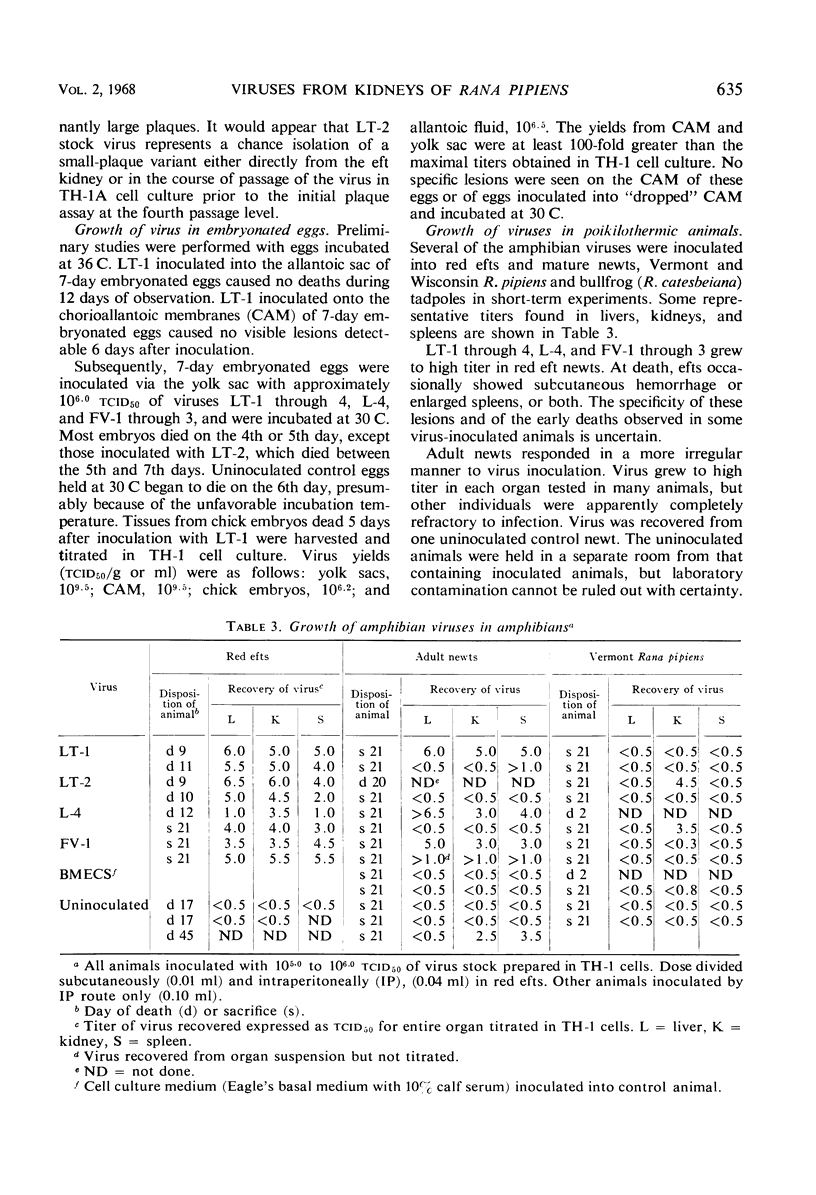
Abstract
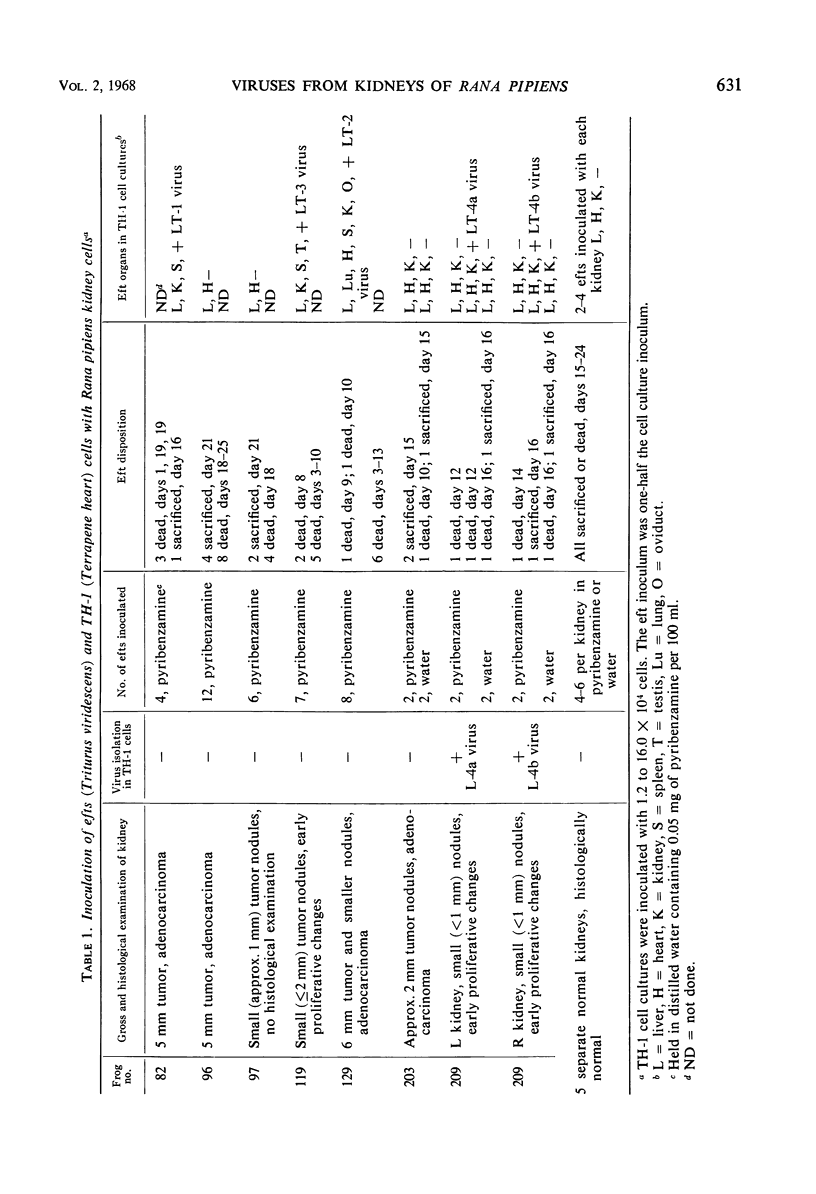
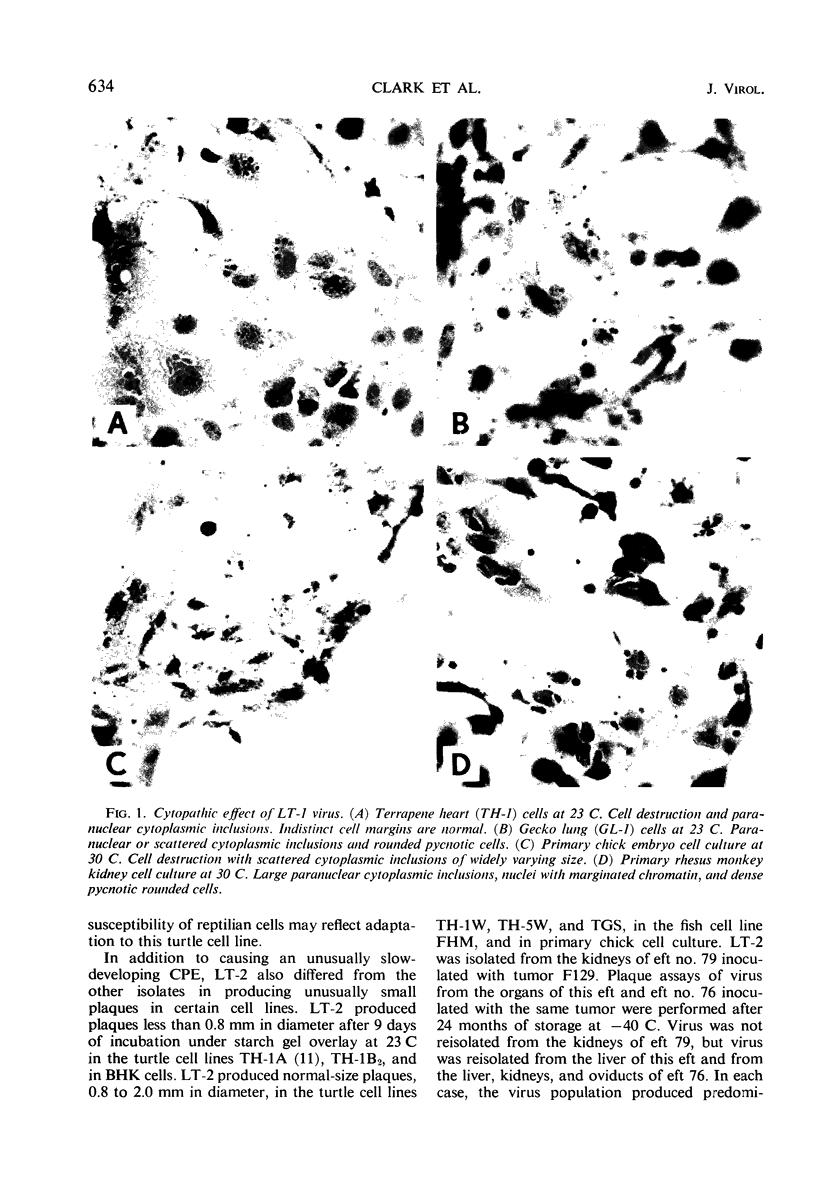
Viruses were isolated from kidneys of normal and renal tumor-bearing Vermont Rana pipiens after subinoculation into red eft newts (Triturus viridescens). Organs of efts inoculated with viable cell suspensions from four of seven tumor-bearing kidneys yielded virus (LT-1, -2, -3, -4) when inoculated into TH-1 (Terrapene heart) cell culture. One tumor-bearing kidney also yielded virus (L-4) by direct inoculation into TH-1 cells. An additional isolate (L-5) was obtained from 1 of 52 normal Vermont frog kidneys inoculated directly into TH-1 cells. LT-1 was propagated with cytopathic effect (CPE) in each of 38 cell types tested, of fish, amphibian, reptilian, avian, and mammalian origin, at 23 or 30 C. LT-1 through LT-4, L-4 and L-5, and FV-1 through FV-3 each induced similar CPE in all cells tested. LT-2, however, induced CPE that progressed at a slower rate than that caused by the other isolates and produced smaller plaques (<0.8 mm) under starch gel overlay. Each of the viruses replicated to high titer in embryonated eggs incubated at 30 C. The viruses also grew in efts and adult newts, but not in bullfrog (Rana catesbeiana) tadpoles or adult leopard frogs. Tumor induction in adult leopard frogs inoculated with LT-1 was not demonstrated. Electron microscopic observations of LT-1 and LT-2 