Abstract
Background
Cystic fibrosis (CF) has multiple effects on the gastrointestinal system, including altered motility. The Cftr knockout mouse model of CF has impaired small intestinal transit but the mechanism is unknown.
Methods
Behavior of circular smooth muscle was studied in an organ bath. Expression levels of prostaglandin (PG) degradative genes was measured by quantitative RT-PCR, and PGE2 levels were measured by enzyme immunoassay.
Key Results
CF circular muscle activity was erratic and had variable frequency of contractions, as compared to WT. The CF tissue was nonresponsive to cholinergic stimulation or direct KCl depolarization. PGE2 and PGF2α are significantly elevated in the CF mouse small intestine, and we hypothesized these contribute to impaired smooth muscle activity. After inhibition of PG synthesis, the CF circular muscle exhibited greater cholinergic responsiveness, which was reversed by exogenous PGE2. PGF2α enhanced activity of CF tissue only after inhibition of PG synthesis. The enteric microbiota was implicated in PGE2 mediated dysmotility because broad spectrum antibiotic treated WT mice, which have slowed transit, exhibit impaired circular muscle activity. This was accompanied by decreased expression of PG degradative genes and increased intestinal PGE2 levels. Furthermore, administration of oral laxative, which eradicates bacterial overgrowth and improves transit in CF mice, increased expression of PG degradative genes, decreased PGE2 levels, and improved CF muscle activity.
Conclusions and Inferences
These results suggest that the enteric microbiota modulates PGE2 levels in a complex manner, which affects enteric smooth muscle activity and contributes to slower small intestinal transit in CF.
Keywords: circular smooth muscle, cystic fibrosis, enteric microbiota, hydroxyprostaglandin dehydrogenase, prostaglandin reductase 1
Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CFTR is a cAMPregulated Cl- channel whose activity is required for proper hydration and regulation of pH in the lumen of various epithelial organs. In CF, the intestinal lumen has decreased fluid volume and an abnormally acidic pH. These conditions cause accumulation of mucus in the intestine (7) which can foster abnormal bacterial colonization of the small intestine, resulting in an innate response that is proposed to alter gut function (8, 13, 24).
The Cftr knockout mouse (Cftrtm1UNC) exhibits symptoms similar to CF patients that include intestinal obstruction by mucofeculant material (35), small intestinal bacterial overgrowth (24), altered innate immune response (8, 26), altered gastrointestinal motility (10), and nutritional deficiencies that cause failure-to-thrive (6, 25, 28).
We have shown that small intestinal transit is dramatically slower in the CF mouse small intestine (10). When CF mice are treated with an osmotic laxative to improve lumen hydration, bacterial overgrowth is eradicated, inflammation is significantly attenuated, and transit is normalized (13). These data are compatible with the model that lumen dehydration fosters abnormal bacterial colonization, leading to an immune response which in turn alters enteric smooth muscle function. Impaired intestinal transit is expected to allow progression to small intestinal bacterial overgrowth.
We showed previously there is a remarkable influx of mast cells into the muscularis externa of the CF mouse small intestine (26) which might affect enteric nerves and smooth muscle function. However, mast cell stabilizing drugs failed to improve small intestinal transit in CF mice (12), so another explanation was sought.
The innate response in the CF mouse small intestine includes altered eicosanoid metabolism which might affect gut motility. Expression of the major prostaglandin (PG) degradative genes [hydroxyprostaglandin dehydrogenase (Hpgd) and prostaglandin reductase 1 (Ptgr1, previously referred to as leukotriene B4 dehydrogenase] is strongly reduced in the CF mouse intestine, resulting in increased PGE2 and PGF2α (11). Expression of prostaglandin synthetic enzyme genes, including the cyclooxygenases 1 and 2, is not altered (11). Among the known functions of PGs are effects on the intestinal muscularis externa (2, 4, 15, 29, 30, 33, 36). In the current study, we tested the hypothesis that elevated PGE2 levels in the CF mouse small intestine are involved in smooth muscle dysfunction.
Materials and Methods
Animals
Cftr(+/-) mice (Cftrtm1UNC), congenic on the C57BL/6J background, were bred to obtain littermate WT [Cftr(+/+)] and CF [Cftr(-/-)] mice as described (26). Mice were kept in a specific pathogen free facility in filter topped cages. Mice of both sexes were used at 6-12 weeks of age. To prevent lethal intestinal obstruction, CF mice were maintained on a complete elemental liquid diet (Peptamen; Nestle, Deerfield, IL), and littermate WT mice were also maintained on this diet (26). For some experiments, broad spectrum antibiotics were added to the liquid diet for a period of three weeks to alter the enteric microbiota (ciprofloxacin, 50 mg/kg per day; and metronidazole, 100 mg/kg per day). In other experiments, CF mice and their control WT littermates were fed solid chow instead of the liquid diet, and their drinking water was replaced with an osmotic laxative solution to prevent intestinal obstruction as previously described (13). All animal work was approved by the Institutional Animal Care and Use Committee.
Organ bath analysis of enteric circular muscle activity
The small intestine was resected into room temperature phosphate-buffered saline (PBS). A ring of small intestine (0.5 cm) was taken 5-7 cm distal to the gastro-duodenal junction and was mounted with stainless steel holders in a 20 ml bath filled with Krebs-Henseleit bicarbonate buffer. The bath was maintained at 37°C and continuously gassed with 95% O2/5% CO2. Isometric force data were recorded using an MP35 System and BSL Pro software (Biopac, Goleta, CA). The tension was adjusted to 0.5 g and the tissue was equilibrated for 1 hr. The buffer was changed every 30 min throughout the experiments. At the end of an experiment the blotted wet weight of the tissue was used to normalize the force data. The thickness of the muscularis externa does not differ significantly in the CF intestine as compared to WT (3, 13) (Supplemental Fig.1C and D).
