Abstract
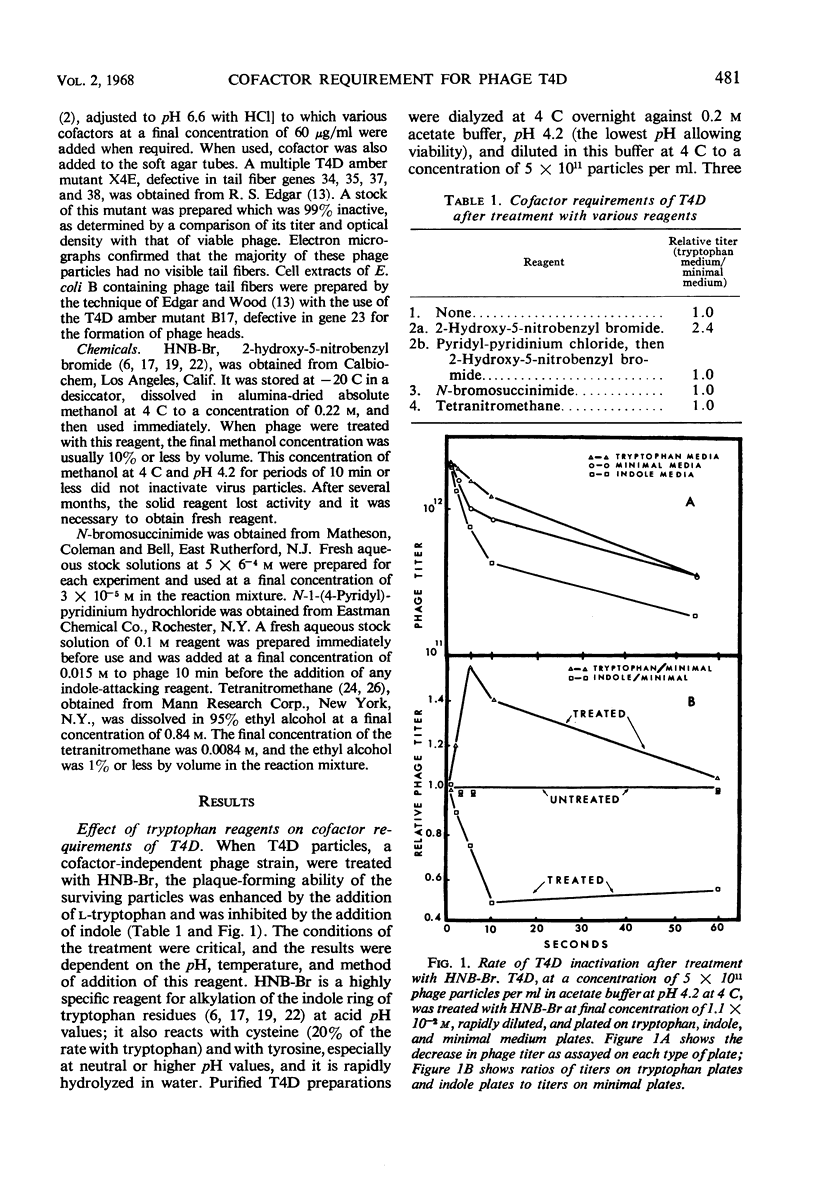
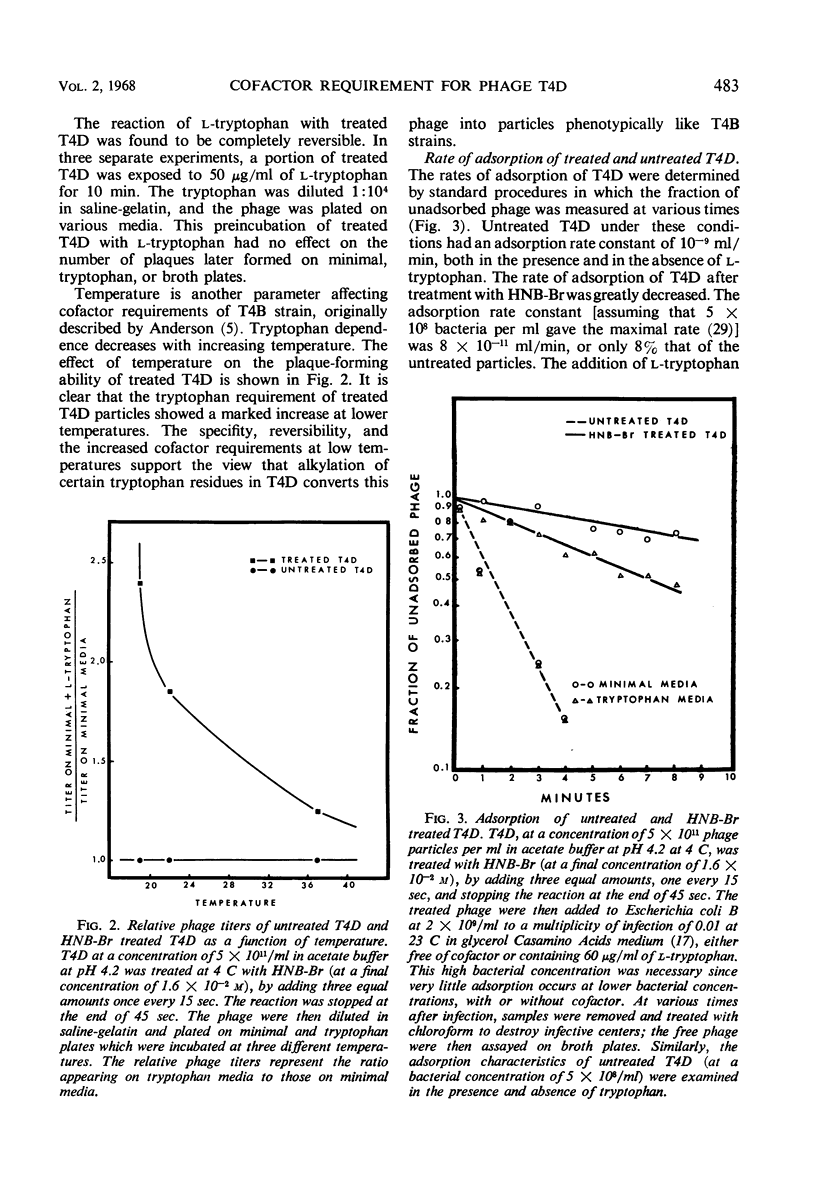
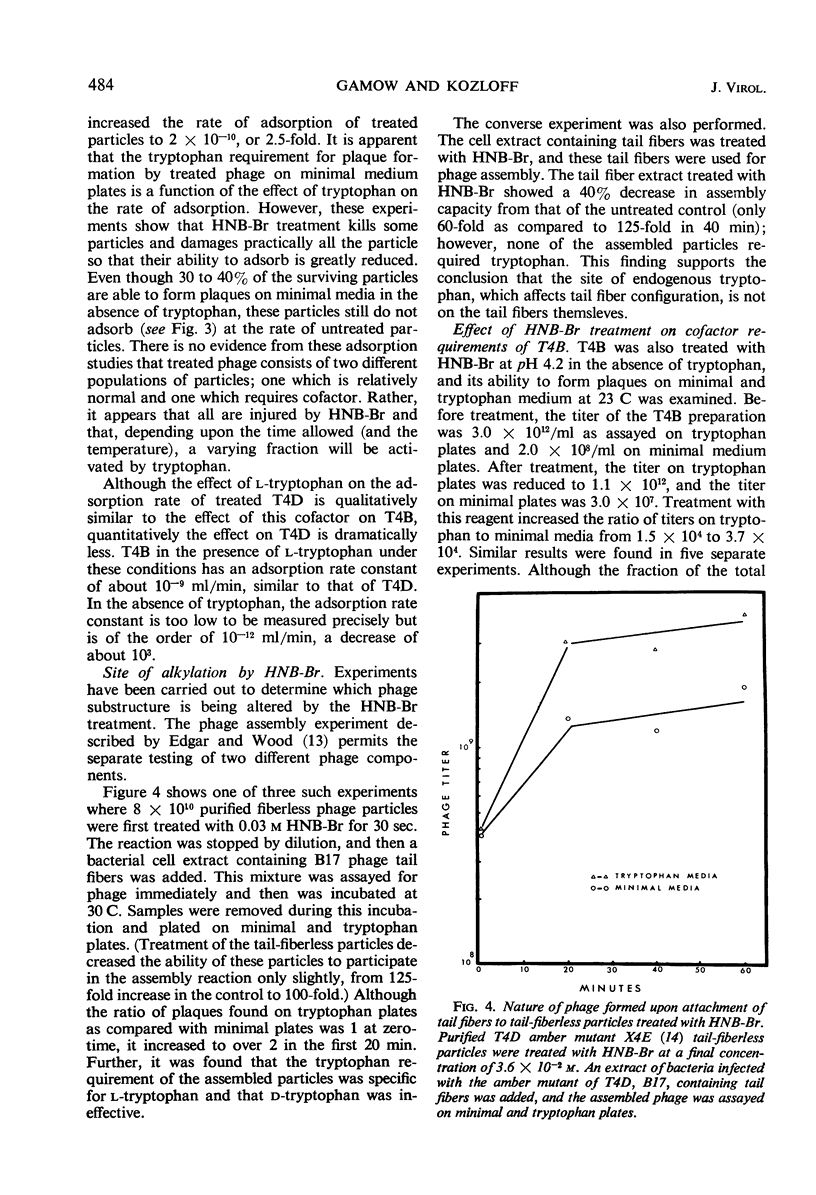
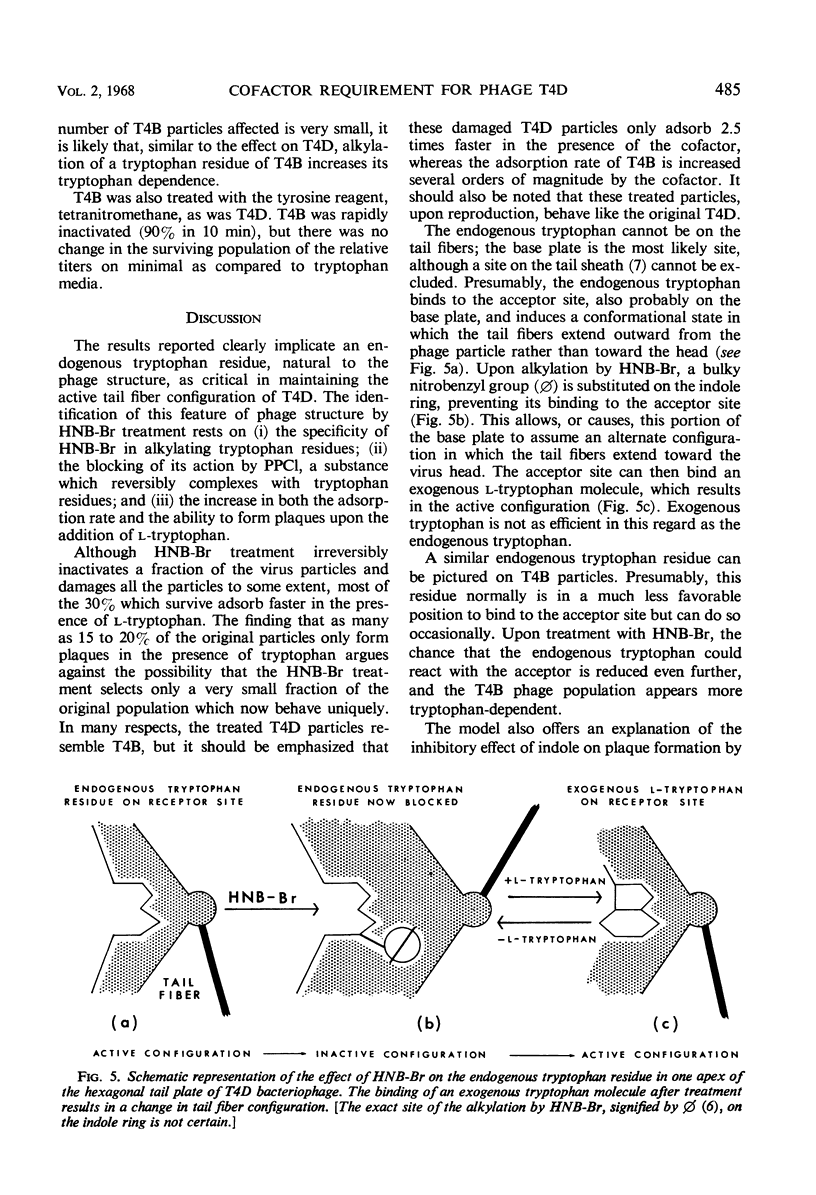
The treatment of bacteriophage T4D with 2-hydroxy-5-nitrobenzyl bromide, a specific reagent for alkylating the indole ring of tryptophan residues, converts these particles from a cofactor-independent form to a cofactor-sensitive form. These treated T4D particles phenotypically resemble T4B particles in certain respects. Their ability to form plaques on minimal medium plates is increased by the addition of l-tryptophan and is inhibited by the addition of indole. In liquid medium, their rate of adsorption is dependent on the presence of the cofactor l-tryptophan. l-Tryptophan-requiring phage have been produced by in vitro assembly of treated tail-fiberless particles of a T4D amber mutant plus untreated tail fiber preparation. When treated tail fibers were used with untreated tail-fiberless particles, the newly assembled particles did not require cofactor. A model of the tail structure of all the T-even bacteriophages is presented which postulates that the active configuration of the tail fibers requires that there be either (i) an endogenous tryptophan residue of the phage particle itself or (ii) an exogenously