viruses revealed cytoplasmic particles, hexagonal in cross section, approximately 120 to 140 mμ in diameter, containing a dense nucleoid. LT-1 and LT-2 viruses were indistinguishable from FV-1 and Tipula iridescent virus. LT-1 was presumed to be a deoxyribonucleic acid virus on the basis of 5-bromodeoxyuridine inhibition. The isolates were ether-sensitive. On the basis of biological, physicochemical, and antigenic similarities, LT-1 through LT-4, L-4, L-5, FV-1 through FV-3, and isolates recently recovered from the bullfrog and the newt may represent strains of the same amphibian cytoplasmic virus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLEM L. W., MOEWUS L., SIGEL M. M. Studies with cells from marine fish in tissue culture. Proc Soc Exp Biol Med. 1961 Dec;108:762–766. doi: 10.3181/00379727-108-27059. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Terrapene heart (TH-1), a continuous cell line from the heart of the box turtle Terrapene carolina. Exp Cell Res. 1967 Nov;48(2):263–268. doi: 10.1016/0014-4827(67)90351-5. [DOI] [PubMed] [Google Scholar]
- DURYEE W. R. The mechanism of virus-induced transformations in tubules of the frog kidney. Acta Unio Int Contra Cancrum. 1959;15:587–594. [PubMed] [Google Scholar]
- Darlington R. W., Granoff A., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. II. Ultrastructural studies and sequential development of virus isolated from normal and tumor tissue. Virology. 1966 May;29(1):149–156. doi: 10.1016/0042-6822(66)90204-2. [DOI] [PubMed] [Google Scholar]