The cholinergic agonist carbachol (CCh) was added to the bath at 2 min intervals in 10-fold steps from 10−8M to 10−4M to stimulate activity. The effect of PGs on muscle contractile behavior was tested by inhibiting PG synthesis with the cyclooxygenase inhibitor indomethacin (10−5 M), added 1 hr before retesting the CCh-response. The effects of exogenous PGE2 (10−6M) and PGF2α (10−6M) (Cayman Chemical, Ann Arbor, MI) were tested before and after pretreatment with indomethacin. In some experiments, the inhibitors tetrodotoxin (10−6M final) and NG-nitro-L-arginine methyl ester (10−4M final) were used as indicated. Contractile activity data were analyzed using Biopac and PeakFit software (Systat, Chicago, IL). Data were averaged from (n) individual animals (given in the Figure Legends) for each experimental condition.
Quantitative RT-PCR (qRT-PCR) measurement of gene expression
Total RNA was prepared from the whole small intestine and used for qRT-PCR as previously described (26). Gene specific primers are listed in the Table (Supplement) and primers for Hpgd and Ptgr1(Ltb4dh) were as previously described (11). qRT-PCR of the mRNA for the ribosomal protein Rpl26 was used for normalization. Data were analyzed by ΔΔCt method with correction for differential PCR efficiencies as previously described (10). Data are expressed relative to the WT control average. Our previous work suggested that alterations in PG degradative genes was responsible for elevated PG levels in the CF intestine (11). Therefore it was decided to use whole thickness intestinal tissue to measure gene expression because, regardless of what tissue layer the degradative genes are normally expressed, their downregulation in CF will result in increased longevity of PGs which in turn will allow greater diffusion to surrounding tissues before the PGs are enzymatically inactivated. The specific tissue layer localization of these enzymes is of less importance under the given circumstances than if synthetic enzymes were upregulated with constant levels of the degradative genes.
Enzyme immunoassay of PGE2
Mice were fasted overnight and the lumen of the small intestine was flushed with 5 ml phosphate buffered saline. PGE2 levels in the flushed fluid were estimated with an enzyme immunoassay that converts PGE2 and all its metabolites to a single compound which is then assayed (Cayman Chemical, Ann Arbor, MI), as previously described (11). Luminal fluid was used for these determinations because (a) the act of resecting the tissue can activate PG synthesis and one might thereby mask an in vivo difference between WT and CF; (b) it is possible there are regional differences along the proximal-distal axis, and flushing the entire small intestine lumen allowed us to get an average of the levels along the small intestine; (c) sampling of luminal contents for assay is frequently used clinically and it is hoped that this work will stimulate study of intestinal PG metabolism in human CF patients and luminal sampling is easily translated to clinical investigations.
Statistics
Data are presented as means ∀ SE. Significance was determined with Systat 11 software (San Jose, CA) by ANOVA followed by a post-hoc Tukey's test. P-values < 0.05 were considered as significant.
Results
To explore the mechanisms by which small intestinal transit is impaired in the CF mouse (10, 13), an organ bath approach was used to measure contractile behavior of the circular smooth muscle, whose activity is important for intestinal transit. The spontaneous tonic contractions of enteric circular muscle of WT mice was regular (Fig.1A) with a narrow distribution of the frequency of contractions (0.630 ∀ 0.006 Hz) (Fig.1C). In contrast, the spontaneous activity of the CF tissue was variable and erratic (Fig.1B). The frequency of spontaneous contractions in the CF tissue (0.518 ∀ 0.022 Hz) was significantly less than in the WT tissue (P<0.00001) and the range of frequencies was broadly distributed (Fig.1D).
Figure 1.
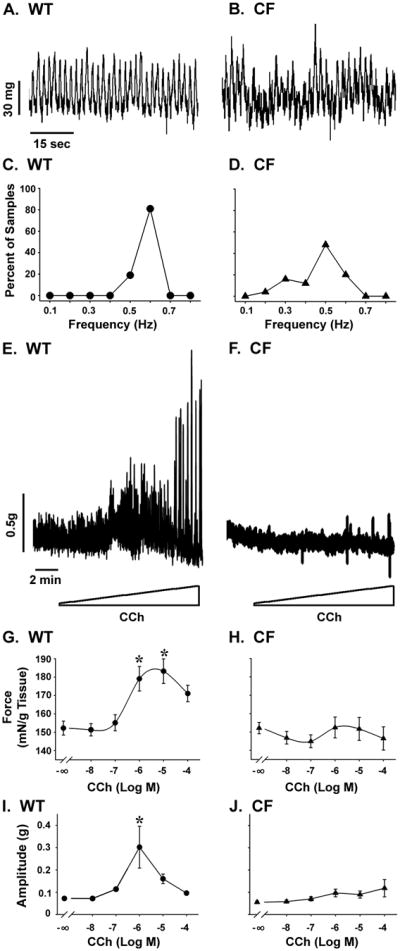
Contractile behavior of WT and CF enteric circular smooth muscle. Tissue rings were mounted in an organ bath and spontaneous (tonic activity in the absence of external stimulus) and cholinergic-stimulated contractility were recorded under isometric conditions. (A) Unstimulated WT tissue has low amplitude regular phasic contractile behavior. (B) Unstimulated CF tissue has variable amplitude irregular phasic contractions. (C) The frequency of spontaneous contractions in WT tissue was narrowly distributed around 0.63 Hz. (D) In the CF tissue the frequency of spontaneous contractions was broadly distributed and was significantly less than in the WT tissue (average of 0.52 Hz; P<0.00001 vs WT). (E) The WT tissue responded to cholinergic stimulation (2 min intervals of stepwise CCh addition from 10−8M through 10−4M) with increased force (G) and amplitude of contractions (I). (F) The CF tissue was non-responsive to cholinergic stimulation with no significant increase in force (H) nor in amplitude of contractions (J). (*) P<0.05 vs unstimulated spontaneous activity by ANOVA with Tukey post-hoc test. (n=16 WT; n=25 CF)
CF enteric circular muscle is nonresponsive to cholinergic stimulation
WT tissue responded to increasing concentrations of carbachol (CCh) (Fig.1E) with increased force (Fig.1G) and amplitude of contractions (Fig.1I). In contrast, the CF tissue was non-responsive to cholinergic stimulation (Fig.1F) and had no significant increase in force (Fig.1H) or in amplitude of contractions (Fig.1J).