added l-tryptophan molecule complexed with a specific tryptophan receptor site, most likely on the phage base plate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. F. The Influence of Temperature and Nutrients on Plaque Formation by Bacteriophages Active on Escherichia coli Strain B. J Bacteriol. 1948 May;55(5):659–665. doi: 10.1128/jb.55.5.659-665.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. F. The Inheritance of Requirements for Adsorption Cofactors in the Bacterial Virus T4. J Bacteriol. 1948 May;55(5):651–658. doi: 10.1128/jb.55.5.651-658.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., CHAMPE S. P., STREISINGER G., BARNETT L. On the interaction of adsorption cofactors with bacteriophages T2 and T4. Virology. 1962 May;17:30–39. doi: 10.1016/0042-6822(62)90078-8. [DOI] [PubMed] [Google Scholar]
- BRENNER S. Genetic control and phenotypic mixing of the adsorption cofactor requirement in bacteriophages T2 and T4. Virology. 1957 Jun;3(3):560–574. doi: 10.1016/0042-6822(57)90010-7. [DOI] [PubMed] [Google Scholar]
- Barman T. E., Koshland D. E., Jr A colorimetric procedure for the quantitative determination of tryptophan residues in proteins. J Biol Chem. 1967 Dec 25;242(23):5771–5776. [PubMed] [Google Scholar]
