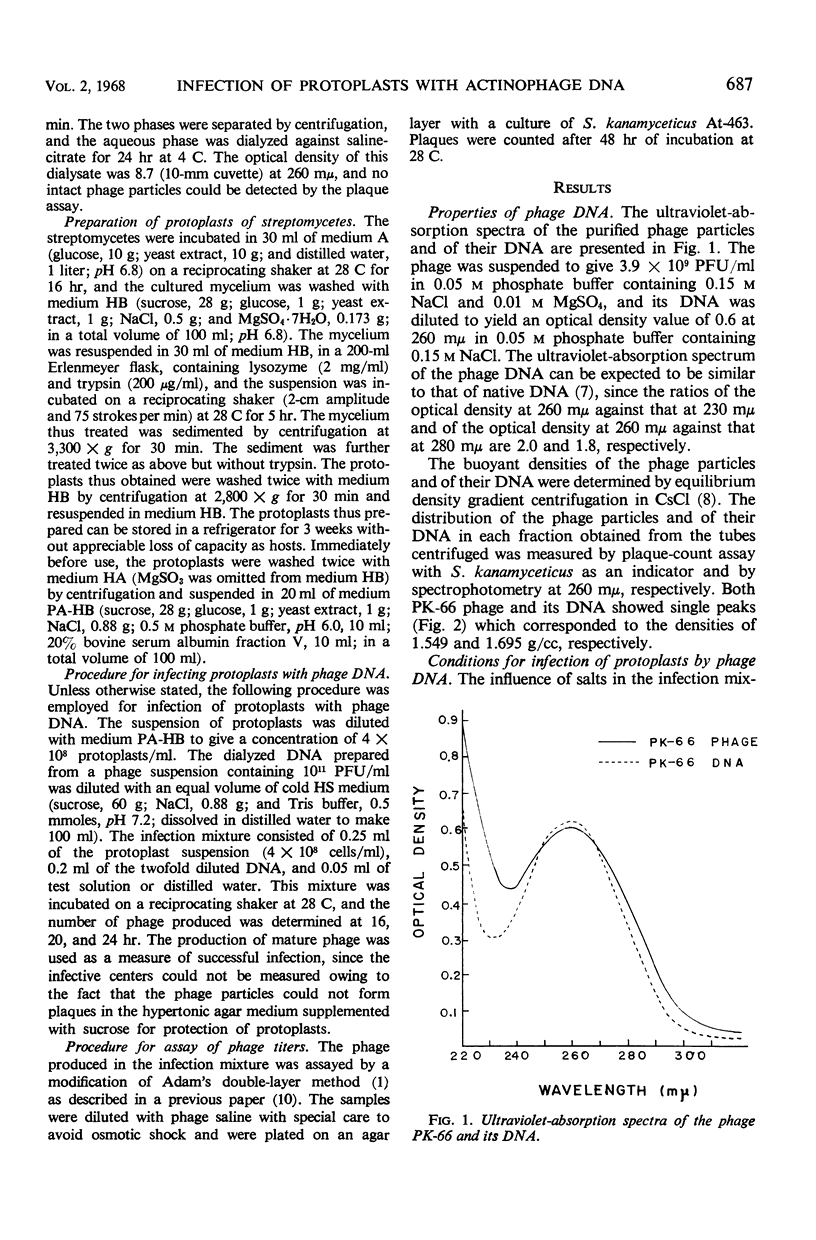
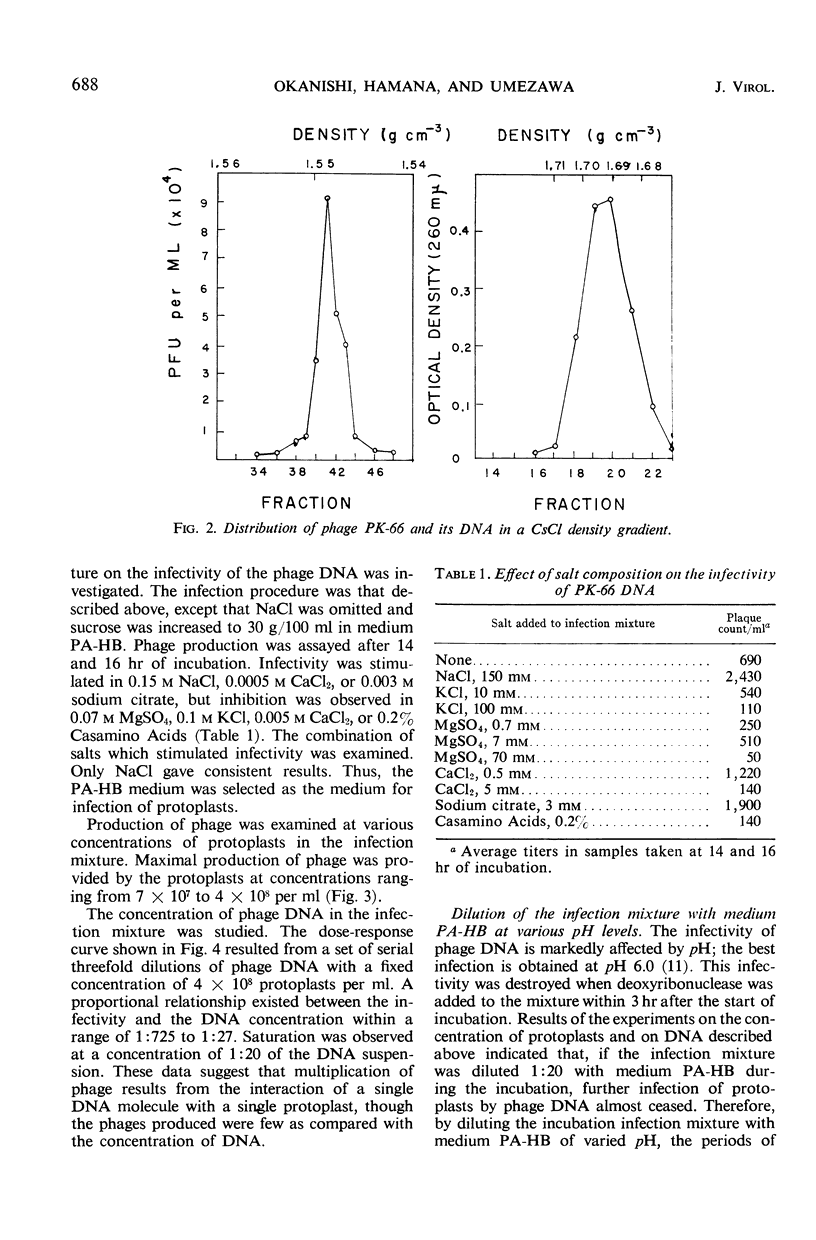
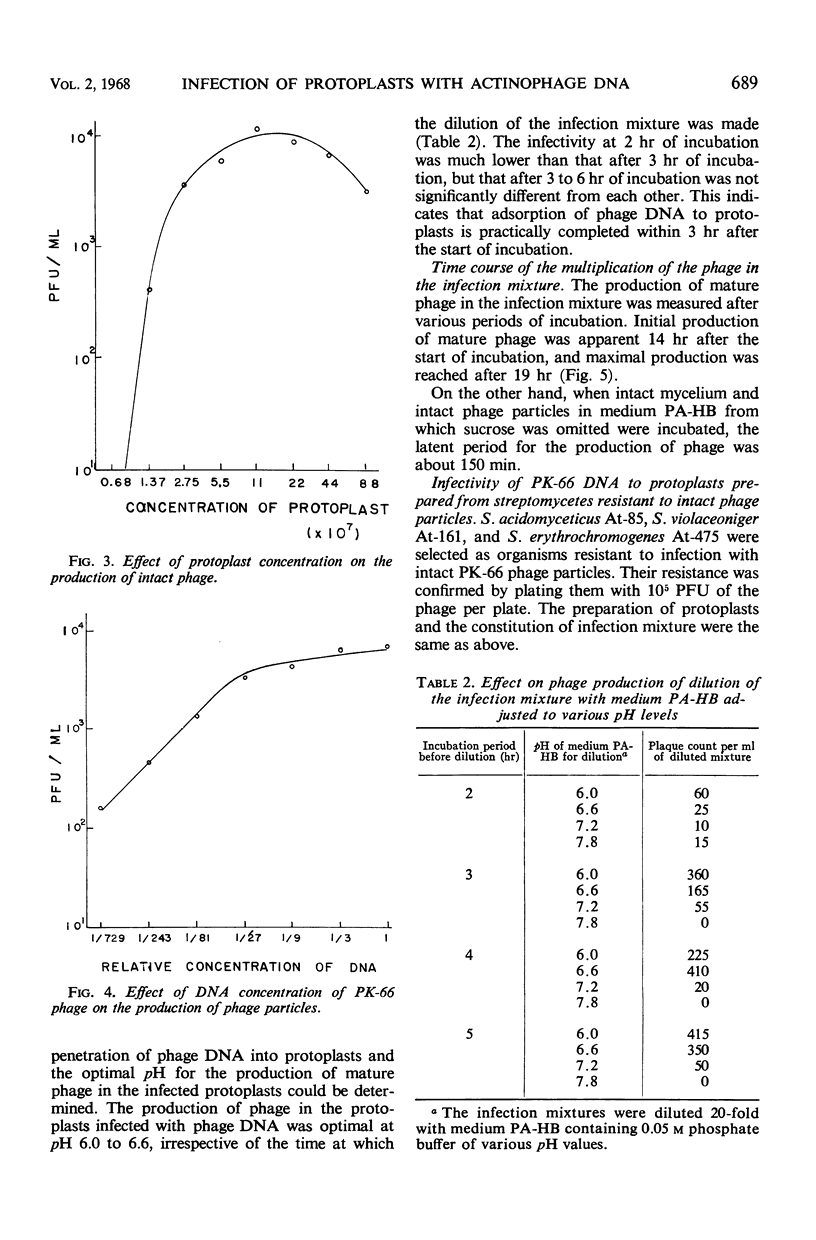
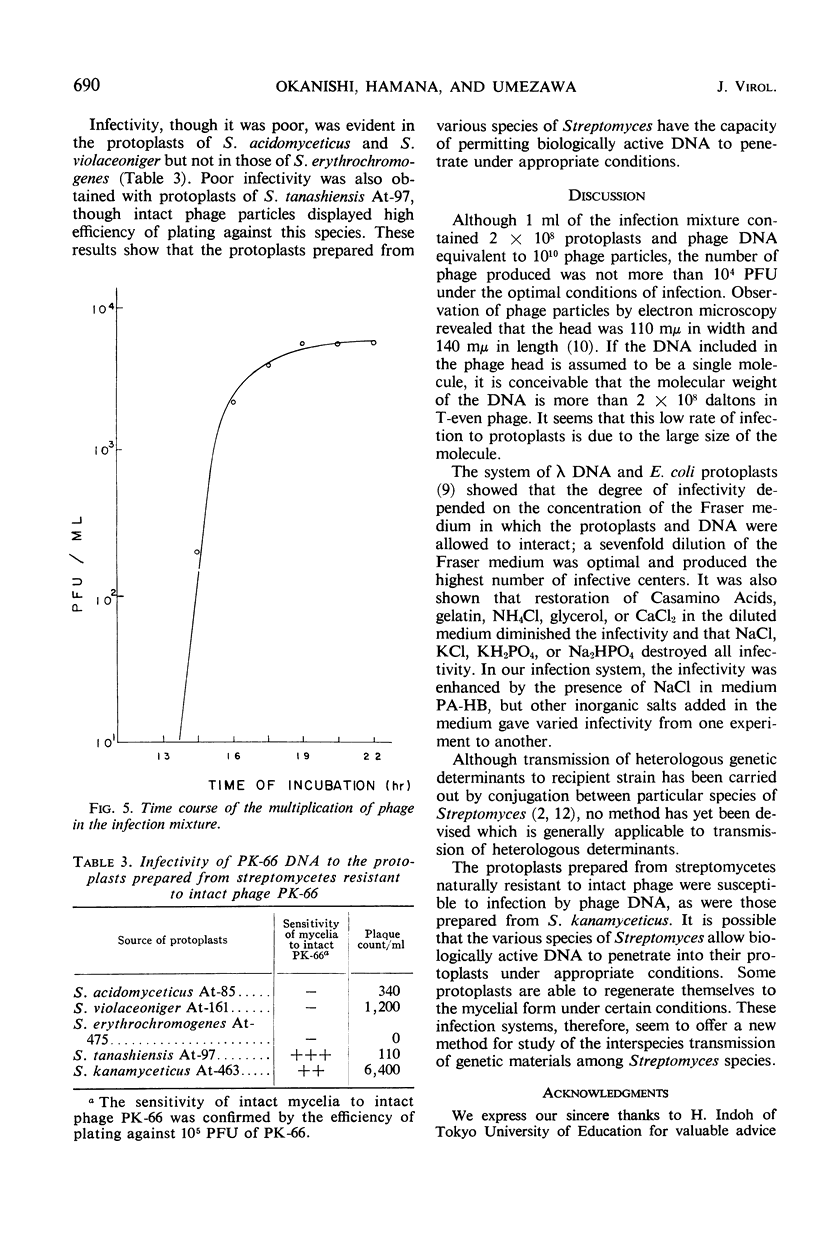
Abstract
To establish a method for transmission of genetic materials in the genus Streptomyces, the conditions of infection of protoplasts of S. kanamyceticus by actinophage PK-66 deoxyribonucleic acid (DNA) were studied. The protoplasts of Streptomyces were prepared by treatments with lysozyme and trypsin. The infectivity of the phage DNA was enhanced by the presence of NaCl in the medium. The optimal concentration of the protoplasts for infection with DNA was 7 × 107 to 