CF muscularis externa is morphologically normal
Because there are many conditions affecting intestinal motility that involve structural changes to the myenteric nerve plexus or the network of interstitial cells of Cajal (ICC), these were examined in CF as compared to WT intestine. There were no noticeable differences comparing WT and CF intestines by acetylcholinesterase histochemistry or neuron specific enolase immunohistochemistry (quantitative morphometry: CF area = 82 ∀ 9% of WT; P>0.05; n=7 WT and 5 CF samples) (Supplemental Fig.1) The labeling pattern of the ICC network by c-kit immunofluorescence also was not different comparing WT and CF muscularis (Supplemental Fig.1).
One of the major smooth muscle inhibitory pathways that can be regulated by inflammation is production of nitric oxide. Therefore, expression of the three nitric oxide synthase (NOS) genes, Nos1 (neuronal NOS/nNOS), Nos2 (inducible NOS/iNOS), and Nos3 (endothelial NOS/eNOS) was measured. By qRT-PCR (Supplemental Fig.2), only nNOS showed altered expression, and the level was modestly but significantly reduced in the CF intestine. By NADPH-diaphorase histochemistry for NOS in whole mounts, there was not a noticeable difference comparing WT and CF tissues (Supplemental Fig.1).
The CF defect is at the level of the smooth muscle
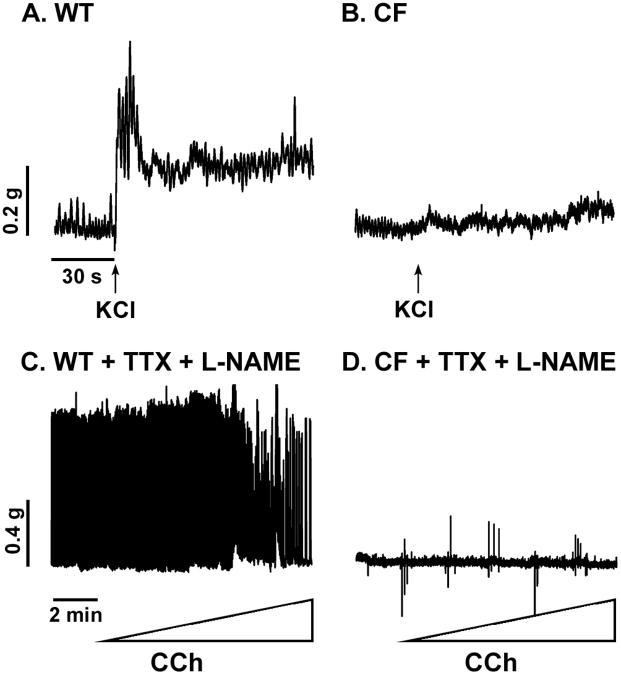
To directly test for smooth muscle dysfunction in the CF intestine, tissues in the organ bath were depolarized with KCl which bypasses membrane receptors and signal transduction pathways to elevate cytosolic calcium. Upon KCl addition, the WT tissue showed a rapid and pronounced increase in tone (Fig.2A). In contrast, the CF tissue had no change when depolarized (Fig.2B).
Figure 2.
Contractile activity of WT and CF enteric circular smooth muscle in response to KCl depolarization, and effects of tetrodotoxin and L-NAME. (A) Response of WT tissue to 50 mM KCl depolarization (added at arrow; (representative of 3 mice). (B) Response of CF tissue to 50 mM KCl depolarization (added at arrow; representative of 7 mice). (C) WT activity without and with CCh stimulation preincubated 10 min in the presence of tetrodotoxin (TTX, 10−6M) and L-NAME (10−4 M). Representative of 2 mice. (D) CF activity without and with CCh stimulation in the presence of TTX and L-NAME. (Representative of 5 mice.)
In the normal intestine, smooth muscle activity is regulated by stimulatory neurotransmitters (acetylcholine and tachykinins) in balance with relaxant effects of the non-adrenergic, non-cholinergic (NANC) neurotransmitters vasoactive intestinal peptide and nitric oxide, and purines. To test for a role of NANC relaxant pathways in CF dysfunction, nerve cell activity was blocked with tetrodotoxin (10−6M), and NG-nitro-L-arginine methyl ester (L-NAME, 10−4M) was used to inhibit production of nitric oxide. These inhibitors strongly increased the activity of WT muscle such that no further increase in the contractile activity was observed with cholinergic stimulation (Fig.2C). In marked contrast, the inhibitors had no effect on the activity of CF tissue without or with cholinergic stimulation (Fig.2D).