- CHENG P. Y. The effect of L-tryptophan on thermal stability of bacteriophage T4, 38. Biochim Biophys Acta. 1956 Dec;22(3):433–442. doi: 10.1016/0006-3002(56)90052-x. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Davidson B., Westley J. Tryptophan in the active site of rhodanese. J Biol Chem. 1965 Nov;240(11):4463–4469. [PubMed] [Google Scholar]
- Delbrück M. Biochemical Mutants of Bacterial Viruses. J Bacteriol. 1948 Jul;56(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILDES P., KAY D. The conditions which govern the adsorption of a tryptophan-dependent bacteriophage to kaolin and bacteria. J Gen Microbiol. 1963 Feb;30:183–191. doi: 10.1099/00221287-30-2-183. [DOI] [PubMed] [Google Scholar]
- FRANKLIN N. C. Serological study of tail structure and function in coliphages T2 and T4. Virology. 1961 Aug;14:417–429. doi: 10.1016/0042-6822(61)90333-6. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- HORTON H. R., KELLY H., KOSHLAND D. E., Jr ENVIRONMENTALLY SENSITIVE PROTEIN REAGENTS. 2-METHOXY-5-NITROBENZYL BROMIDE. J Biol Chem. 1965 Feb;240:722–724. [PubMed] [Google Scholar]
- HORTON H. R., KOSHLAND D. E., Jr A HIGHLY REACTIVE COLORED REAGENT WITH SELECTIVITY FOR THE TRYPTOPHAN RESIDUE IN PROTEINS. 2-HYDROXY-5-NITROBENZYL BROMIDE. J Am Chem Soc. 1965 Mar 5;87:1126–1132. doi: 10.1021/ja01083a033. [DOI] [PubMed] [Google Scholar]
