In this study reggie-1/flotillin-2 is identified as a component of the tubulovesicular sorting and recycling compartment, where it interacts with and controls the activity of Rab11a and SNX4. Evidence is given that reggie-1 expression is necessary for the proper recycling of transferrin receptor and E-cadherin in HeLa and A431 cells, respectively.
Abstract
The lipid raft proteins reggie-1 and -2 (flotillins) are implicated in membrane protein trafficking but exactly how has been elusive. We find that reggie-1 and -2 associate with the Rab11a, SNX4, and EHD1–decorated tubulovesicular recycling compartment in HeLa cells and that reggie-1 directly interacts with Rab11a and SNX4. Short hairpin RNA–mediated down-regulation of reggie-1 (and -2) in HeLa cells reduces association of Rab11a with tubular structures and impairs recycling of the transferrin–transferrin receptor (TfR) complex to the plasma membrane. Overexpression of constitutively active Rab11a rescues TfR recycling in reggie-deficient HeLa cells. Similarly, in a Ca2+ switch assay in reggie-depleted A431 cells, internalized E-cadherin is not efficiently recycled to the plasma membrane upon Ca2+ repletion. E-cadherin recycling is rescued, however, by overexpression of constitutively active Rab11a or SNX4 in reggie-deficient A431 cells. This suggests that the function of reggie-1 in sorting and recycling occurs in association with Rab11a and SNX4. Of interest, impaired recycling in reggie-deficient cells leads to de novo E-cadherin biosynthesis and cell contact reformation, showing that cells have ways to compensate the loss of reggies. Together our results identify reggie-1 as a regulator of the Rab11a/SNX4-controlled sorting and recycling pathway, which is, like reggies, evolutionarily conserved.
INTRODUCTION
Reggie-1 and reggie-2 (flotillin-2 and flotillin-1, respectively) are lipid raft proteins expressed in virtually every cell type and in organisms as distant as flies and humans (Stuermer, 2010). Although this might suggest that reggies subserve basic cellular functions, such roles have not been clearly defined. Reggies form oligomers and clusters of <100 nm at the cytoplasmic face of the plasma membrane (PM) and at membranes of various types of vesicles (Stuermer, 2010). They are implicated in endocytosis of the glycosylphosphatidylinositol-anchored protein CD59 and claimed to constitute a specific clathrin-independent endocytic route (Glebov et al., 2006). This view, however, is controversial (Stuermer, 2010; Otto and Nichols, 2011), although it is now widely accepted that reggies are involved in cargo trafficking. For instance, A. Saltiel and colleagues demonstrated in adipocytes a role of reggie-2 in the translocation of glucose transporter 4 (Glut4) from a perinuclear (reggie-positive; Fecchi et al., 2006) store to the PM, a process involving the adapter protein CAP, the Cdc42-related GTPase TC10 (Baumann et al., 2000; Kioka et al., 2002; Chang et al., 2007), and the exocyst (Kawase et al., 2006). Reggies were later shown to participate in trafficking of the cholesterol transporter NPC1L1 (Ge et al., 2011) and the dopamine transporter DAT (Cremona et al., 2011) and to promote the clathrin-dependent uptake of the amyloid precursor protein (Schneider et al., 2008) and NPC1L1 (Ge et al., 2011). Of interest, reggies strikingly accumulate at cell–cell contact sites of many cells (Stuermer et al., 2004; Solis et al., 2010), where they are colocalized with E-cadherin in epithelial cells (Málaga-Trillo et al., 2009; Solis et al., 2012). More recently, reggies were shown to be functionally involved in adherens junction formation and dynamics in A431 epithelial cells. In these cells, reggie down-regulation by specific short hairpin RNAs (shRNAs) increased epidermal growth factor receptor signaling (by interfering with its uptake) and accelerated macropinocytosis (Solis et al., 2012), which is recognized as the pathway responsible for junctional E-cadherin internalization in MCF7 cells (Bryant et al., 2007). Junctional E-cadherin, in turn, is subject to rapid turnover and recycling (Hong et al., 2010). This and the finding that internalized E-cadherin is apparently trafficked in reggie-decorated vesicles (Solis et al., 2012) suggested that reggie might function in E-cadherin or generally in cargo recycling (Stuermer, 2010). This hypothesis received support from our work in neurons, which fail to extend their axon and are unable to regenerate axons after optic nerve lesion in zebrafish when reggie is down-regulated (Munderloh et al., 2009). Growth cone elongation and axon regeneration require the constant turnover and redelivery of membrane and membrane proteins (Shao et al., 2002; Falcone et al., 2006; Stuermer, 2010), a process that appears to be somehow regulated by reggie (Bodrikov et al., 2011; Koch et al., 2012).
To obtain a better understanding of whether and how reggies contribute to membrane protein trafficking and recycling, we decided to examine the role of reggie in simpler model cells. We used A431 cells for the analysis of how reggie might regulate E-cadherin trafficking and HeLa cells, which can be easily transfected and are therefore commonly used to explore the intricate network controlling cargo transport through the endosomal system. Moreover, HeLa cells exhibit a tubulovesicular trafficking system supported by proteins with membrane-deforming properties such as eps15 homology domain (EHD) family members (Naslavsky and Caplan, 2011) and sorting nexins (SNX; Worby and Dixon, 2002; Cullen, 2008). SNX4, in particular, has been identified as participant in the sorting of the transferrin (Tf) receptor (TfR) away from lysosomal degradation and into the Rab11a recycling pathway (Traer et al., 2007). It has been proposed that tubules provide an expansion of the endomembrane compartments for extensive cargo sorting through the endosome network which is assisted by GTPases of the Rab, Ras, and Rho families (Grant and Donaldson, 2009; Stenmark, 2009). Rab11a, for instance, defines the segment that receives cargo sorted away from degradation pathways and destined for recycling (Ullrich et al., 1996; Takahashi et al., 2012). The redelivery of recycling cargo to the PM typically involves components of the exocyst. The exocyst binds Rab11a (Zhang et al., 2004; Wu et al., 2005) and together with the GTPases TC10 and RalA promotes the targeted recycling of specific cargo (Chen et al., 2006; Stuermer, 2010). TC10 and exo70 are known to interact with reggie (Baumann et al., 2000; Chang et al., 2007; Bodrikov et al., 2011) for reinsertion into the PM of Glut4 and N-cadherin, respectively.
This information, together with our present finding that reggies decorate a widely ramified tubulovesicular compartment indicative of sorting and recycling, led us to examine whether reggies interact with SNX4 and Rab11a and contribute to TfR recycling in HeLa cells. We also explore whether reggies might participate in E-cadherin recycling in connection with Rab11a and SNX4 in A431 cells. This is addressed by using the so-called Ca2+ switch assay (Chitaev and Troyanovsky, 1998; Pertz et al., 1999). We thus analyze whether reggies, being evolutionarily conserved and present in basically every cell type, constitute a new member of the equally well-conserved Rab11a/SNX4-mediated recycling route.