The above data suggest that the difference in the CF intestine is at the level of the smooth muscle cells rather than the other tissues that support and regulate contractility. Since there was no response to cholinergic stimulation by the CF tissue, expression levels of the two major intestinal muscarinic acetylcholine receptors, Chrm2 (muscarinic receptor 2) and Chrm3 (muscarinic receptor 3), were measured. Expression of levels of these genes was not significantly different in the CF tissue as compared to WT (Supplemental Fig.2).
Contribution of PGE2 to CF muscle dysfunction
Associated with the innate response to bacterial overgrowth in the CF mouse intestine, there is downregulation of the two major PG degradative genes, Hpgd and Ptgr1(11), both of which are expressed in the muscularis. As a consequence, the CF small intestine has elevated PGE2 and PGF2α levels (11). Because PGs are known to modulate enteric smooth muscle behavior, we examined whether the elevated levels of PGE2 and PGF2α have roles in the dysfunction of the CF enteric circular muscle.
First, the cyclooxygenase (COX) inhibitor indomethacin was used to inhibit prostaglandin synthesis. In WT tissue, inhibition of PG synthesis significantly increased the magnitude of cholinergic stimulated force generation (Fig.3A and C), as well as increasing the amplitude of contractions (Fig.3E). In CF tissue, inhibition of PG synthesis also significantly increased force generation in response to CCh stimulation (Fig.3B and D), and the amplitude of stimulated contractions (Fig.3F) compared to controls (Fig.1H and J, respectively). Previous work showed that protein levels of COX1 and COX2 were not altered in the CF small intestine as compared to WT (11). To test if one of these enzymes is of preferential importance in CF smooth muscle dysfunction, the selective inhibitors SC-560 (COX1 selective) and nimesulide (COX2 selective) were used. Inhibition of COX1 increased activity in 3 of 8 CF samples, whereas inhibition of COX2 increased activity in 7 of 10 CF samples (data not shown). When combined, SC-560 and nimesulide had a similar effect as indomethacin (data not shown).
Figure 3.
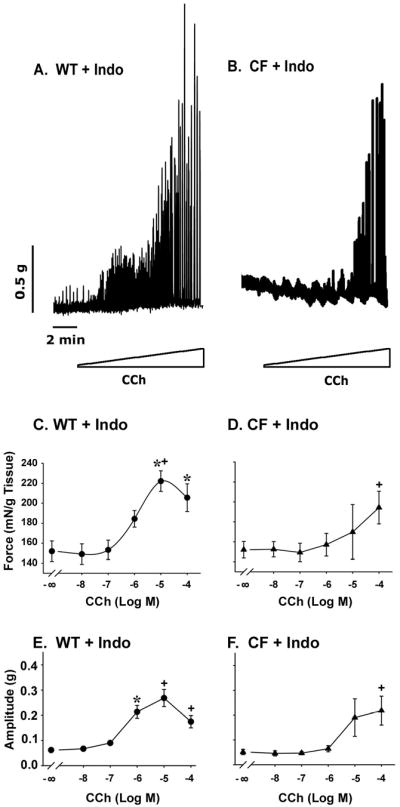
Effect of inhibition of PG synthesis on contractile behavior of WT and CF enteric circular smooth muscle. Tissues were preincubated in the organ bath for 1 hr with indomethacin (Indo, 10-5M). (A) WT and (B) CF tissues show increased responses to cholinergic stimulation (stepwise CCh addition from 10−8M through 10−4M) after inhibition of PG synthesis. There was significantly greater force (C, E) and amplitude of contractions (D, F) in both WT and CF tissues, respectively, after indomethacin preincubation. (n=13 WT; n=20 CF) (*) P<0.05 vs unstimulated activity; (+) P<0.05 vs tissue without indomethacin (Fig.1) at same [CCh] by ANOVA with Tukey post-hoc test.
Next, it was tested how addition of PGE2 and PGF2α, which are both elevated in the CF small intestine (11), would affect the contractile behavior of tissues. Addition of exogenous PGE2 to WT tissue after inhibition of PG synthesis largely abolished the contractile response to cholinergic stimulation (Fig.4A and C). Similarly, addition of exogenous PGE2 to CF tissue after inhibition of PG synthesis resulted in total loss of response to CCh stimulation (Fig.4B and D).
Figure 4.
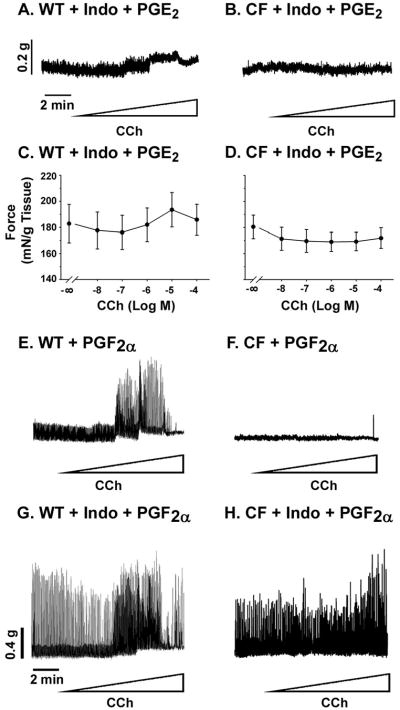
Effect of exogenous PGs on contractile response of WT and CF enteric circular smooth muscle, with and without inhibition of PG synthesis. Where indicated, tissues were preincubated in the organ bath for 1 hr with indomethacin (Indo, 10−5M) to inhibit prostaglandin synthesis. (A, C) Addition of PGE2 (10−6M) to WT tissue preincubated with indomethacin caused loss of response to cholinergic stimulation (stepwise CCh addition from 10−8M through 10−4M). (B, D) Addition of PGE2 (10-6M) to CF tissue preincubated with indomethacin caused loss of response to cholinergic stimulation. (n=4 WT; n=5 CF) (E) Addition of exogenous PGF2α (10−6M) to WT tissue increased contractile activity. (F) CF tissue contractile activity was not affected by addition of PGF2α. (G) In WT tissue, addition of PGF2α after inhibition of prostaglandin synthesis resulted in even greater contractile activity. (H) In CF tissue, addition of PGF2α after inhibition of prostaglandin synthesis increased contractile activity. (Representative of 2 WT and 3 CF mice).