- Duryee W. R. Factors influencing development of tumors in frogs. Ann N Y Acad Sci. 1965 Aug 10;126(1):59–84. doi: 10.1111/j.1749-6632.1965.tb14268.x. [DOI] [PubMed] [Google Scholar]
- FAWCETT D. W. Electron microscope observations on intracellular virus-like particles associated with the cells of the Lucké renal adenocarcinoma. J Biophys Biochem Cytol. 1956 Nov 25;2(6):725–741. doi: 10.1083/jcb.2.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. J., Rosenfeld S. J. Frog renal adenocarcinoma: cytological studies in situ and in vitro. Ann N Y Acad Sci. 1965 Aug 10;126(1):99–114. doi: 10.1111/j.1749-6632.1965.tb14270.x. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Rafferty K. A., Jr The isolation and properties of viruses from Rana pipiens: their possible relationship to the renal adenocarcinoma of the leopard frog. Ann N Y Acad Sci. 1965 Aug 10;126(1):237–255. doi: 10.1111/j.1749-6632.1965.tb14278.x. [DOI] [PubMed] [Google Scholar]
- Gravell M., Malsberger R. G. A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci. 1965 Aug 10;126(1):555–565. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- LUNGER P. D. THE ISOLATION AND MORPHOLOGY OF THE LUCK'E FROG KIDNEY TUMOR VIRUS. Virology. 1964 Oct;24:138–145. doi: 10.1016/0042-6822(64)90096-0. [DOI] [PubMed] [Google Scholar]
