Abstract
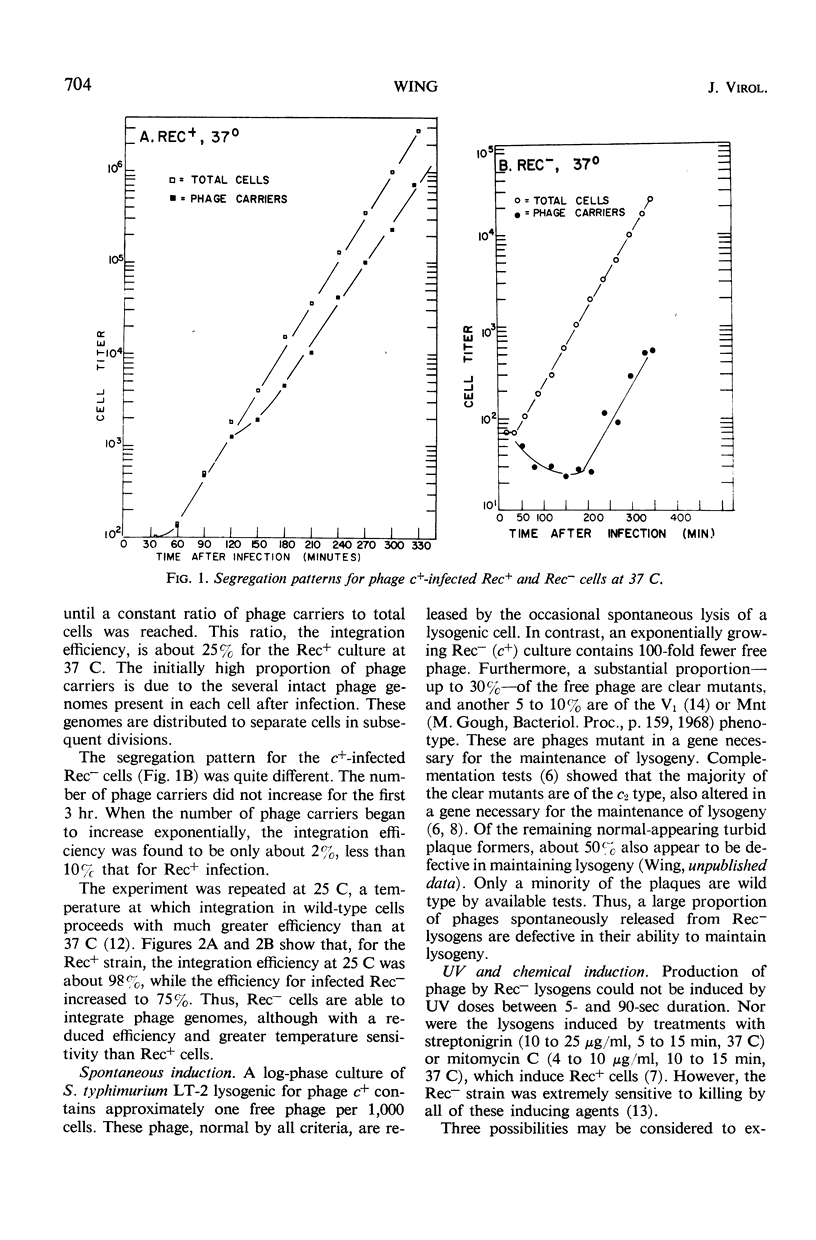
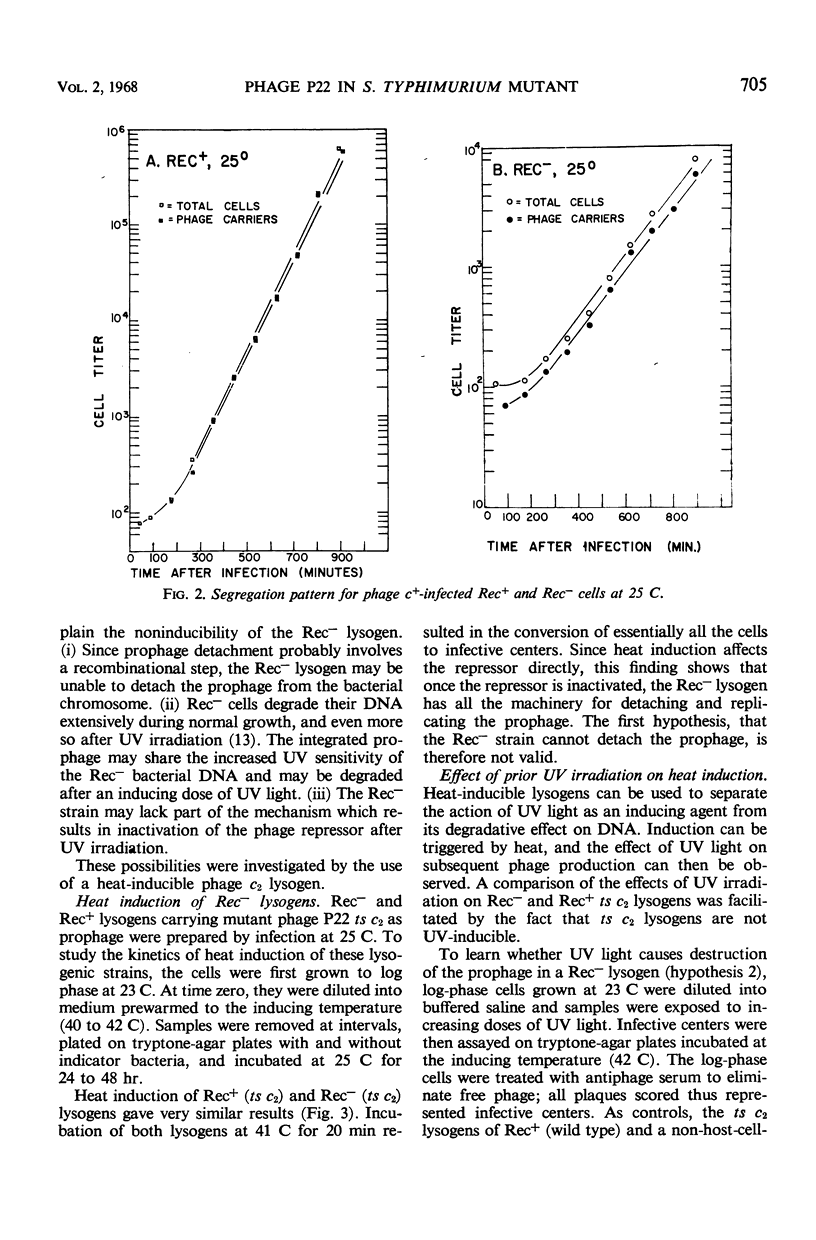
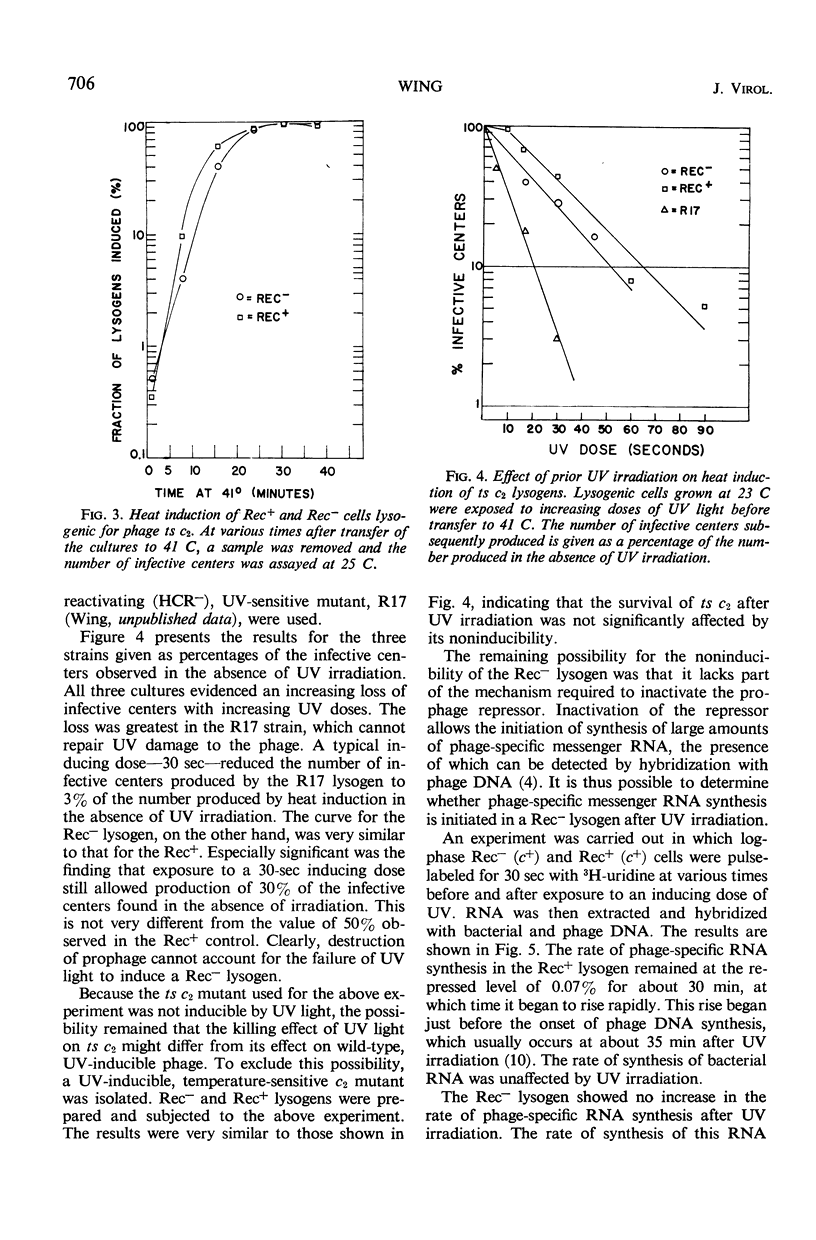
Phage P22 can integrate as prophage into a recombination-deficient (Rec−) strain of Salmonella typhimurium. At 37 C, the integration efficiency is only 10% that in Rec+ infection, but at 25 C the efficiencies in Rec− and Rec+ hosts are similar. Rec− lysogens cannot be induced by ultraviolet irradiation or by treatments with the chemical inducing agents streptonigrin or mitomycin C. Heat induction of Rec− cells lysogenic for a temperature-sensitive c2 mutant (ts c2) is normal, showing that the Rec− cell has the machinery necessary for prophage excision. Ultraviolet irradiation of Rec− (ts c2) lysogens prior to heat induction does not prevent the formation of infective centers after temperature shift. Thus, the noninducibility of Rec− lysogens is not due to destruction of the prophage as a result of ultraviolet irradiation. Deoxyribonucleic acid-ribonucleic acid (RNA) hybridization experiments demonstrate that no increase in phage-specific RNA synthesis occurs after ultraviolet irradiation of a Rec− (c+) lysogen. The Rec− mutant appears to lack part of the mechanism required to destroy the phage repressor and allow the initiation of early phage functions such as messenger RNA synthesis. A similar conclusion was reached previously for an Escherichia coli Rec− strain.