Addition of exogenous PGF2α increased the cholinergic response of WT tissue (Fig.4E) but did not affect the CF tissue (Fig.4F). Next, tissues were preincubated with indomethacin to reduce endogenous PG levels, followed by addition of exogenous PGF2α. After inhibition of PG synthesis, PGF2α induced in WT tissue increased phasic contractile activity even in the absence of CCh (Fig.4G). Similarly, the CF tissue had greatly increased activity in response to exogenous PGF2α after inhibition of PG synthesis (Fig.4H).
Perturbation of the gut microbiota with antibiotics impairs circular smooth muscle activity
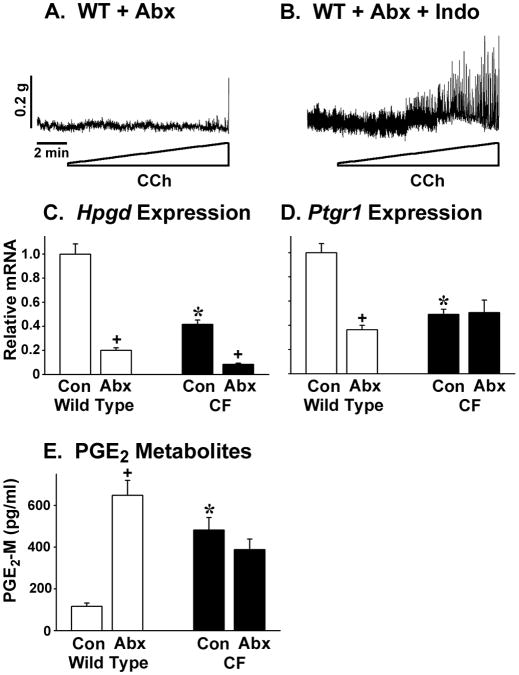
We recently reported that mice treated with broad spectrum antibiotics have slowed small intestinal transit as compared to control mice (10). We hypothesized that the dramatic perturbation of the intestinal microbiota caused by long term administration of antibiotics (24) could alter enteric muscle function and account for the slowed transit. When WT mice were treated with broad spectrum antibiotics (see Materials and Methods), the circular muscle activity was strongly impaired and there was little response to cholinergic stimulation (Fig.5A). As a control for potential acute effects of the antibiotics, WT tissue was tested for CCh responsiveness after incubation with the antibiotics. Acute treatment of the tissue with antibiotics did not inhibit the response to CCh stimulation (Supplemental Figure 3).
Figure 5.
Effect of antibiotics on WT enteric circular muscle activity, PG degradative gene expression, and PGE2 levels. Mice were treated for 3 weeks with ciprofloxacin (50 mg/kg per day) and metronidazole (100 mg/kg per day). (A) Circular muscle from antibiotic (Abx) treated mice was not responsive to cholinergic stimulation (Representative of 4 mice). (B) Activity was restored by preincubation in the organ bath for 1 hr with indomethacin (Indo, 10−5M) to inhibit prostaglandin synthesis. (C) Antibiotics significantly reduced intestinal expression of Hpgd in both WT and CF mice (n=10 each WT and CF samples). (D) Antibiotics significantly reduced intestinal expression of Ptgr1 in WT mice (n=10 each WT and CF samples). (E) Antibiotics significantly increased PGE2 levels (measured as PGE2 and its metabolites) in the WT intestine. (n=14 WT, 10 WT + Abx, 8 CF, 9 CF + Abx) (*) P<0.05 CF vs WT control (Con); (+) P<0.05 vs control of same genotype by ANOVA with Tukey post-hoc test.
To test for an involvement of PGs in this altered behavior, we preincubated tissue from the antibiotic treated WT mice with indomethacin and then assessed contractile activity. After inhibition of PG synthesis, the WT circular muscle from antibiotic treated mice now responded to stimulation (Fig.5B). Because the mechanism of increased PGs in CF mice appears to be due largely to decreased expression of PG degradative genes (11), we measured gene expression levels of Hpgd and Ptgr1. Both Hpgd and Ptgr1 were significantly reduced in the intestine of antibiotic treated WT mice (Fig.5C and D, respectively). Expression levels in antibiotic treated WT mice for these genes was similar to or less than that of untreated CF mice (Fig.5C and D). To determine whether changes in expression of these PG degradative genes in antibiotic-treated WT mice affects PGE2 levels, we measured PGE2 and its metabolites by enzyme immunoassay. As shown in Fig.5E, PGE2 was very strongly increased in the intestine of antibiotic treated WT mice. PGE2 levels were not further increased in CF mice as compared to control CF mice (Fig.5E).