- Lehane D. E., Jr, Clark H. F., Karzon D. T. A plaque method for titration of frog viruses using starch gel overlay. Proc Soc Exp Biol Med. 1967 May;125(1):50–54. doi: 10.3181/00379727-125-32010. [DOI] [PubMed] [Google Scholar]
- Lunger P. D., Darlington R. W., Granoff A. Cell-virus relationships in the Lucké renal adenocarcinoma: an ultrastructure study. Ann N Y Acad Sci. 1965 Aug 10;126(1):289–314. doi: 10.1111/j.1749-6632.1965.tb14281.x. [DOI] [PubMed] [Google Scholar]
- NORDSIEK F. W., KOPAC M. J. NUCLEOLAR ABERRATIONS IN FROG KIDNEY TUMOUR CELLS. Nature. 1964 Feb 22;201:840–841. doi: 10.1038/201840a0. [DOI] [PubMed] [Google Scholar]
- ROSE S. M., ROSE F. C. Tumor agent transformations in Amphibia. Cancer Res. 1952 Jan;12(1):1–12. [PubMed] [Google Scholar]
- Rafferty K. A., Jr The cultivation of inclusion-associated viruses from Lucké tumor frogs. Ann N Y Acad Sci. 1965 Aug 10;126(1):3–21. doi: 10.1111/j.1749-6632.1965.tb14266.x. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Habas J. A. Relation of infection to tissue temperature in mice infected with Mycobacterium marinum and Mycobacterium leprae. J Bacteriol. 1967 Mar;93(3):790–796. doi: 10.1128/jb.93.3.790-796.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. AMPHIBIAN CELL CULTURE: PERMANENT CELL LINE FROM THE BULLFROG (RANA CATESBEIANA). Science. 1964 Jun 26;144(3626):1578–1580. doi: 10.1126/science.144.3626.1578. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]