RESULTS
Reggies are associated with the tubulovesicular sorting and recycling system
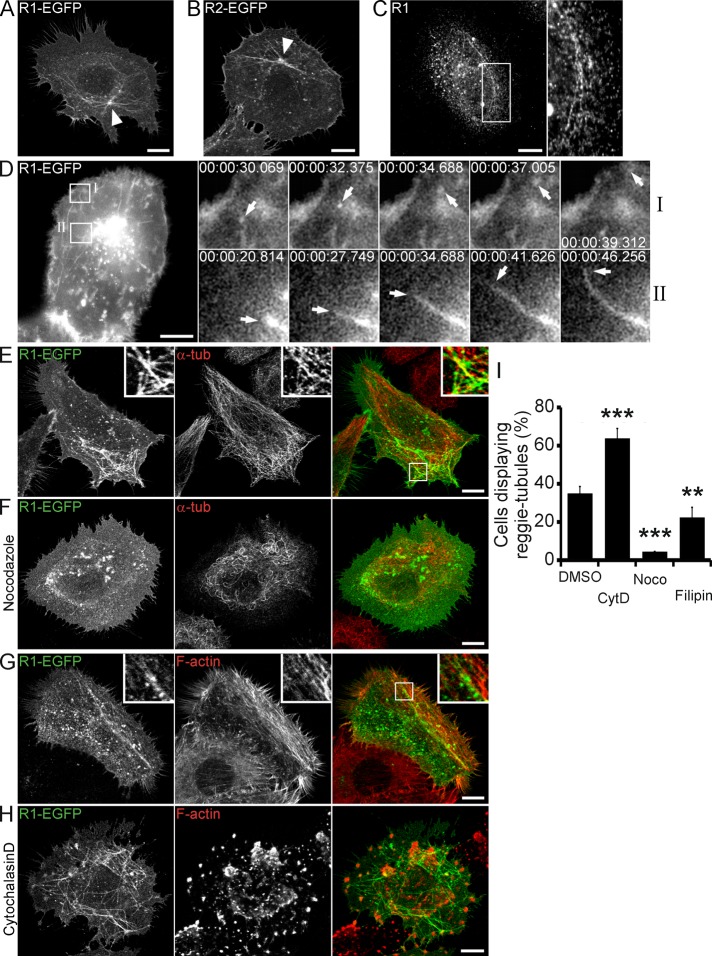
In our attempt to understand the contribution of reggie to the intracellular trafficking and recycling of membrane proteins (TfR in HeLa and E-cadherin in A431 cells), we transfected HeLa cells with reggie-1–enhanced green fluorescent protein (EGFP) or reggie-2–EGFP. Of interest, expression of either reggie-1 or -2 led to the formation of prominent reggie-positive tubular structures, which emerged from the perinuclear recycling compartment and reached to the vicinity of the PM (Figure 1, A and B). Such tubules were also apparent after immunostaining with a reggie-1–specific antibody (Ab) consisting of conspicuous rows of orderly aligned puncta and centered on the recycling compartment (Figure 1C). In addition, reggie-1–specific Abs labeled small and larger vesicles (the latter representing lysosomes; Stuermer et al., 2001). In further experiments aimed at characterizing of tubulovesicular system, we focused on reggie-1 and determined by immunostaining analyses that tubules decorated by reggie-1 (hereafter reggie-tubules) are not constituents of the endoplasmic reticulum, Golgi, and mitochondrial endomembrane systems (Supplemental Figure S1, A–C). Reggie-tubules appeared highly dynamic, with vesicles deriving from and merging with tubules and moving toward and away from the PM (Figure 1D and Supplemental Movie S1). Although less prominent, dynamic reggie-tubules were also observed in A431 cells (Supplemental Figure S1D and Supplemental Movie S2).
FIGURE 1:
Characterization of reggie-tubules in HeLa cells. (A–C) Confocal microscopy of HeLa cells shows reggie-decorated tubules after expression of either reggie-1-EGFP (R1-EGFP; A) or reggie-2-EGFP (R2-EGFP; B) emanating from the perinuclear recycling compartment (arrowheads). Immunostaining of endogenous reggie-1 (R1; C) shows its localization at tubular structures and vesicles. Boxed area indicates adjacent enlargement. (D) Real-time images of HeLa cells expressing R1-EGFP were recorded for 2 min. In the first time-lapse recording (I; left, boxed region), a vesicle emerges from the end of a reggie-tubule and moves toward the PM (arrows). The second time-lapse recording (II; left, boxed region) shows the elongation of a reggie-tubule toward the PM (arrows). (E–H) R1-EGFP–decorated tubules in HeLa cells partially colocalize with the α-tubulin microtubules (α-tub; E) and the F-actin cytoskeleton (G). Exposure to nocodazole (F) or cytochalasin D (H) caused collapse and abnormal number and organization of reggie-tubules, respectively. Boxed areas are enlarged in insets. (I) Quantification of dimethyl sulfoxide (control), nocodazole (Noco), cytochalasin D (CytD), and filipin effects on reggie-tubule formation in HeLa cells (n = 3, **p < 0.01, ***p < 0.001, one-way ANOVA; error bars, SEM). Scale bars, 10 μm.
As typical for tubulovesicular systems in earlier studies (Grant and Donaldson, 2009), the reggie-tubules extended along microtubules and collapsed when cells were exposed to the microtubule-polymerization blocker nocodazole (Figure 1, E, F, and I). Reggies also colocalized with filamentous actin (Langhorst et al., 2007). When cells were treated with cytochalasin D to inhibit actin polymerization, the number of cells with reggie-tubules increased, but tubules appeared highly disorganized (Figure 1, G–I). In contrast, the number of cells exhibiting reggie-tubules decreased significantly when cells were incubated with the cholesterol-sequestering drug filipin (Figure 1I), which is consistent with the notion that reggies are preferentially associated with cholesterol-enriched membrane domains (Roitbak et al., 2005; Langhorst et al., 2008). Reggies possess a cholesterol recognition amino acid consensus (CRAC) motif in their head domain (also known as the stomatin, prohibitin, flotillin, HFLK/C [SPFH] domain). The head domain and its acylation sites are required for the interaction of reggies with membranes (Neumann-Giesen et al., 2004; Liu et al., 2005; Langhorst et al., 2008), whereas the α-helical coiled-coil tail (flotillin) domain promotes homo- and hetero-oligomerization (Solis et al., 2007).
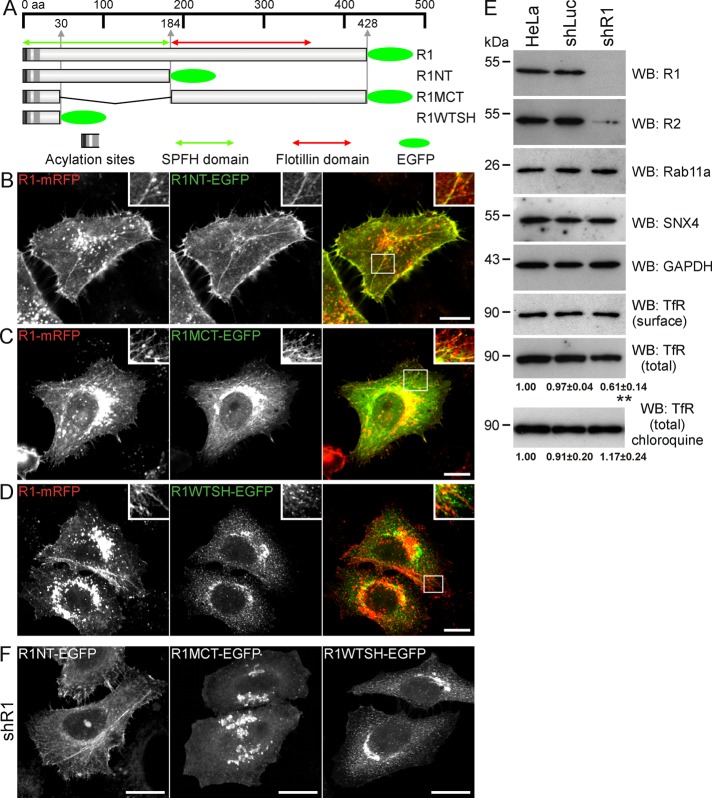
To evaluate which domain is responsible for the localization at tubules, we cotransfected reggie-1 full-length and its membrane-associated deletion constructs (Figure 2A; Langhorst et al., 2008). The construct containing the N-terminal SPFH domain (R1NT) and the one comprising the C-terminal tail (R1MCT) domain localized at reggie-tubules (Figure 2, B and C), whereas the construct lacking both regions (R1WTSH) but including the first 30 amino acids with the acylation sites of reggie-1 did not (Figure 2D). The R1MCT construct comprising the tail domain might localize to reggie-tubules by its interaction with the reggie-1 full-length protein. Therefore we generated shR1 HeLa cells to express the mutant proteins in reggie-depleted cells (Figure 2E). In shR1 cells, the R1MCT deletion construct (as well as the R1WTSH mutant) no longer bound to tubules (Figure 2F). The R1NT construct, however, still exhibited tubular structures in reggie-depleted cells (Figure 2F). These data indicate that the head domain is necessary for the association of reggie-1 with tubules, whereas the tail domain is dispensable. Of interest, the R1NT mutant was absent from large intracellular vesicles decorated by reggie-1 (Figure 2B), that is, lysosomes (Langhorst et al., 2008), suggesting that the tail domain might be necessary for lysosomal targeting.