Osmotic laxative improves circular smooth muscle function in CF mice
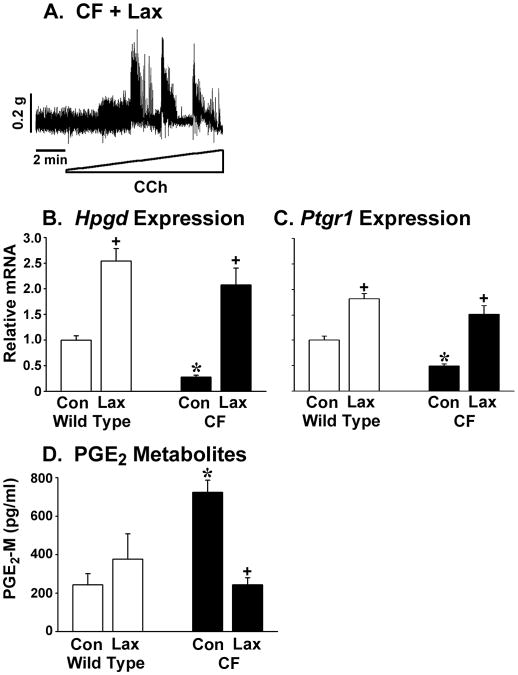
Treatment of CF mice with osmotic laxative dramatically improves the intestinal phenotype and this includes eradication of bacterial overgrowth and normalization of small intestinal transit (13). Therefore, we tested whether laxatives administered for 3 weeks would improve enteric smooth muscle function. In 3 of 4 CF mice treated with laxative, circular smooth muscle activity was improved (Fig.6A). Supporting an important role for the PG degradative genes in this effect, laxative treatment upregulated expression of Hpgd and Ptgr1 genes in the CF mice (Fig.6B and C, respectively). Laxative treatment also significantly increased Hpgd and Ptgr1 gene expression in WT mice (Fig.6B and C). PGE2 metabolite levels were significantly reduced in CF mice after laxative treatment, but were unaffected in treated WT mice (Fig.6E).
Figure 6.
Effect of laxative on CF enteric circular muscle activity, PG degradative gene expression, and PGE2 levels. Mice were treated for 3 weeks with an osmotic laxative (see Materials and Methods). (A) Circular muscle from CF mice treated with laxative (Lax) were responsive to cholinergic stimulation (representative of 3 of 4 treated CF mice; tissue from the fourth mouse was not responsive). (B) Laxative significantly increased intestinal expression of Hpgd in both WT and CF mice (n=10 WT control, 12 WT + Lax, 11 CF control, 6 CF + Lax). (C) Laxative significantly increased intestinal expression of Ptgr1 in both WT and CF mice (n=10 WT control, 12 WT + Lax, 11 CF control, 6 CF + Lax). (D) Laxative significantly decreased PGE2 levels in the CF intestine (n=4 each WT, WT + Lax, CF, CF + Lax) (*) P<0.05 CF vs WT control; (+) P<0.05 vs control (Con) of same genotype by ANOVA with Tukey post-hoc test.
Discussion
In this work, we sought a mechanistic explanation for impaired small intestinal transit in the CF mouse (10). We found that the circular smooth muscle from CF mice has a profound lack of activity and is nonresponsive to cholinergic simulation. It might seem surprising that mice with such a profound dysfunction of enteric smooth muscle can survive. However, there is a precedent in that mice deficient in the major enteric muscarinic receptors (M2/M3 double knockout), have severely impaired intestinal motility, but they survive with only a mild phenotype (23).
Because the CF mouse has an innate immune response in the small intestine (26), and inflammatory processes can damage enteric nerves and/or ICC which could result in muscle dysfunction, we assessed these morphologically. We found no evidence of damage to the enteric nervous system, nor to the ICC network. This morphological analysis does not rule out subtle changes in nerves and ICCs in the CF intestine, but there are no dramatic changes in the CF intestine as there are in other motility disorders.
Blockage of the major NANC pathways with TTX and L-NAME failed to restore activity to the CF circular muscle, whereas these treatments strongly enhanced activity of WT muscle. Because the CF tissue could not be activated by direct KCl-mediated depolarization, which strongly contracted the WT muscle, these data suggest that the defect in the CF tissue is most likely myogenic, rather than neurogenic or due to damage to the ICCs.
As part of the innate immune response of the CF mouse small intestine, eicosanoid metabolism is altered, resulting in elevated levels of PGE2 and PGF2α (11). There are several effects of various PGs on enteric smooth muscle function (5, 29, 30, 36). Therefore, we hypothesized that PGs were involved in the CF enteric muscle dysfunction. Treatment of CF tissue to block PG synthesis resulted in restoration of circular muscle activity. Inhibition of PG synthesis did not fully restore activity to the CF circular muscle, which may be due to the fact that several PGs affect the smooth muscle and that COX inhibitors will reduce levels of them all.
It is known that PGE2 relaxes circular smooth muscle, and that PGF2α causes activation (5, 29, 30, 36). Interestingly, the effect of PGE2 is dominant over that of PGF2α (15) which may explain the partial restorative effect by inhibition of PG synthesis on CF circular smooth muscle activity. In support of this, we found there was a robust response in the CF circular muscle to exogenous PGF2α but only after PG synthesis inhibition, which will also decrease levels of the relaxant PGE2. Furthermore, the activity of the CF tissue after inhibiting PG synthesis could be reversed by addition of exogenous PGE2. Even though the stimulatory prostaglandin PGF2α is elevated in the CF small intestine, the net effect is loss of activity and a dramatic decrease in small intestinal transit in vivo. This is likely due to the fact that the relaxant effect of PGE2 dominates the stimulatory effect of PGF2α.