Full text
PDF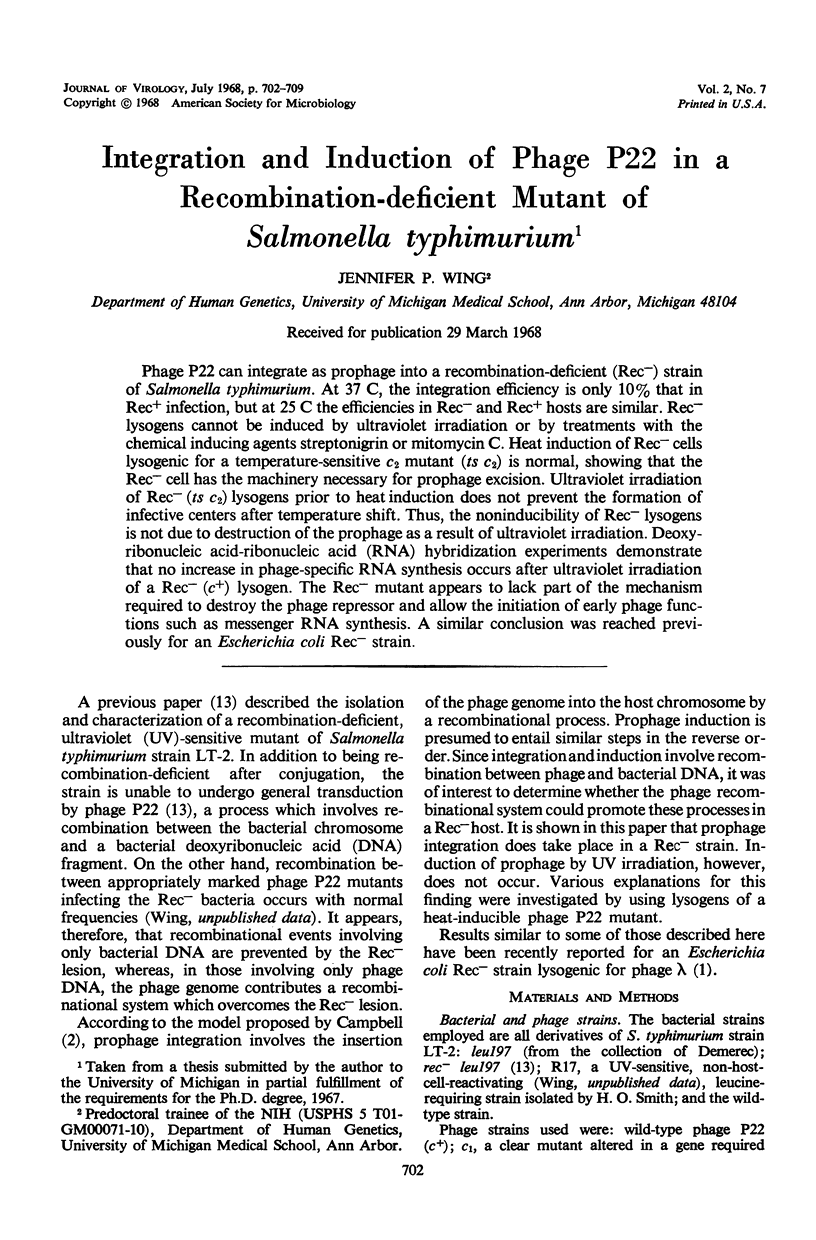



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- Green M. H. Inactivation of the prophage lambda repressor without induction. J Mol Biol. 1966 Mar;16(1):134–148. doi: 10.1016/s0022-2836(66)80268-1. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- LEVINE M., BORTHWICK M. THE ACTION OF STREPTONIGRIN ON BACTERIAL DNA METABOLISM AND ON INDUCTION OF PHAGE PRODUCTION IN LYSOGENIC BACTERIA. Virology. 1963 Dec;21:568–574. doi: 10.1016/0042-6822(63)90228-9. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- LEVINE M., SMITH H. O. SEQUENTIAL GENE ACTION IN THE ESTABLISHMENT OF LYSOGENY. Science. 1964 Dec 18;146(3651):1581–1582. doi: 10.1126/science.146.3651.1581. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Defective phage formation by lysogens of integration deficient phage P22 mutants. Virology. 1968 Feb;34(2):203–223. doi: 10.1016/0042-6822(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Wing J. P., Levine M., Smith H. O. Recombination-deficient mutant of Salmonella typhimurium. J Bacteriol. 1968 May;95(5):1828–1834. doi: 10.1128/jb.95.5.1828-1834.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]