FIGURE 2:
Reggie-1 head (SPFH) domain is necessary for its localization at tubules in HeLa cells. (A) Schematic representation of the C-terminal, EGFP-tagged reggie-1 constructs used in this study. Reggie-1 full-length (R1) and its membrane-associated deletion constructs lacking the tail (flotillin) domain (R1NT), the head domain (R1MCT), or both domains (R1WTSH). The EGFP tags are depicted as green ovals. (B–D) Confocal images of HeLa cells expressing wild-type reggie-1-mRFP (R1-mRFP) and reggie-1 deletion mutants revealed that R1NT-EGFP (B) and R1MCT-EGFP (C) decorate reggie-tubules, whereas the R1WTSH-EGFP (D) construct was not observed at tubules. Boxed areas are enlarged in insets. (E) Expression levels of reggie-1 (R1), reggie-2 (R2), Rab11a, SNX4, TfR, and glyceraldehyde-3-phosphate dehydrogenase as loading control were analyzed by Western blots (WBs) from extracts of shRNA stably transfected and untransfected HeLa cells. shRNA against reggie-1 (shR1) strongly reduced reggie-1 and reggie-2 expression compared with control transfected shRNA (shLuc) and HeLa cells, whereas no effects were observed on the levels of Rab11a and SNX4. Biotinylation analysis showed that TfR surface expression was not affected in shR1 cells. Total TfR expression level was significantly reduced in shR1 cells compared with shLuc and HeLa cells. This effect was rescued by blocking lysosomal degradation with 50 μM chloroquine (n = 4, **p < 0.01, one-way ANOVA, mean ± SEM). (F) Expression of the reggie-1 deletion constructs in shR1 HeLa cells revealed the formation of reggie-tubules by the construct containing the head domain (R1NT-EGFP). The reggie-1 deletion mutants lacking this domain (R1MCT and R1WTSH) were not observed at tubules. Scale bars, 10 μm.
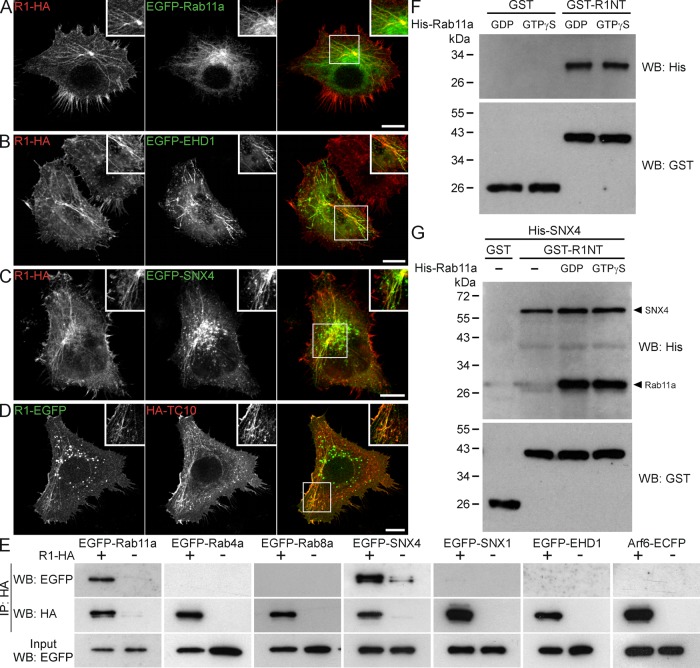
Tubulovesicular systems often serve in membrane protein sorting and recycling (Grant and Donaldson, 2009). To determine whether reggie-1 is a component of the recycling system, we cotransfected HeLa cells with reggie-1 and Rab11a. Both proteins strongly colocalized at the perinuclear region, as well as at tubular structures emerging from this area (Figure 3A). In agreement with these observations, reggie-1 and Rab11a (identified by double immunogold staining and electron microscope analysis) were detected together at elongated tubular structures adjacent to the PM of HeLa and PC12 cells (Supplemental Figure S2, A–D). Both were also coclustered at tubular structures extending along parallel bundles of what were most likely microtubules (Supplemental Figure S2B).
FIGURE 3:
Reggie-tubules belong to the recycling compartment. (A) Reggie-1-HA (R1-HA) and EGFP-Rab11a are colocalized at tubules, as well as at the recycling compartment in the perinuclear region, as seen in the merged image (right). (B–D) Reggie-1 also colocalized at tubules with EGFP-EHD1 (B) and EGFP-SNX4 (C), as well as with the exocyst-regulating GTPase TC10 (HA-TC10; D). Boxed areas are enlarged in insets. Scale bars, 10 μm. (E) Immunoprecipitation (IP) and Western blot (WB) analyses from cotransfected HeLa cell extracts show that reggie-1-HA (R1-HA) specifically interacts with EGFP-Rab11a and EGFP-SNX4 but not with EGFP-tagged Rab4a, Rab8a, SNX1, EHD1, or Arf6-ECFP. (F, G) Western blot analysis shows that recombinant His6-tagged Rab11a (His-Rab11a; F) loaded with GDP or GTPγS and SNX4 (His-SNX4; G) is efficiently pulled down by a GST-fusion construct of the head/SPFH domain of reggie-1 (GST-R1NT) but not by GST used as control. No competition is observed for the interaction of GST-R1NT with His6-tagged Rab11a and SNX4 (G).
In addition to Rab11a, numerous small GTPases have been reported to reside at the recycling compartment (Grant and Donaldson, 2009). Accordingly, the reggie-positive perinuclear compartment and tubules also contained the GTPases associated with recycling: Arf6 and Rab8a (Supplemental Figure S3, A and B), as well as with EHD1 and SNX4 (Figure 3, B and C), but not with the retromer component SNX1 (Supplemental Figure S3C). No significant colocalization with the early endosomal markers EEA1 and Rab4a was observed (Supplemental Figure S3, D and E).
The exocyst complex participates in membrane protein targeting from the recycling compartment to the PM (Grant and Donaldson, 2009). Consequently we found that reggie-tubules colocalized with the exocyst subunit Exo70, whereas Sec5 was associated with the reggie-positive perinuclear compartment (Supplemental Figure S4, A and B). Reggie-tubules also colocalized with the exocyst-regulating GTPases TC10 and RalA (Figure 3D and Supplemental Figure S4C). This connects reggie-tubules to reggie‘s role in Glut4 and N-cadherin trafficking to the PM, which requires TC10 and the exocyst (Baumann et al., 2000; Chen et al., 2006; Chang et al., 2007; Bodrikov et al., 2011).
To examine whether reggie-1 might interact with components of the tubulovesicular system specifically involved in recycling, we performed coimmunoprecipitation experiments on transfected HeLa cells. As shown in Figure 3E, immunoprecipitation experiments with reggie-1–hemagglutinin (HA) specifically coprecipitated EGFP-tagged Rab11a and SNX4 but not EHD1, Rab4a, Rab8a, SNX1, or Arf6-ECFP. To analyze whether reggie-1 directly interacts with Rab11a and SNX4, we carried out in vitro pull-down assays using recombinant proteins. Because the SPFH domain of reggie-1 seems to be responsible for its localization in tubules (Figure 2F), we generated a glutathione S-transferase (GST)-fusion construct (GST-R1NT) of this domain, excluding the hydrophobic stretch within the first 30 amino acids to avoid unspecific binding. Of note, GST-R1NT was able to pull down recombinant hexahistidine (His6)-tagged SNX4 and Rab11a independently of its loading with GDP or GTPγS (Figure 3, F and G). In addition, no competition was observed for the interaction of GST-R1NT with Rab11a and SNX4 (Figure 3G). Because a GST-Rab11a construct was unable to pull down His6-SNX4 (Supplemental Figure S4D), these results indicate that reggie-1 might be necessary for the coordination of SNX4 and Rab11a in the recycling compartment.
Together these data identify reggie-1 as a component of the tubulovesicular system involved in the regulation of recycling.
