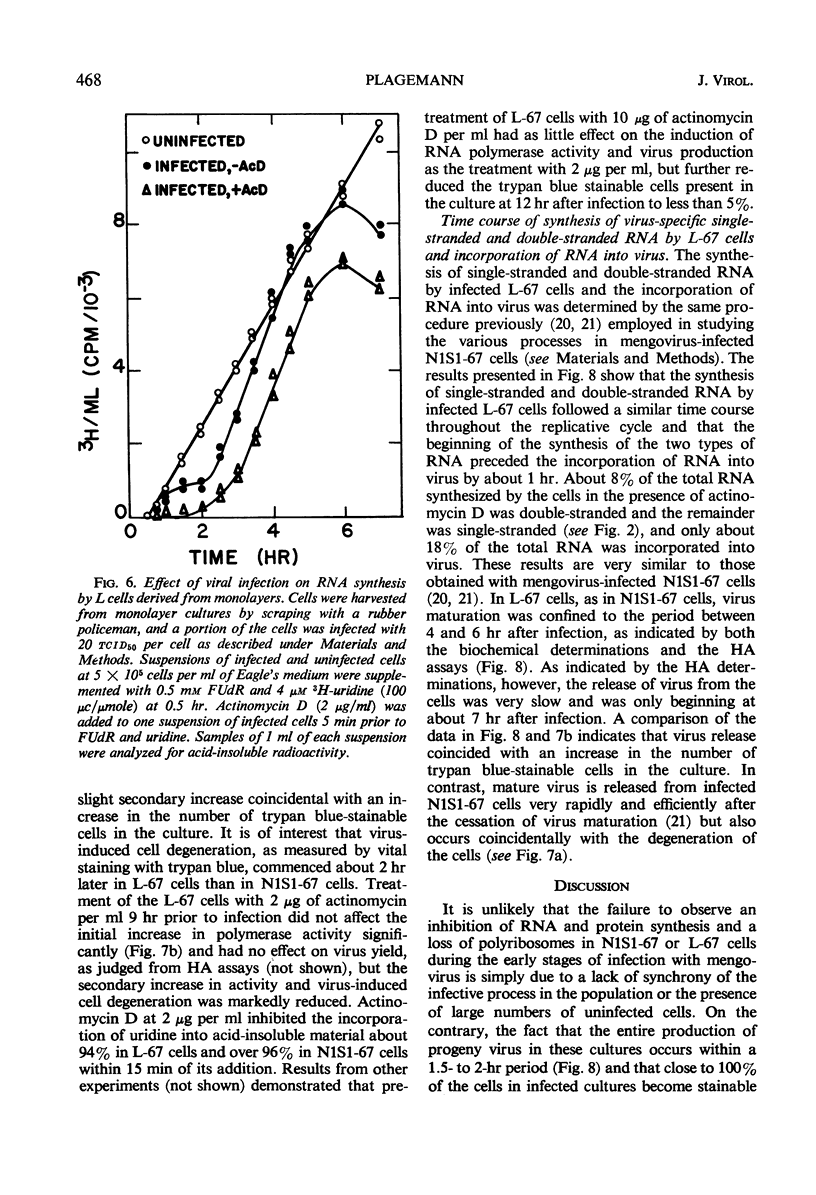
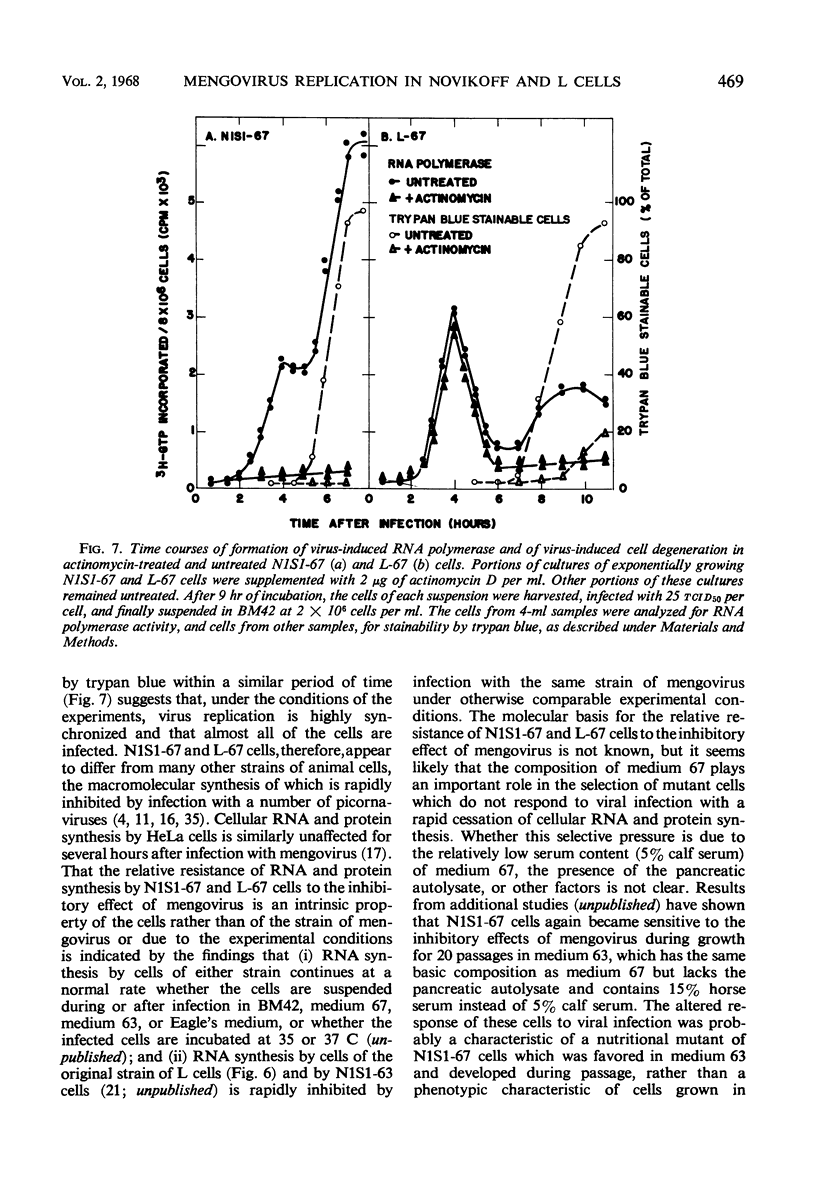
Novikoff cells (strain N1S1-67) and L-67 cells, a nutritional mutant of the common strain of mouse L cells which grows in the same medium as N1S1-67 cells, were infected with mengovirus under identical experimental conditions. The synthesis of host-cell ribonucleic acid (RNA) by either type of cell was not affected quantitatively or qualitatively until about 2 hr after infection, when viral RNA synthesis rapidly displaced the synthesis of cellular RNA. The rate of synthesis of protein by both types of cells continued at the same rate as in uninfected cells until about 3 hr after infection, and a disintegration of polyribosomes occurred only towards the end of the replicative cycle, between 5 and 6 hr. The time courses and extent of synthesis of single-stranded and double-stranded viral RNA and of the production of virus were very similar in both types of cells, in spite of the fact that the normal rate of RNA synthesis and the growth rate of uninfected N1S1-67 cells are about three times greater than those of L-67 cells. In both cells, the commencement of viral RNA synthesis coincided with the induction of viral RNA polymerase, as measured in cell-free extracts. Viral RNA polymerase activity disappeared from infected L-67 cells during the period of production of mature virus, but there was a secondary increase in activity in both types of cells coincidental with virus-induced disintegration of the host cells. Infected L-67 cells, however, disintegrated and released progeny virus much more slowly than N1S1-67 cells. The two strains of cells also differed in that replication of the same strain of mengovirus was markedly inhibited by treating N1S1-67 cells with actinomycin D prior to infection; the same treatment did not affect replication in L-67 cells.