We also performed experiments to further support the proposed role of elevated PGE2 in intestinal dysmotility and to test for a link between smooth muscle dysfunction and the enteric microbiota. Long term (3 weeks) administration of broad spectrum antibiotics to WT mice, which lowers the bacterial load and also likely results in a new stable microbiota, slows in vivo intestinal transit (10). In the current work we found that circular smooth muscle from antibiotic treated mice has impaired activity in vitro. Accompanying this dysfunction was a decrease in the major PG degradative genes, Hpgd and Ptgr1, as well as a significant increase in intestinal PGE2 levels. As a corollary, we also tested the effects of osmotic laxative, which improves in vivo intestinal transit in CF mice (13). Laxative treatment of CF mice improved in vitro circular smooth muscle function and this was accompanied by increased expression of Hpgd and Ptgr1, and decreased PGE2 levels. Together, these data provide strong evidence that the microbiota can modulate PG levels, principally by control of their degradation and that this can be an important factor in normal and dysregulated enteric circular smooth muscle function.
The intestinal microbiota is known to affect motility. Germ free rodents have slow small intestinal transit and conventionalization of their microbiota normalizes their transit rates (18). Furthermore, gnobiotic animals show different effects with regard to transit depending on the bacterium used for colonization. E.coli gnobiotic animals have slow transit, whereas those colonized by probiotic-type bacteria (e.g., lactobacillus) show increased rates of transit. The mechanism linking the enteric microbiota to motility is not understood. Our data point to changes in expression of PG degradative genes which are somehow modulated by the microbiota. CF mice are overgrown with mostly E.coli and other Enterobacteriacae, which based on gnobiotic rodent studies is expected to lead to slow transit. Antibiotic treated WT mice have levels of bacteria lower than control WT mice, which may be similar to the germ free state where transit is also slow. Both very low levels of bacteria (or the reduction of specific species) in antibiotic treated WT mice as well as overgrowth with Enterobacteriacae in control CF mice are associated with altered PG metabolism, and there is a concomitant loss of circular smooth muscle responsiveness. What is the common mechanism between the microbiota and PG metabolic gene expression remains to be determined.
Gastrointestinal dysmotility has been reported to occur in up to half of CF patients (1, 9, 14, 19, 20, 22, 32). The interdigestive migrating motor complex is one of the principal mechanisms to maintain a low bacterial load in the proximal small intestine, and bacterial overgrowth may be common in CF patients (16, 20, 27), although the sensitivity and accuracy of the techniques used in human patients to demonstrate intestinal overgrowth cast some uncertainty on those results. Normal GI motility is also important for proper digestion, and nutrition is impaired in CF. Malnutrition in CF is strongly associated with the progression of airway disease (7, 21). Bacterial overgrowth can contribute to poor nutrition (17, 34) as well as other aspects of the gut phenotype in CF (7).
The mechanisms of altered gut motility in CF, until now, have not been explored. It has been known for a long time that PGs modulate enteric smooth muscle activity and there are several examples of gut dysmotility where PGE2 is implicated. Investigations of experimentally induced post-operative ileus have shown that the PG synthetic enzyme COX2 is upregulated in an endotoxin-dependent manner by intestinal manipulation, suggesting that elevated PGs play a role in dysmotility in this condition (33, 37). It has also been demonstrated that activated macrophages in the muscularis externa are associated with increased PGE2 levels and a decreased cholinergic response in rats (31). Other work in dogs has shown that PGE2 decreases activity of the migrating motor complex and that administration of indomethacin could restore motility (36). Thus, altered PGE2 levels cause intestinal dysmotility in a number of conditions, to which CF may now be added.
In summary, the enteric circular smooth muscle of the CF mouse small intestine has dramatically reduced activity. Elevated PGE2 levels are implicated in the smooth muscle inactivity. PGE2 mediated dysmotility appears to be caused by perturbations in the enteric microbiota. Once begun, dysmotility in the CF intestine likely contributes to progression to bacterial overgrowth which contributes to poor nutrition in this disease. These novel data demonstrate the importance of the PG degradative pathway and PGE2 levels in intestinal motor dysfunction.
Supplementary Material
Acknowledgments
We thank Eileen Roach for performing morphometric analyses and figure preparation, and Maureen Flynn for assistance with histological preparations. Supported by a grant from the Cystic Fibrosis Foundation and by a pilot project as part of National Institutes of Health P20RR024214 grant from the National Center for Research Resources. Portions of this work have been published in abstract form: Meldi L, De Lisle RC. Impaired Enteric Circular Muscle Activity in the Cystic Fibrosis Mouse Small Intestine: Role of Prostaglandin E2 [abstract]. Gastroenterology 2008; 134 (Suppl 1): 479.
Footnotes
Disclosures: The authors have no other disclosures to make.