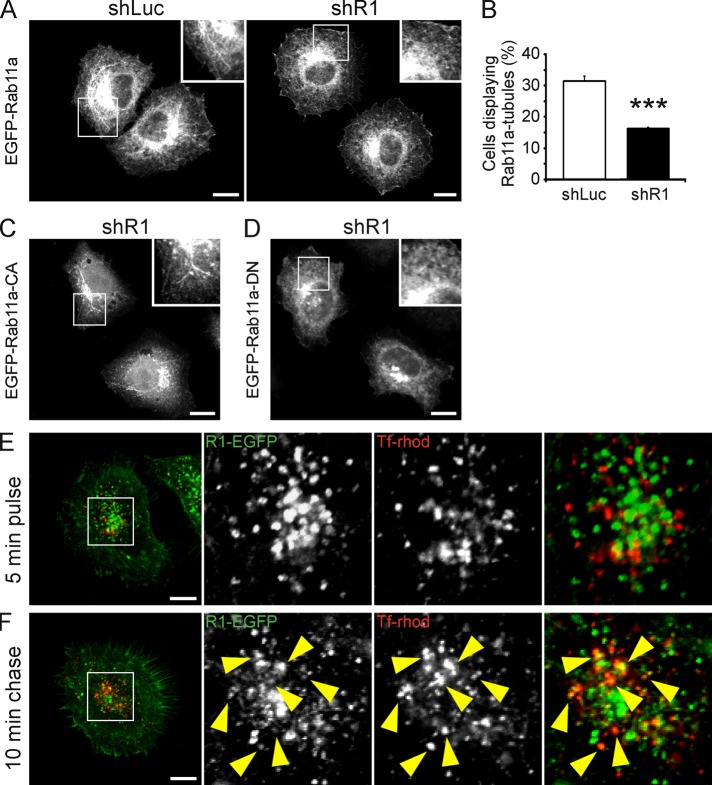
To evaluate whether reggie-1 controls the formation of the tubular recycling system, we analyzed the cellular distribution of Rab11a and EHD1 after depletion of reggie-1. Down-regulation of reggie-1 causes the proteasomal degradation of reggie-2 (Solis et al., 2007), so that cells have reduced levels of both reggies (Figure 2E). Rab11a in tubules was significantly reduced in shR1 cells as well as in HeLa cells treated with a specific small interfering RNA (siRNA) against reggie-1 compared with shLuc and control siRNA–transfected cells (Figure 4, A and B, and Supplemental Figure S5, A–C). Conversely, the pattern of EHD1-decorated tubules was normal in both shR1 and shLuc cells (Supplemental Figure S5D). Thus, although reggie-1 seems to regulate Rab11a localization in tubules, it is most probably not required for the formation of tubules per se. To study whether the presence of Rab11a in tubules depends on its activity, we analyzed the subcellular localization of constitutively active (CA) and dominant-negative (DN) mutants of Rab11a (Q70L and N25S, respectively; Ullrich et al., 1996). The Rab11a-CA mutant clearly localized at tubules in shR1 and shLuc control cells (Figure 4C), but the Rab11a-DN construct did not (Figure 4D). The absence of Rab11a-DN from tubules points to a relation between Rab11a localization and activity.
FIGURE 4:
Reggie-1 colocalizes with internalized Tf-rhod at the recycling compartment. (A) Localization of Rab11a (EGFP-Rab11a) at tubules is significantly reduced in reggie-depleted (shR1) cells compared with controls (shLuc). (B) Quantification of the effect of reggie-1 down-regulation on Rab11a-tubule formation in HeLa cells (n = 3, ***p < 0.001, paired t test; error bars, SEM). (C, D) shR1 HeLa cells showed that the constitutively active mutant of Rab11a (EGFP-Rab11a-CA; C) localizes at tubules, whereas its dominant-negative variant (EGFP-Rab11a-DN; D) is absent from tubules. (E, F) Tf-rhod–containing endosomes after a 5-min pulse do not significantly colocalize with reggie-1–labeled (R1-EGFP) structures at the perinuclear compartment (E). Tf-rhod colocalization with reggie-1, however, increased at the perinuclear recycling compartment (arrowheads; F) after a 5-min pulse followed by a 10-min chase. Boxed areas are magnified at right. Scale bars, 10 μm.
Taken together, our present data show that reggie-1 is a component of the tubulovesicular recycling system and might be involved in Rab11a activation during recycling.
The role of reggie-1 in TfR recycling
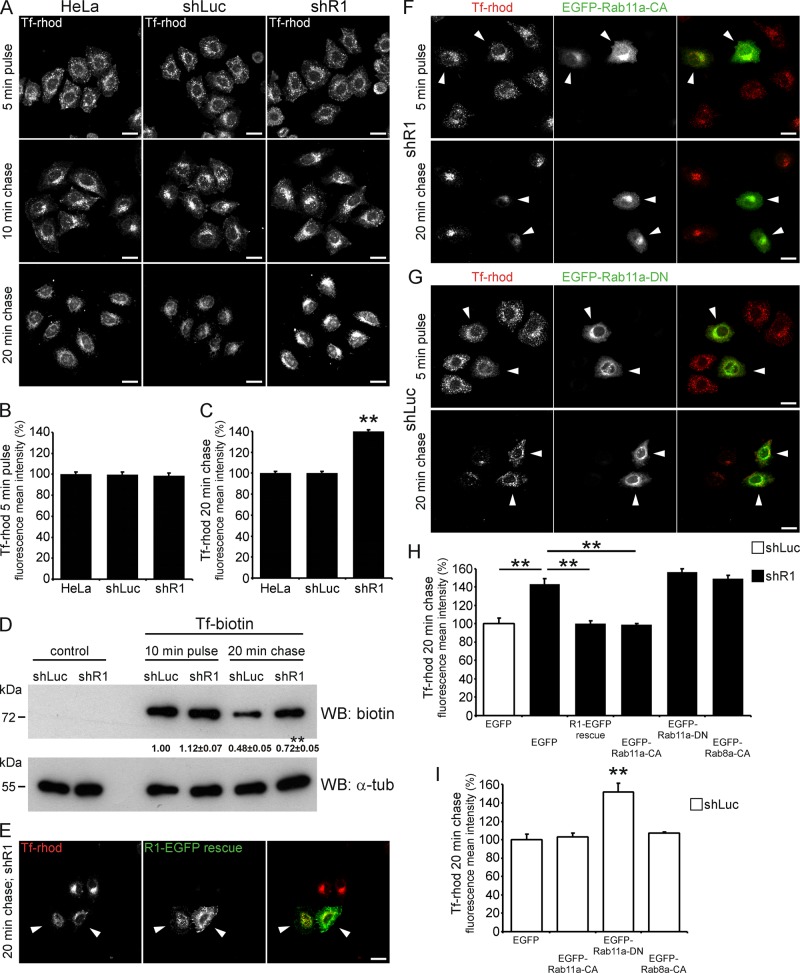
To determine whether reggie-1 is indeed involved in cargo recycling, we analyzed the trafficking of rhodamine-labeled transferrin (Tf-rhod) in HeLa cells expressing reggie-1–EGFP. No apparent colocalization between Tf-rhod and reggie-positive perinuclear structures was observed after 5 min of incubation (Figure 4E), suggesting that reggie-1 is not involved in Tf endocytosis. In a pulse-chase experiment, HeLa cells were incubated for 5 min with Tf-rhod (pulse), washed, and left for 10 min (chase) to allow Tf trafficking to the recycling compartment. As expected, Tf-rhod accumulated at the reggie-positive perinuclear recycling compartment (Figure 4F). Accordingly, quantification of the Pearson's r revealed a twofold increase in the colocalization of Tf-rhod and reggie-1-EGFP after a 10-min chase (0.21 ± 0.02 for a 5-min pulse and 0.42 ± 0.04 for a 5-min pulse/10-min chase; p < 0.001). Moreover, the TfR also accumulated at the perinuclear compartment in a similar pulse-chase experiment and colocalized with endogenous reggie-1 (Supplemental Figure S5E), suggesting that reggies may be involved in TfR recycling. How reggies affect Tf trafficking was examined using the pulse-chase method in shR1 cells. The amount and distribution of incorporated Tf-rhod did not differ between shR1, shLuc, and untransfected HeLa cells after a 5-min pulse (Figure 5, A and B). After a 10-min chase, cells showed similar accumulation of Tf-rhod at the perinuclear compartment (Figure 5A), excluding a major role of reggies in the endocytosis of Tf-rhod and its transport from early endosomes to the recycling compartment. Of importance, however, the perinuclear accumulation of Tf-rhod increased ∼40% in shR1 cells after a 20-min chase compared with shLuc and untransfected HeLa cells (Figure 5, A and C). Immunostainings also revealed increased accumulation of TfR at the perinuclear compartment in shR1 cells after a 20-min chase (Supplemental Figure S5, F and G). Therefore the absence of reggies seems to impair TfR recycling. Biochemical analysis of pulse-chase experiments using biotinylated Tf confirmed that down-regulation of reggies did not affect Tf endocytosis but significantly delayed its recycling after a 20-min chase (Figure 5D). The specificity of this phenotype was supported by a rescue experiment in which the shR1 cells were transfected with a shRNA-resistant reggie-1 construct (Solis et al., 2007). After a 20-min chase, the Tf-rhod accumulation at the perinuclear region was reduced to the normal level in cells in which reggie-1 was reintroduced (Figure 5, E and H) but not in untransfected shR1 cells.