Full text
PDF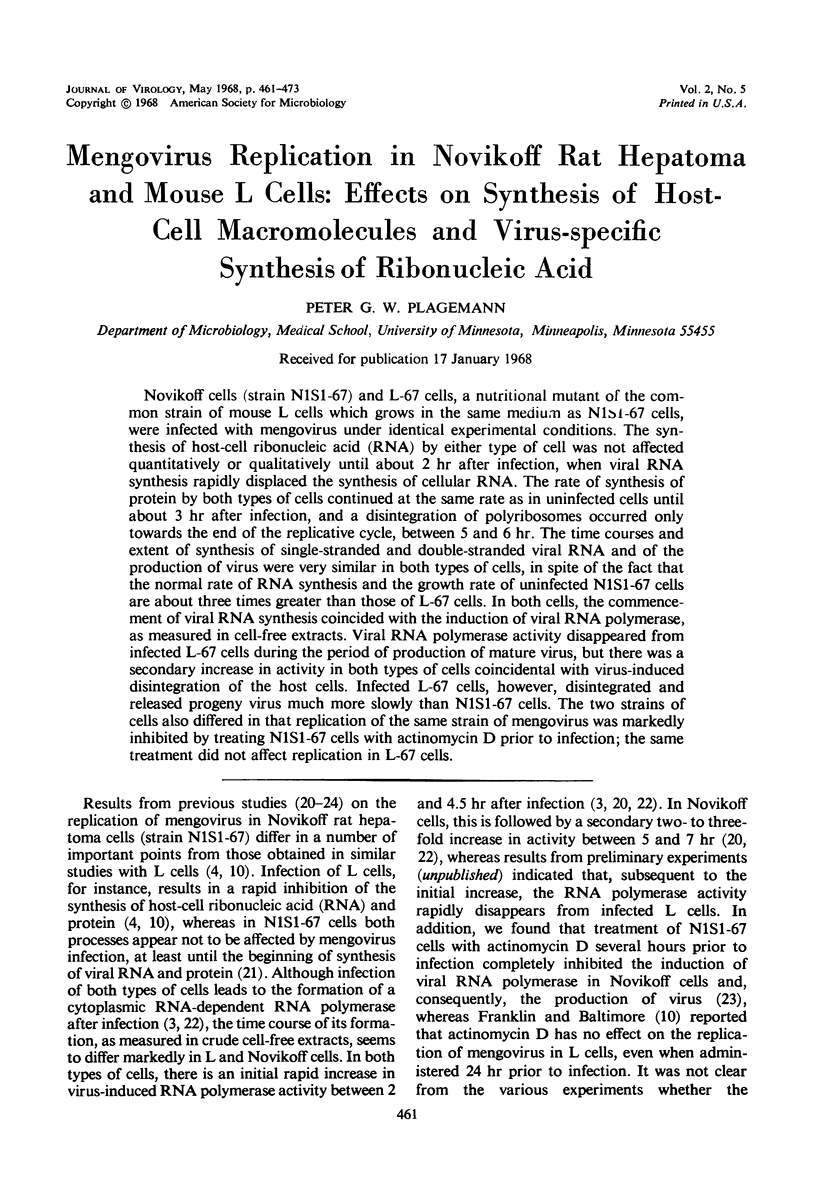

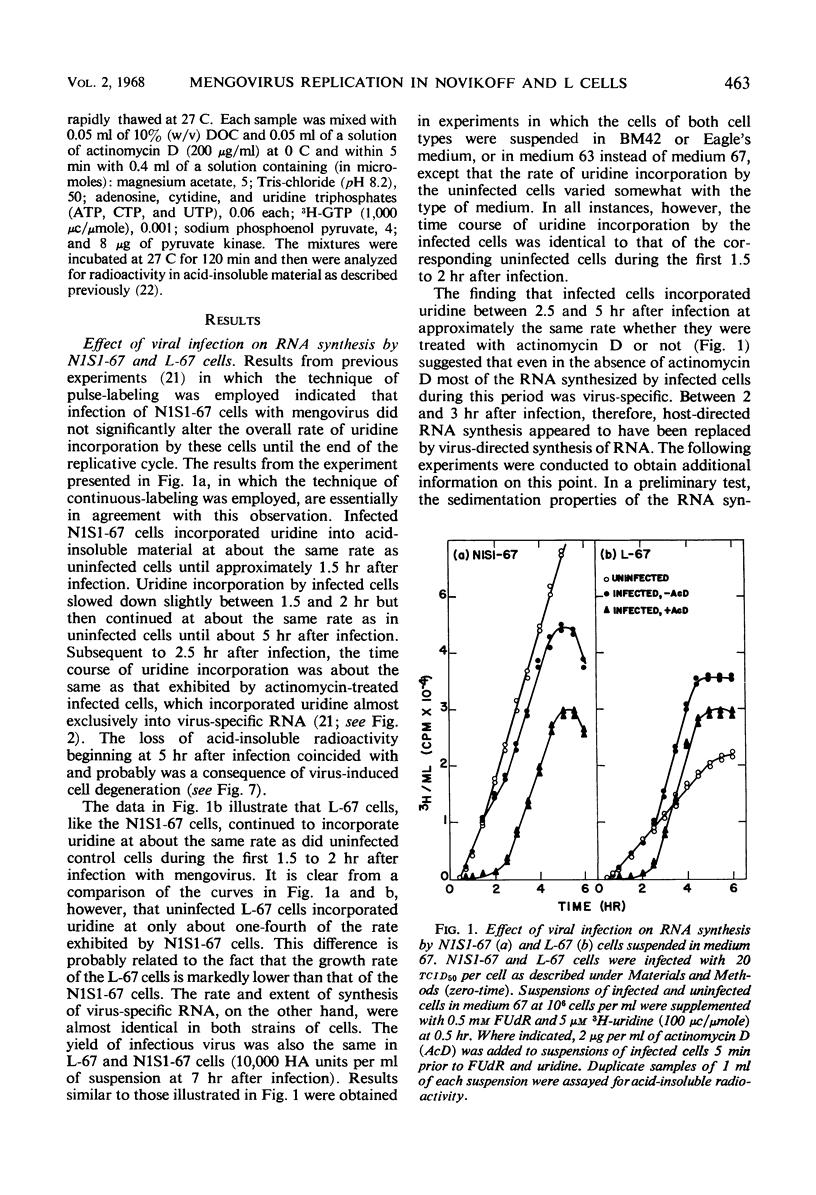
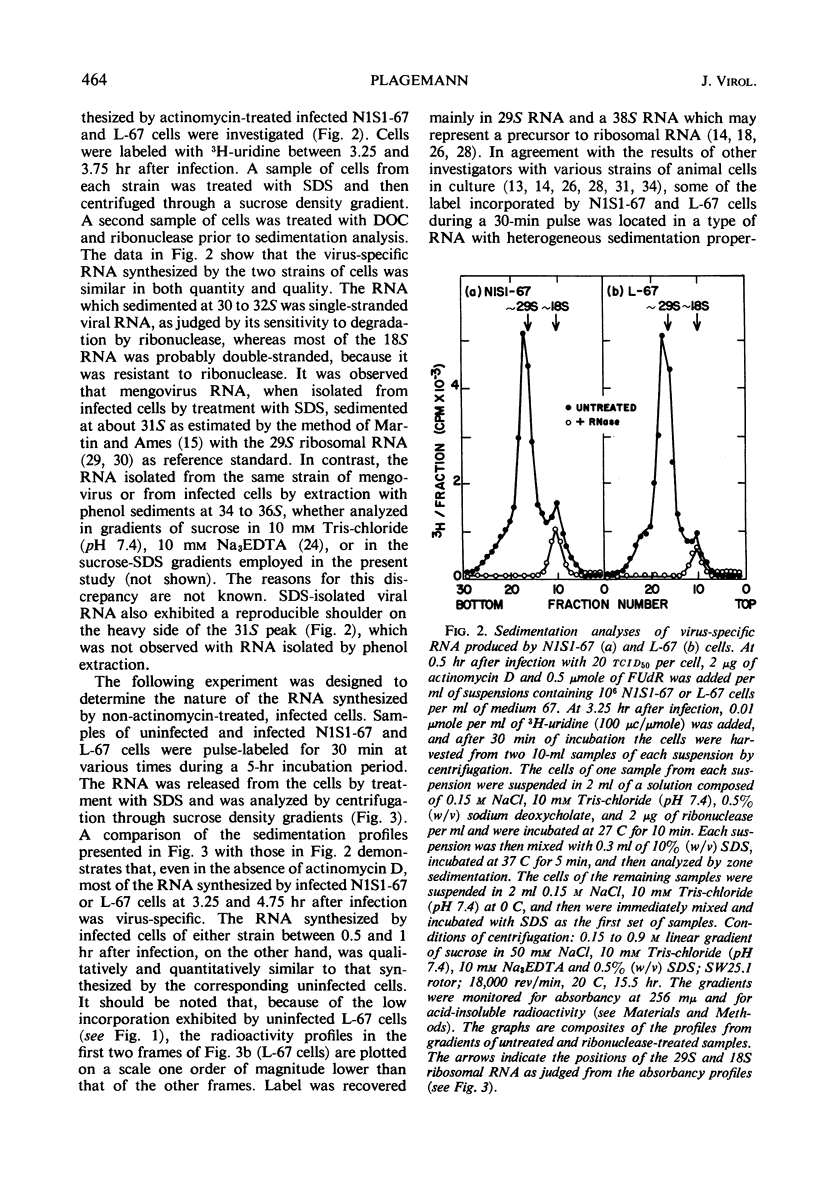
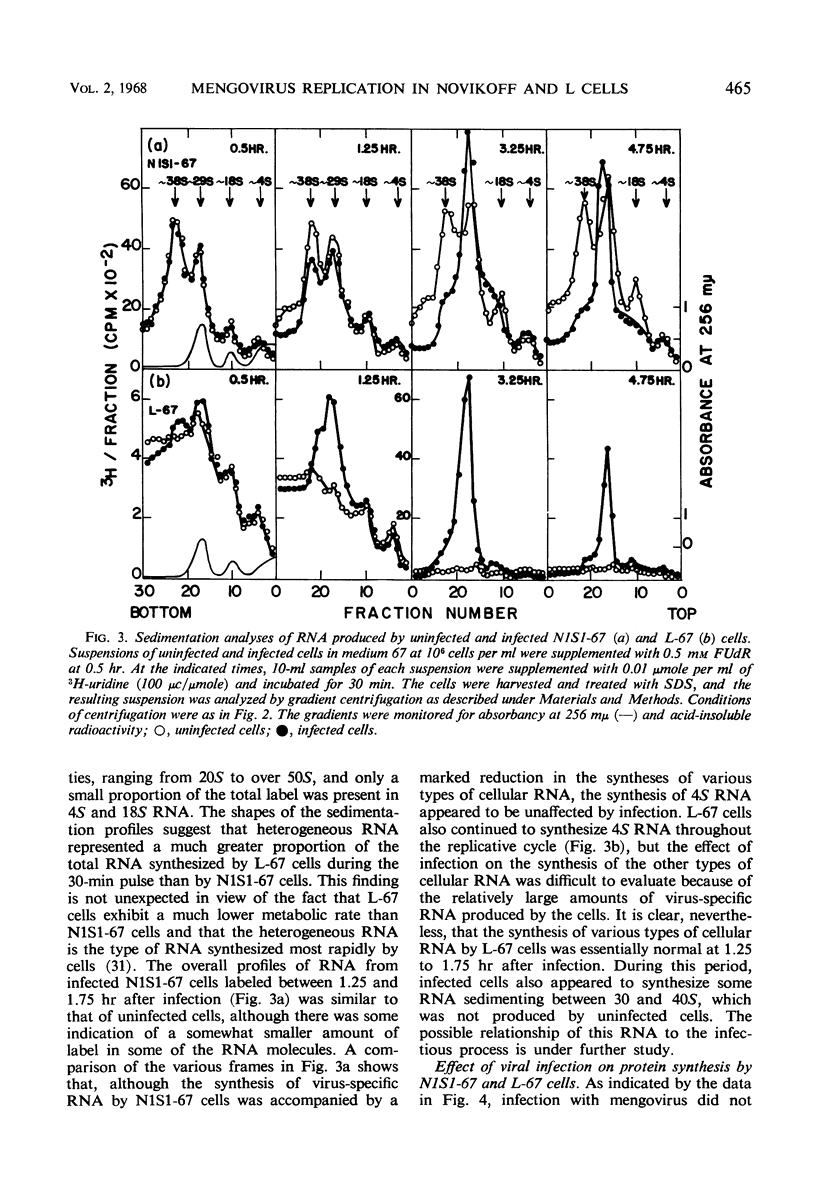



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., EGGERS H. J., FRANKLIN R. M., TAMM I. Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proc Natl Acad Sci U S A. 1963 Jun;49:843–849. doi: 10.1073/pnas.49.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M. A NEW RIBONUCLEIC ACID POLYMERASE APPEARING AFTER MENGOVIRUS INFECTION OF L-CELLS. J Biol Chem. 1963 Oct;238:3395–3400. [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M., CALLENDER J. MENGOVIRUS-INDUCED INHIBITION OF HOST RIBONUCLEIC ACID AND PROTEIN SYNTHESIS. Biochim Biophys Acta. 1963 Nov 22;76:425–430. [PubMed] [Google Scholar]
- BRAND K. G., SYVERTON J. T. Results of species-specific hemagglutination tests on "transformed," nontransformed, and primary cell cultures. J Natl Cancer Inst. 1962 Jan;28:147–157. [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Cooper P. D. The inhibition of poliovirus growth by actinomycin D and the prevention of the inhibition by pretreatment of the cells with serum or insulin. Virology. 