References
- 1.Bali A, Stableforth DE, Asquith P. Prolonged small-intestinal transit time in cystic fibrosis. Br Med J (Clin Res Ed) 1983;287:1011–1013. doi: 10.1136/bmj.287.6398.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20(1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 3.Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, Freedman SD, Durie PR. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G839–G848. doi: 10.1152/ajpgi.00582.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bennett A, Eley KG, Stockley HL. The effects of prostaglandins on guinea-pig isolated intestine and their possible contribution to muscle activity and tone. Br J Pharmacol. 1975;54:197–204. doi: 10.1111/j.1476-5381.1975.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett A, Hensby CN, Sanger GJ, Stamford IF. Metabolites of arachidonic acid formed by human gastrointestinal tissues and their actions on the muscle layers. Br J Pharmacol. 1981;74:435–444. doi: 10.1111/j.1476-5381.1981.tb09989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijvelds MJ, Bronsveld I, Havinga R, Sinaasappel M, De Jonge HR, Verkade HJ. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol. 2005;288:G646–G653. doi: 10.1152/ajpgi.00295.2004. [DOI] [PubMed] [Google Scholar]
- 7.Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon PJ, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 8.Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE, Shull GE, Tanabe H, Ouellette AJ, Franklin CL, Walker NM. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1050–G1058. doi: 10.1152/ajpgi.00393.2003. [DOI] [PubMed] [Google Scholar]
- 9.Dalzell AM, Freestone NS, Billington D, Heaf DP. Small intestinal permeability and orocaecal transit time in cystic fibrosis. Arch Dis Child. 1990;65:585–588. doi: 10.1136/adc.65.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Lisle RC. Altered Transit and Bacterial Overgrowth in the Cystic Fibrosis Mouse Small Intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–G111. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 11.De Lisle RC, Meldi L, Flynn M, Jansson K. Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine. J Pediatr Gastroenterol Nutr. 2008;47:406–416. doi: 10.1097/MPG.0b013e31817e0f2c. [DOI] [PubMed] [Google Scholar]
- 12.De Lisle RC, Meldi L, Roach E, Flynn M, Sewell R. Mast cells and gastrointestinal dysmotility in the cystic fibrosis mouse. PLos ONE. 2009;4:e4283. doi: 10.1371/journal.pone.0004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 14.Escobar H, Perdomo M, Vasconez F, Camarero C, del Olmo MT, Suarez L. Intestinal permeability to 51Cr-EDTA and orocecal transit time in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1992;14:204–207. doi: 10.1097/00005176-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Frantzides CT, Lianos EA, Wittmann D, Greenwood B, Edmiston CE. Prostaglandins and modulation of small bowel myoelectric activity. Am J Physiol. 1992;262:G488–G497. doi: 10.1152/ajpgi.1992.262.3.G488. [DOI] [PubMed] [Google Scholar]
- 16.Fridge JL, Conrad C, Gerson L, Castillo RO, Cox K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;44:212–218. doi: 10.1097/MPG.0b013e31802c0ceb. [DOI] [PubMed] [Google Scholar]
- 17.Husebye E. Gastrointestinal motility disorders and bacterial overgrowth. J Intern Med. 1995;237:419–427. doi: 10.1111/j.1365-2796.1995.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 18.Husebye E, Hellstrom PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 19.King SJ, Button BM, Kelly MJ, Roberts SK, Nyulasi IB, Kotsimbos T, Wilson JW. Delayed gastric emptying is common in adults with cystic fibrosis and is associated with lower body mass index. Pediatr Pulmonol Suppl. 2006;29:373–(abstract). [Google Scholar]
- 20.Lewindon PJ, Robb TA, Moore DJ, Davidson GP, Martin AJ. Bowel dysfunction in cystic fibrosis: importance of breath testing. J Paediatr Child Health. 1998;34:79–82. doi: 10.1046/j.1440-1754.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 21.Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol. 2006;41:35–49. doi: 10.1002/ppul.20286. [DOI] [PubMed] [Google Scholar]
- 22.Mascarenhas MR, Mondick JT, Barrett JS, Zhuang H, Edwards K, Schall JI. Comparison of gastric emptying and small bowel transit in subjects with CF and healthy controls. Pediatr Pulmonol Suppl. 2006;29:377–(abstract). [Google Scholar]
- 23.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–6049. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norkina O, De Lisle RC. Potential genetic modifiers of the cystic fibrosis intestinal inflammatory phenotype on mouse chromosomes 1, 9, and 10. BMC Genet. 2005;6:29. doi: 10.1186/1471-2156-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–G1041. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien S, Mulcahy H, Fenlon H, O'Broin A, Casey M, Burke A, FitzGerald MX, Hegarty JE. Intestinal bile acid malabsorption in cystic fibrosis. Gut. 1993;34:1137–1141. doi: 10.1136/gut.34.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59:191–195. doi: 10.1203/01.pdr.0000196720.25938.be. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM. Evidence that prostaglandins are local regulatory agents in canine ileal circular muscle. Am J Physiol. 1984;246:G361–G371. doi: 10.1152/ajpgi.1984.246.4.G361. [DOI] [PubMed] [Google Scholar]
- 30.Sanger GJ, Bennett A. Regional differences in the responses to prostanoids of circular muscle from guinea-pig isolated intestine. J Pharm Pharmacol. 1980;32:705–708. doi: 10.1111/j.2042-7158.1980.tb13043.x. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Torihashi S, Hori M, Nasu T, Ozaki H. Phagocytotic activation of muscularis resident macrophages inhibits smooth muscle contraction in rat ileum. J Vet Med Sci. 2007;69:1053–1060. doi: 10.1292/jvms.69.1053. [DOI] [PubMed] [Google Scholar]
- 32.Schappi MG, Roulet M, Rochat T, Belli DC. Electrogastrography reveals post-prandial gastric dysmotility in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2004;39:253–256. doi: 10.1097/00005176-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 34.Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep. 2003;5:365–372. doi: 10.1007/s11894-003-0048-0. [DOI] [PubMed] [Google Scholar]
- 35.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 36.Thor P, Konturek JW, Konturek SJ, Anderson JH. Role of prostaglandins in control of intestinal motility. Am J Physiol. 1985;248:G353–G359. doi: 10.1152/ajpgi.1985.248.3.G353. [DOI] [PubMed] [Google Scholar]
- 37.Türler A, Schnurr C, Nakao A, Togel S, Moore BA, Murase N, Kalff JC, Bauer AJ. Endogenous endotoxin participates in causing a panenteric inflammatory ileus after colonic surgery. Ann Surg. 2007;245:734–744. doi: 10.1097/01.sla.0000255595.98041.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.