FIGURE 5:
Down-regulation of reggie-1 impairs Tf recycling in HeLa cells. (A) Wild-type and shRNA stably transfected HeLa cells were pulsed with Tf-rhod for 5 min and then chased for 10 and 20 min. Reggie-depleted (shR1) cells showed no defects in Tf-rhod uptake (5-min pulse) and transport from early endosomes to the recycling compartment (10-min chase) compared with control transfected (shLuc) and untransfected HeLa cells. After a 20-min chase, however, the accumulation of Tf-rhod was retained at the perinuclear compartment in the majority of shR1 cells but reduced in shLuc and HeLa cells. (B, C) Quantification of the effect of reggie-1 down-regulation on Tf-rhod uptake (B) and recycling (C) in HeLa cells (n = 3, **p < 0.01, one-way ANOVA; error bars, SEM). (D) Western blot (WB) analysis of pulse-chase experiments in shLuc and shR1 cells using biotinylated Tf (Tf-biotin) confirmed that Tf recycling was delayed in reggie-depleted cells after a 20-min chase. No significant difference was observed in Tf-biotin uptake upon reggie down-regulation (n = 4, **p < 0.01, paired t test, mean ± SEM). α-Tubulin (α-tub) was used as loading control. (E) Expression of a shRNA-resistant reggie-1 construct (R1-EGFP rescue) rescued the Tf-rhod recycling defects observed after a 20-min chase in transfected (arrowheads) but not in untransfected shR1 cells. (F, G) Pulse-chase experiments were performed in shR1 (F) and control shLuc (G) cells expressing a Rab11a constitutively active (EGFP-Rab11a-CA) and dominant-negative (EGFP-Rab11a-DN) mutant, respectively. Whereas the Rab11a-CA construct was able to rescue the Tf-rhod recycling defects in shR1 cells without affecting its uptake (arrowheads; F), in shLuc cells the Rab11a-DN mutant impaired both Tf-rhod uptake and recycling (arrowheads; G). (H, I) Quantification of Tf-rhod perinuclear accumulation from pulse-chase experiments in E–G. A constitutively active mutant of Rab8a (EGFP-Rab8a-CA) was not able to rescue or mimic the effects of reggie depletion in shR1 or shLuc cells, respectively (n = 3, **p < 0.01, one-way ANOVA; error bars, SEM). Scale bars, 10 μm.
Further biochemical characterization of reggie-deprived cells revealed that the levels of Rab11a and SNX4 were unchanged in shR1 cells compared with untreated HeLa and control shLuc cells (Figure 2E). The total level of TfR, however, was significantly reduced in shR1 cells compared with controls (Figure 2E). The Tf uptake was not affected in shR1 cells (Figure 5, A, B, and D), nor was the cell surface expression of TfR diminished in shR1 cells, as shown in a biotinylation assay (Figure 2E). Thus reduction of TfR in reggie-depleted cells is likely caused by missorting of the endocytosed receptor into the lysosomal degradation pathway, as reported after down-regulation of SNX4 (Traer et al., 2007). In agreement with this view, shR1 cells showed striking colocalization of Tf-rhod with the lysosomal marker Lamp-2 after a 20-min chase, which is rarely observed in shLuc control cells (Supplemental Figure S5H). In addition, the total protein level of TfR was restored in shR1 cells when lysosomal degradation was blocked by chloroquine (Figure 2E).
If down-regulation of reggie-1 indeed affected activation of Rab11a, reduction of Tf recycling should be rescued by forced Rab11a activation in shR1 cells. Therefore we expressed the Rab11a-CA mutant in shR1 cells and analyzed Tf recycling in pulse-chase experiments. The level of incorporated Tf-rhod after a 5-min pulse was unaffected by the Rab11a-CA construct in shR1 cells (Figure 5F). After a 20-min chase, perinuclear accumulation of Tf-rhod was significantly reduced in cells that expressed Rab11a-CA but not in untransfected shR1 cells (Figure 5, F and H). Thus the CA mutant of Rab11a was able to rescue the recycling defects induced by reggie-1 down-regulation. Conversely, the Rab11a-DN and Rab8a-CA mutants were unable to rescue this phenotype (Figure 5H). Moreover, the defect in Tf recycling was partially mimicked in control shLuc cells by the expression of the Rab11a-DN mutant but not by the CA constructs of Rab11a and Rab8a (Figure 5, G and I). Expression of Rab11a-DN, however, in shLuc cells seemed to reduce Tf-rhod uptake, and its accumulation after a 20-min chase was observed not only at the perinuclear region, but also at peripheral areas of transfected cells (Figure 5G; Takahashi et al., 2012).
Together our data indicate that reggies might regulate TfR sorting and recycling by coordinating the activity of Rab11a and SNX4.
Reggie-1 regulates E-cadherin recycling in A431 cells
We recently showed that reggies are involved in E-cadherin–mediated cell adhesion and adherens junction dynamics (Solis et al., 2012). We also demonstrated that a substantial fraction of E-cadherin is trafficked in reggie-decorated vesicles and tubules at the level of adherens junctions (Solis et al., 2012), suggesting that reggies regulate aspects of E-cadherin transport.
To determine whether E-cadherin recycling to the PM occurs in conjunction with the tubulovesicular system discussed earlier, we coexpressed reggie-1–monomeric red fluorescent protein (mRFP) and E-cadherin–EGFP and followed protein trafficking by live imaging. We confirmed that E-cadherin was localized to the reggie-decorated tubulovesicular system and that both proteins traffic in tubules (in a Ca2+ switch experiment, described later; Supplemental Figure S6A and Supplemental Movie S3). To ascertain that tubules are not artifacts of reggie-1 overexpression, we performed immunostainings to visualize the distribution of the endogenous proteins. E-cadherin colocalized with endogenous reggie-1 at the perinuclear compartment and in tubular structures (Supplemental Figure S6B), indicating that E-cadherin is recycled in association with reggie-1 in A431 cells.
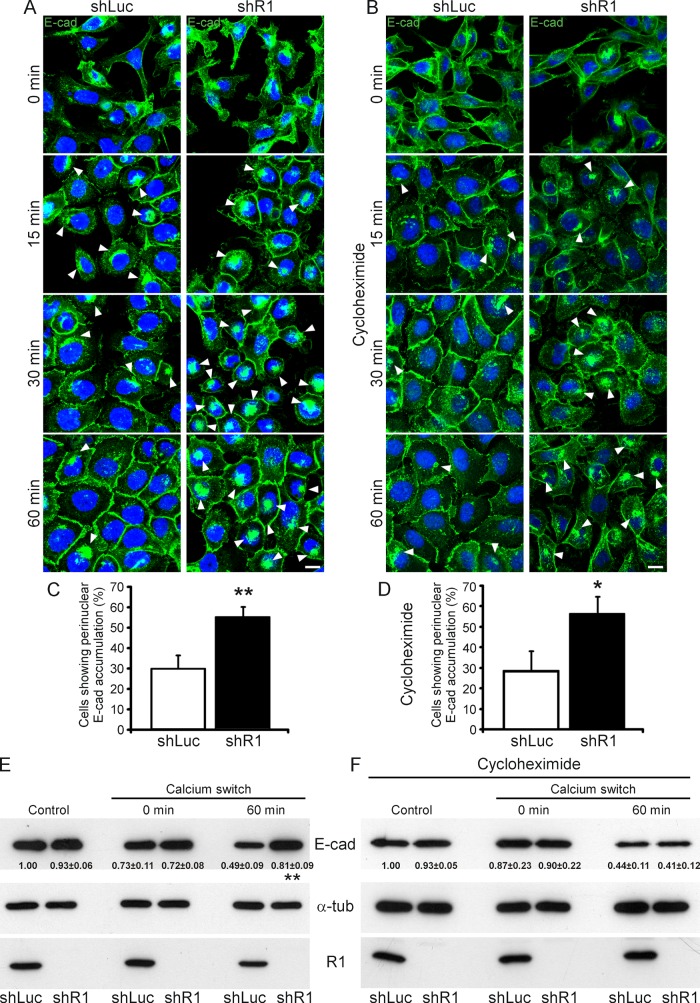
To induce E-cadherin endocytosis and recycling, we subjected shLuc and shR1 A431 cells to chelation by ethylene glycol tetraacetic acid (EGTA) in the Ca2+ switch experiment (causing E-cadherin internalization and loss of cell adhesion; Le et al., 1999). After Ca2+ depletion, E-cadherin became clustered in the perinuclear compartment in both shLuc and shR1 cells (Figure 6A) and partially colocalized with Rab11a and TfR (Supplemental Figure S6C), indicating that internalized E-cadherin accumulates at the recycling compartment in A431 cells. Perinuclear E-cadherin, however, also colocalized with the lysosomal marker Lamp-2 (Supplemental Figure S6C), suggesting that a fraction of E-cadherin undergoes degradation once it is endocytosed (see later discussion). We also confirmed by using a blocker of dynamin (MiTMAB) that the perinuclear accumulation resulted from E-cadherin endocytosis via the classic clathrin- and dynamin-dependent uptake pathways (Le et al., 1999; Supplemental Figure S6D). Perinuclear E-cadherin accumulation was still observed in shLuc and shR1 cells at 15 min after Ca2+ replenishment (Figure 6A). After 30 and 60 min, however, E-cadherin accumulation disappeared from the majority of the shLuc control cells but remained in the majority of the shR1 cells (Figure 6, A and C). This was quantified by counting cells with perinuclear E-cadherin accumulation of ≥6 μm2 after 30 min of Ca2+ replenishment (which is the average size of endocytic E-cadherin clusters; Figure 6C). The increased number of cells retaining E-cadherin clusters indicates that reggie-depleted A431 cells have defects in recycling E-cadherin to the PM.