1966 Apr;28(4):663–678. doi: 10.1016/0042-6822(66)90251-0. [DOI] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Flanagan J. F. Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J Bacteriol. 1966 Feb;91(2):789–797. doi: 10.1128/jb.91.2.789-797.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- Horton E., Liu S. L., Martin E. M., Work T. S. Properties of a virus-induced RNA polymerase in ascites cells infected with encephalomyocarditis virus. J Mol Biol. 1966 Jan;15(1):62–76. doi: 10.1016/s0022-2836(66)80209-7. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. IV. The localization of metabolic changes within subcellular fractions of Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1961 Dec;81:514–520. doi: 10.1042/bj0810514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCormick W., Penman S. Inhibition of RNA synthesis in HeLa and L cells by Mengovirus. Virology. 1967 Jan;31(1):135–141. doi: 10.1016/0042-6822(67)90017-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Hodnett J. L., Busch H. Base composition of fractions of nuclear and nucleolar ribonucleic acid obtained by sedimentation and chromatography. J Biol Chem. 1966 Apr 10;241(7):1544–1550. [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Reversible inhibition of induction of mengovirus RNA polymerase and of virus maturation in Novikoff rat hepatoma cells by phenethyl alcohol. Virology. 1968 Feb;34(2):319–330. doi: 10.1016/0042-6822(68)90242-0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. II. General properties of the viral-induced ribonucleic acid polymerase. J Bacteriol. 1966 Jun;91(6):2327–2332. doi: 10.1128/jb.91.6.2327-2332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Symposium on replication of viral nucleic acids. 3. Replication of mengovirus ribonucleic acid. Bacteriol Rev. 1966 Jun;30(2):288–308. doi: 10.1128/br.30.2.288-308.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatnick J., Arlinghaus R. B. Foot-and-mouth disease virus-induced ribonucleic acid polymerase in baby hamster kidney cells. Virology. 1967 Apr;31(4):601–608. doi: 10.1016/0042-6822(67)90188-2. [DOI] [PubMed] [Google Scholar]
- RAKE A. V., GRAHAM A. F. KINETICS OF INCORPORATION OF URIDINE-C14 INTO L CELL RNA. Biophys J. 1964 Jul;4:267–284. doi: 10.1016/s0006-3495(64)86782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Gordon M. Differential inhibitory effects of actinomycin D among strains of poliovirus. J Bacteriol. 1966 Jun;91(6):2309–2316. doi: 10.1128/jb.91.6.2309-2316.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz E., Noll H. Characterization of cytoplasmic and chloroplast polysomes in plants: evidence for three classes of ribosomal RNA in nature. Proc Natl Acad Sci U S A. 1967 Mar;57(3):774–781. doi: 10.1073/pnas.57.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology. 1966 Nov;30(3):355–367. doi: 10.1016/0042-6822(66)90114-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M. The nature of the two rapidly labelled ribonucleic acid components of animal cells in culture. Biochim Biophys Acta. 1966 Jul 20;123(1):91–101. doi: 10.1016/0005-2787(66)90162-6. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]