- Hillcoat B. L., Perkins J. P., Bertino J. R. Dihydrofolate reductase from the L1210 R murine lymphoma. Further studies on the binding of substrates and inhibitors to the enzyme. J Biol Chem. 1967 Oct 25;242(20):4777–4781. [PubMed] [Google Scholar]
- KANNER L. C., KOZLOFF L. M. THE REACTION OF INDOLE AND T2 BACTERIOPHAGE. Biochemistry. 1964 Feb;3:215–223. doi: 10.1021/bi00890a013. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M. Folic acid, a structural component of T4 bacteriophage. J Mol Biol. 1965 Jul;12(3):780–792. doi: 10.1016/s0022-2836(65)80327-8. [DOI] [PubMed] [Google Scholar]
- STENT G. S., WOLLMAN E. L. On the two step nature of bacteriophage absorption. Biochim Biophys Acta. 1952 Mar;8(3):260–269. doi: 10.1016/0006-3002(52)90041-3. [DOI] [PubMed] [Google Scholar]
- STENT G. S., WOLLMAN E. L. Studies on activation of T4 bacteriophage by cofactor. II. The mechanism of activation. Biochim Biophys Acta. 1950 Nov;6(2):307–316. doi: 10.1016/0006-3002(50)90104-1. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- VISWANATHA T., LAWSON W. B., WITKOP B. The action of N-bromosuccinimide on trypsinogen and its derivatives. Biochim Biophys Acta. 1960 May 20;40:216–224. doi: 10.1016/0006-3002(60)91345-7. [DOI] [PubMed] [Google Scholar]