FIGURE 6:
Down-regulation of reggie-1 impairs E-cadherin recycling in A431 cells. (A, B) E-cadherin endocytosis and recycling were induced in shRNA stably transfected A431 cells by incubation with EGTA for 3 h, followed by a Ca2+ recovery for 0, 15, 30, or 60 min in the absence (A) or presence of cycloheximide (B). Depletion of Ca2+ and E-cadherin internalization causes cells to give up cell–cell contacts (0 min). Ca2+ repletion (15, 30, and 60 min) allows cell–cell contact reformation. Immunostaining using an E-cadherin (E-cad) antibody showed its strong perinuclear accumulation (arrowheads) at 0- and 15-min recovery in both cycloheximide-treated and nontreated reggie-depleted (shR1) and control (shLuc) A431 cells. Perinuclear E-cad accumulation, however, was strongly reduced after 30- and 60-min recovery in shLuc cells but maintained in the majority of shR1 cells independent of cycloheximide treatment (arrowheads). Incomplete formation of E-cadherin–mediated cell contacts during Ca2+ recovery in cycloheximide-treated shR1 cells was evident (B). Scale bars, 10 μm. (C, D) Quantification of the effect of reggie down-regulation on E-cadherin recycling in normal (C) and cycloheximide-treated (D) A431 cells (n = 3, *p < 0.05, **p < 0.01, paired t test; error bars, SEM). (E, F) Expression levels of E-cadherin, reggie-1 (R1), and α-tubulin (α-tub) as loading control were analyzed by Western blots from extracts of shLuc and shR1 A431 cells in absence (E) or presence of cycloheximide (F). shLuc and shR1 A431 cells (control) showed similar E-cadherin expression levels, which were slightly reduced after Ca2+ chelation (0 min) independently of cycloheximide treatment. After 60-min recovery, however, shR1 cells presented a significantly higher E-cadherin expression level than control shLuc cells (E). This effect was abolished upon cycloheximide treatment (F; n = 4, **p < 0.01, paired t test, mean ± SEM).
As in HeLa cells, the recycling defects observed in A431 cells were not caused by reduced levels of Rab11a or SNX4 in shR1 cells (Supplemental Figure S6E). To determine whether reggie controls Rab11a activity during E-cadherin recycling to the same extent as during TfR recycling, we transfected shR1 A431 cells with Rab11a-CA and subjected them to the Ca2+ switch experiment. The majority of the shR1 cells with Rab11a-CA lost the abnormal perinuclear E-cadherin accumulation, indicating that Rab11a-CA was able to rescue the blocked recycling (Figure 7, A and B). Expression of Rab11a-DN, by contrast, mimicked the reggie-knockdown phenotype in control shLuc cells (Figure 7, A and B). Disappearance of E-cadherin accumulation in shR1 cells (rescue) was also achieved by overexpression of SNX4 but not by EHD1 or the Rab8a-CA mutant (Figure 7, A and B). These results suggest that reggie-1 participates in E-cadherin recycling and operates in pathways that codepend on Rab11a and SNX4.
FIGURE 7:
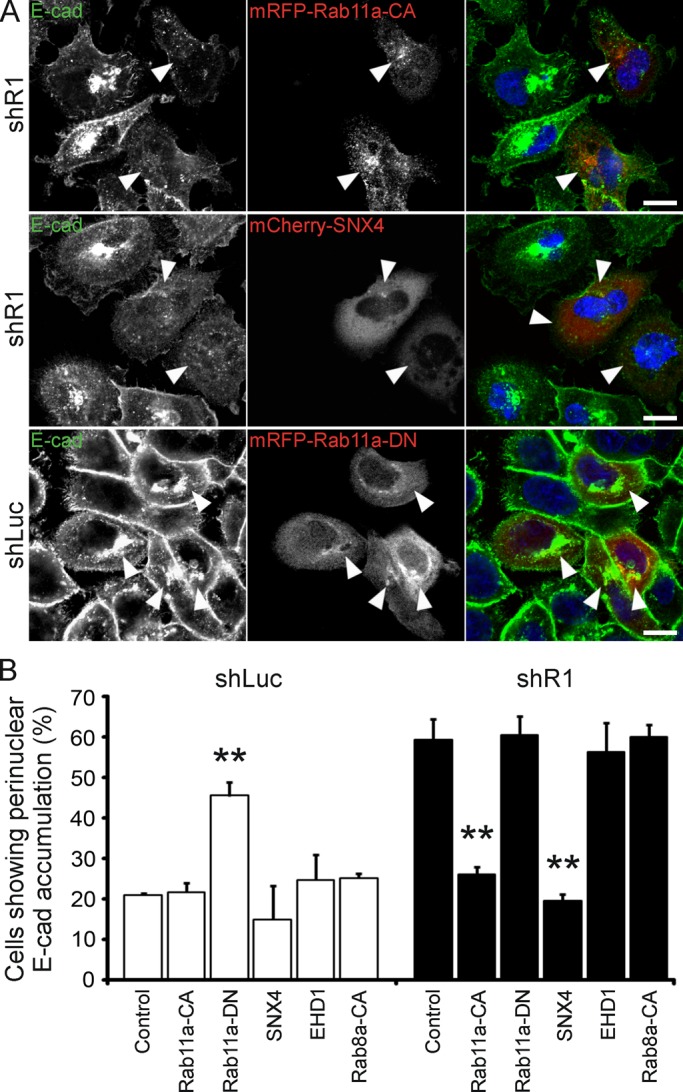
Rab11a and SNX4 rescue defects in E-cadherin recycling in reggie-depleted A431 cells. (A) A Ca2+ switch experiment in reggie-down-regulated (shR1) A431 cells revealed that the perinuclear E-cadherin (E-cad) accumulation was released in cells expressing the Rab11a constitutively active mutant (mRFP-Rab11a-CA) or SNX4 (mCherry-SNX4) after 30-min recovery. In a similar experiment, the E-cadherin recycling phenotype was mimicked in control shLuc A431 cells by the expression of the dominant-negative mutant of Rab11a (mRFP-Rab11a-DN). Scale bars, 10 μm. (B) Quantification of the effects of SNX4 and the Rab11a-CA and -DN mutants on E-cadherin recycling in shLuc and shR1 A431 cells. Expression of EGFP-EHD1 (EHD1) or mRFP-Rab8a-CA mutant (Rab8a-CA) was not able to mimic or rescue this phenotype in shLuc or shR1 cells, respectively (n = 4, **p < 0.01, one-way ANOVA; error bars, SEM).
In the Ca2+ switch experiment we noted that shR1 A431 cells reformed E-cadherin–labeled contacts almost to the same extent as their shLuc counterparts (Figure 6A) and despite persisting intracellular accumulation and thus impaired E-cadherin recycling. When protein levels were examined in Western blot analyses, the E-cadherin concentration was equal in shR1 and shLuc cells at 0 min but was significantly increased in shR1 cells after 60 min of Ca2+ replenishment (Figure 6E). To analyze whether this effect results from de novo synthesis of E-cadherin, we treated the cells with cycloheximide (blocking protein synthesis) and examined them again at 0 and 60 min in the Ca2+ switch experiment. Under this condition E-cadherin expression was equal in shLuc and shR1 cells at 60 min (Figure 6F), indicating that the increase in E-cadherin in shR1 cells results from up-regulation of its de novo synthesis. Both shR1 and shLuc cells, however, showed decreased E-cadherin at 60 min compared with 0 min, probably due to E-cadherin degradation (Figure 6F; Shen et al., 2008). Of note, treatment of cells with cycloheximide did not reduce the number of cells with increased perinuclear E-cadherin accumulation, and shR1 cells showed a delay in formation of E-cadherin–dependent cell contacts (Figure 6, B and D), indicating that reggie depletion impairs E-cadherin recycling to the PM and thus cell contact formation.
Together our findings identify reggie-1 as a component of the tubulovesicular recycling system and a regulator of TfR and E-cadherin recycling via its interaction with Rab11a and SNX4.