4 × 108/ml. A proportional relationship was found between the infectivity and the DNA concentration within a certain range. The maximal production of mature phage was achieved after 19 hr of incubation. The number of phage propagated in the infection mixture reached 104 plaque-forming units per ml under the appropriate conditions. The phage DNA infected not only protoplasts prepared from S. kanamyceticus but also those prepared from S. violaceoniger and S. acidomyceticus, which were resistant to intact phage PK-66.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY S. G., LEDERBERG J. Heterokaryosis in Streptomyces. J Bacteriol. 1956 Aug;72(2):219–225. doi: 10.1128/jb.72.2.219-225.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H., Ikeda Y. Genetic Recombination of Transforming Deoxyribonucleic Acid Molecules with the Recipient Genome and Among Themselves in Protoplasts of Bacillus subtilis. J Bacteriol. 1966 Aug;92(2):455–463. doi: 10.1128/jb.92.2.455-463.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- MEYER F., MACKAL R. P., TAO M., EVANS E. A., Jr Infectious deoxyribonucleic acid from gamma bacteriophage. J Biol Chem. 1961 Apr;236:1141–1143. [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Utahara R., Okami Y. Infection of the protoplasts of Streptomyces kanamyceticus with deoxyribonucleic acid preparation from actinophage PK-66. J Bacteriol. 1966 Dec;92(6):1850–1852. doi: 10.1128/jb.92.6.1850-1852.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsinelli M., Beretta M. Genetic Recombination in Crosses Between Streptomyces aureofaciens and Streptomyces rimosus. J Bacteriol. 1966 Jan;91(1):63–68. doi: 10.1128/jb.91.1.63-68.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKETO A., TAKAGI Y. An infective deoxyribonucleic acid from bacteriophage phi-X174. Biochim Biophys Acta. 1960 Dec 4;45:199–200. doi: 10.1016/0006-3002(60)91443-8. [DOI] [PubMed] [Google Scholar]