DISCUSSION
Our results identify reggie-1 as a binding partner of Rab11a and SNX4 and show that reggies function as regulators of the Rab11a- and SNX4-mediated sorting and recycling of TfR in HeLa and of E-cadherin in A431 cells. This corresponds to the association of reggie-1 and -2 with the tubulovesicular recycling compartment decorated with Rab11a, SNX4, and EHD1 and several other GTPases required for cargo trafficking. The central role that reggie-1 plays in recycling was recognized in reggie-deficient HeLa cells, which retain the TfR in a perinuclear compartment instead of redelivering it to the PM. Cell transfection with Rab11a-CA rescues the blocked recycling in reggie-deficient cells so that the TfR returned to the PM. Similarly, in A431 cells reggie-1 is needed for the redelivery of E-cadherin from the perinuclear Rab11a-positive compartment to the PM in the Ca2+ switch experiment. Again, Rab11a-CA as well as SNX4 overexpression rescues E-cadherin recycling. Together with the evidence that reggie-1 specifically binds and interacts with Rab11a and SNX4, our results imply that reggie-1 associates with a complex of Rab11a and SNX4 and coordinates their activities. As in previous studies, reggie down-regulation had no significant effect on the largely clathrin- and dynamin-dependent endocytosis of either TfR or E-cadherin, nor did reggie colocalize to any significant extent with EEA1 and vesicles of early endocytosis (Langhorst et al., 2008; Schneider et al., 2008; Cremona et al., 2011; Solis et al., 2012), although some staining of Rab5a at reggie-tubules occurred. By promoting recycling, reggie-1 apparently contributes, together with Rab11a and SNX4 (Traer et al., 2007), to divert TfR from lysosomal degradation in HeLa cells, since TfR was markedly reduced in shR1 cells. Of interest, A431 cells in the Ca2+ switch experiment responded to the impaired recycling caused by reggie down-regulation with E-cadherin de novo synthesis to promote the reformation of cell contacts. Together our results identify reggie-1 as a new and important regulator of membrane protein trafficking along the Rab11a- and SNX4-mediated sorting and recycling route.
The role of reggie in Rab11a- and SNX4-mediated recycling is almost certainly not confined to TfR and E-cadherin, as reggies were reported to affect aspects of trafficking of Glut4 (Baumann et al., 2000; Fecchi et al., 2006), DAT (Cremona et al., 2011), NPC1L1 (Ge et al., 2011), N-methyl-d-aspartate receptor (Swanwick et al., 2009), adhesion molecules (Hoehne et al., 2005; Swanwick et al., 2010; Bodrikov et al., 2011), Wnt and Hedgehog (Katanaev et al., 2008; Solis et al., 2013), and the T-cell receptor (Stuermer et al., 2004) in many different cell types and species. Whether the formation of a protein complex between reggie-1, SNX4, and Rab11a directly activates the GTPase or is involved in the trafficking of these molecules remains to be tested.
Our finding that the reggie proteins are constituents of the tubulovesicular system is consistent with their proposed role in the targeted delivery of membrane proteins such as guidance and growth factor receptors and adhesion and transporter proteins to specific sites of the PM such as cell contacts, leading edge of migratory cells, and growth cones (Stuermer, 2010). These represent regions with highly efficient recycling activities of membrane and associated proteins. This membrane protein turnover is absolutely required for cell migration and axon growth (Shao et al., 2002) and is likewise needed for adherens junction (Hong et al., 2010) and focal adhesion formation (Caswell et al., 2009). Membrane protein turnover, whether at cell–cell or cell–substrate contact sites or at the leading edge or growth cones, typically depends on Rab11a (Lock and Stow, 2005; Eva et al., 2010) and members of the SNX and EHD families (Shao et al., 2002; Worby and Dixon, 2002; Cullen, 2008; Naslavsky and Caplan, 2011). Thus our present results can indirectly account for the earlier findings that identified reggies as crucial elements for growth cone elongation and axon regeneration (Munderloh et al., 2009; Bodrikov et al., 2011; Koch et al., 2012). In vertebrates, reggies are present in all cells analyzed so far, yet apparently they are more important in certain cell types (neurons) than in others, particularly when the polarized transport of specific cargo molecules is concerned.
The function of reggies in the targeted recycling of cargo appears to be evolutionary conserved. Reggie down-regulation or misexpression causes defects in the deployment of specific proteins in species as distant as Drosophila and mammals (Hoehne et al., 2005; Katanaev et al., 2008), where Rab11a and SNX family members also have important roles. The striking degree of sequence identity between Drosophila and mammalian reggies (Rivera-Milla et al., 2006) further suggests that the domains for membrane binding and protein–protein interactions inherent to the head and tail domains are conserved.
Of interest, our results show, furthermore, that A431 cells compensate for the loss of reggie by increasing E-cadherin biosynthesis. Only then are the cells able to reestablish proper contacts, a process of great significance for tissue integrity and prevention of metastasis (Gavard and Gutkind, 2008). This observation emphasizes the importance of reggies in cargo recycling and can explain why down-regulation or even knockout of reggies gives subtle or no apparent phenotypes (Ludwig et al., 2010; Banning et al., 2012; Berger et al., 2012). We suspect that cells activate compensatory mechanisms—such as de novo synthesis of E-cadherin—in the absence of reggie to guarantee the targeted delivery of important membrane proteins.
Cells can possess a sophisticated tubulovesicular recycling system equipped with coat proteins in which cargo proteins can be sorted away for the delivery to specific sites of the cell (Jovic et al., 2009). This group of cargo proteins includes E-cadherin (Grant and Donaldson, 2009) and Glut4 and β-integrins, both representing membrane proteins whose trafficking was suggested to be influenced by reggie (Fecchi et al., 2006; Schrock et al., 2009; Stuermer, 2010). The expansion of the sorting and recycling compartment into tubules is considered to promote sorting and depends on high packing density of proteins with membrane-deforming properties, such as members of the EHD and SNX families (van Weering et al., 2010; Naslavsky and Caplan, 2011). Reggies seem to represent a new type of coat protein. The analysis of reggie domains suggests that reggies may possess membrane-deforming properties on their own, and therefore their overexpression would favor tubule formation. On the other hand, tubules persist in the absence of reggie, indicating that they are not absolutely required for tubule formation. Reggies cluster preferentially in cholesterol-rich domains and possess a CRAC motif in their head domain (Roitbak et al., 2005). Reggie association with tubules and the cholesterol depletion experiment that disrupts tubules suggest that these endomembranes have a specific lipid raft composition favored by the reggie proteins (Morrow and Parton, 2005; Langhorst et al., 2008). Thus reggies appear to create or demarcate membrane domains of specific lipid composition, probably like EHD1 and SNX4, which are associated with phosphatidylinositol-4-phosphate and phosphatidylinositol-4,5-bisphosphate (Jovic et al., 2009; Cullen, 2011).
Together our results show that reggie-1 binds Rab11a and SNX4 and contributes to Rab11a- and SNX4-dependent sorting and recycling needed for the redelivery of TfR and E-cadherin to the PM. This evidence, together with published data (ours and those of other groups), agrees with the idea that reggies regulate recycling and targeted redelivery of several (or even many) membrane proteins, implying that this activity might represent the function of reggie, which had for so long been ill defined.
MATERIALS AND METHODS
Reagents and antibodies
Cell culture reagents were purchased from Life Technologies (Carlsbad, CA). Monoclonal antibodies (mAbs) against reggie-1/flotillin-2 (ESA), flotillin-1, GM130, Rac1, E-cadherin, and RalA were from BD Biosciences (San Diego, CA), mAbs against HA and GFP from Roche (Indianapolis, IN), mAbs against CoxIV and PDI and polyclonal antibody (pAb) against E-cadherin and EEA1 from Cell Signaling Technology (Beverly, MA), mAb against TfR and pAb against Rab11a from Invitrogen (Carlsbad, CA), and pAb against reggie-1/flotillin-2 and mAb against biotin from Sigma-Aldrich (St. Louis, CA). pAbs against Sec5, Exo70, and SNX4 were from Santa Cruz Biotechnology (Santa Cruz, CA), mAb against RGS-His from Qiagen (Valencia, CA), and pAbs against α-tubulin and glyceraldehyde-3-phosphate dehydrogenase from Abcam (Cambridge, MA). Horseradish peroxidase–conjugated pAb against GST was from GE Healthcare (Piscataway, NJ), and mAb against Lamp-2 was from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Phalloidin–Alexa 568, rhodamine-conjugated transferrin, and biotinylated transferrin were from Invitrogen. Secondary Abs for immunostaining and Western blots were from Jackson ImmunoResearch (West Grove, PA).
Plasmids
The R1-HA, R1-EGFP, R1-EGFP rescue, R1-mRFP, R2-EGFP, R1NT-EGFP, R1MCT-EGFP, and R1WTSH-EGFP constructs were described previously (Langhorst et al., 2007; Solis et al., 2007, 2012). E-cadherin–EGFP vector was generously provided by Vann Bennett (Duke University Medical Center, Durham, NC), EGFP-Rab4a by Marci Scidmore (Cornell University, Ithaca, NY), EGFP-SNX4 by Kirsten Sandvig (University of Oslo, Oslo, Norway), EGFP-SNX1, mCherry-SNX4 by Peter Cullen (University of Bristol, Bristol, United Kingdom), EGFP-EHD1 by Juan S. Bonifacino (National Institutes of Health, Bethesda, MD), HA-TC10 by Alan Saltiel (University of Michigan, Ann Arbor, MI), EGFP-Rab8a WT and Q67L by Johan Peränen (University of Helsinki, Helsinki, Finland), and EGFP-Rab11a Q70L and S25N mutants by Stephen Ferguson (University of Western Ontario, London, Canada). Arf6-ECFP (11382) and EGFP-Rab11a WT (12674) were from Addgene (Cambridge, MA). mRFP-Rab11a mutants were obtained by replacing the EGFP open reading frame (ORF) with the mRFP sequence derived from the pmRFP-C1 plasmid. Arf6-DsRed was cloned by replacing the ECFP ORF with the DsRed sequence from the pDsRed-monomer-N1 vector (Clontech, Mountain View, CA). The reggie-1 head/SPFH domain (amino acids 31–183) was cloned into the pGEX-KG vector (Abdesselem et al., 2009) to produce a recombinant GST-fusion protein. Rab11a cDNA was cut from the EGFP-Rab11a plasmid and cloned into the vectors pGEX-4T-1 (GE Healthcare) and pQE30 (Qiagen) to generate GST- and His6-tagged constructs, respectively. His6-tagged SNX4 was generated by inserting the corresponding cDNA derived from the EGFP-SNX4 plasmid into the pQE30 vector. His6-tagged and GST-fusion proteins were expressed in Escherichia coli BL21-CodonPlus (DE3)-RIPL (Stratagene, Santa Clara, CA).
Cell cultures
HeLa and A431 cells were cultured in MEM and DMEM, respectively, supplemented with 10% fetal calf serum, l-glutamine, and penicillin/streptomycin. Vector transfections were carried out with FugeneHD (Roche) and siRNA transfections with Nanofectin siRNA transfection reagent (PAA, Linz, Austria) according to manufacturer's instructions. Alexa Fluor 546–labeled siRNA duplexes against reggie-1 (R1.0) and firefly luciferase (GL2) were obtained from Qiagen using previously described target sequences (Solis et al., 2007). Stably transfected HeLa cells were generated using shRNA plasmids previously reported (Solis et al., 2012) and by selection in normal media supplemented with 10 μg/ml puromycin. shRNA stably transfected A431 cells were previously described (Solis et al., 2012).
Immunofluorescence and microscopy
HeLa and A431 cells were washed and paraformaldehyde fixed at 37°C for better preservation of tubular structures. Staining of A431 and HeLa cells was done as previously described (Langhorst et al., 2008; Solis et al., 2012). Cells were analyzed with α-Plan-Apochromat 63×/1.4 oil or Apochromat 40×/1.2 water objective at a confocal microscope (LSM510 Meta) equipped with an AxioCamHRm (all Carl Zeiss, Jena, Germany).
Toxin treatments
HeLa cells grown on poly-l-lysine (pLys)-coated coverslips were transfected with R1-EGFP for 48 h and incubated for 30 min at 37°C in MEM supplemented with 10 μM cytochalasin D, 10 μM nocodazole, and 10 μg/ml filipin (all Calbiochem, La Jolla, CA) or dimethyl sulfoxide as control. After fixation and staining, ∼600 transfected cells per condition from three independent experiments were analyzed by counting R1-EGFP–expressing cells with tubular structures. The total amount of cotransfected cells was set to 100% (one-way analysis of variance [ANOVA] test for statistical analysis).
Quantification of Rab11a-tubules
For quantification of Rab11a-tubules after reggie-1 knockdown, HeLa cells were transfected with siRNA against reggie-1 or control GL2 for 48 h and then transfected with the EGFP-Rab11a vector for additional 24 h before fixation. Alternatively, shRNA stably transfected HeLa cells were transfected with the EGFP-Rab11a vector on pLys-coated coverslips and grown for 24 h before fixation. Approximately 600 cells per condition from three independent experiments were analyzed by comparing cells exhibiting or not Rab11a-positive tubules. The total amount of transfected cells was set to 100% (paired t test for statistical analysis).
Biochemical and biotinylation analyses
HeLa and A431 cells were lysed with ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 2 mM EDTA, 1% Triton X-100, 10% glycerin) supplemented with protease and phosphatase inhibitor cocktails (Thermo Scientific, Waltham, MA). Extracts were cleared by centrifugation and boiled at 95°C for 5 min or used for coimmunoprecipitation analyses. Briefly, lysates were incubated with 1 μg of Ab against HA epitope for 1 h at 4°C. Then 20 μl of protein G–agarose (Roche) was added and incubated overnight at 4°C. The beads were washed and prepared for SDS–PAGE and Western blots. TfR protein levels were analyzed from total cell extracts of parental and shRNA stably transfected HeLa cells nontreated or treated overnight with 50 μM chloroquine (Sigma-Aldrich) in normal medium to block lysosomal degradation. Quantification of blots was done using ImageJ (National Institutes of Health, Bethesda, MD) from four independent experiments. One-way ANOVA or paired t test was used for statistical analysis. Biotinylation of cell surface proteins was carried out as described previously (Solis et al., 2012).
Transferrin uptake and pulse-chase assays
The transferrin uptake and pulse-chase experiments were carried out as described previously (Jovic et al., 2009). Briefly, HeLa cells were starved in MEM lacking serum and supplemented with 0.5% bovine serum albumin (MEM-BSA) for 1 h at 37°C. Cells were incubated with MEM-BSA supplemented with 2 μg/ml Tf-rhod or Tf-biotin for the time indicated in the corresponding figures. Then cells were either fixed as described or subjected to a chase of 10 or 20 min in complete MEM at 37°C, followed by fixation. Fluorescence mean intensities of total and perinuclear accumulation of Tf-rhod were scored from roughly 200 cells per group (three independent experiments) using LSM Image Browser software (Carl Zeiss) for uptake and pulse-chase experiments (one-way ANOVA test for statistical analysis). Colocalization of R1-EGFP and Tf-rhod was quantified from confocal images by Pearson's r using the JACoP plug-in for ImageJ (Bolte and Cordelieres, 2006). The thresholds were determined automatically using Costes automatic thresholding, and Pearson's r was scored from at least 100 cells per condition from two independent experiments (paired t test for statistical analysis). For immunoblotting, HeLa cells were starved for 1 h in 0.2% MEM-BSA, followed by a 10-min pulse with 20 μg/ml Tf-biotin in MEM-BSA. After several washing steps with acidic buffer (0.2 M acetic acid, 0.5 M NaCl) to remove surface bound Tf, cells were either lysed immediately or subjected to a chase for 20 min in complete MEM at 37°C and then lysed and prepared for SDS–PAGE and Western blots using Abs against biotin and α-tubulin as control. Quantification of blots was done from four independent experiments (paired t test for statistical analysis).
Pull down of recombinant proteins
GST-fusion proteins were isolated from bacteria cell extracts using glutathione Sepharose 4B beads (GE Healthcare) according to manufacturer's instructions. GST-fusion proteins immobilized to glutathione Sepharose beads were used to pull down His6-tagged proteins. Briefly, bacterial extracts containing His6-tagged Rab11a were incubated with 1 mM GDP or 100 μM GTPγS in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, supplemented with 150 mM NaCl and 10 mM MgCl2 for 1 h at room temperature to allow guanine nucleotide loading. Equal volumes of GDP- or GTPγS-loaded Rab11a extracts and/or extracts containing His6-tagged SNX4 were applied to GST-coupled beads and incubated for 3 h at 4°C. Beads were repeatedly washed, and bound proteins were eluted with 40 mM reduced glutathione in 50 mM Tris-HCl, pH 8.0, and 150 mM NaCl. Samples were prepared for SDS–PAGE, and Western blots were carried out using anti-GST and anti–RGS-His Abs.
Calcium switch experiments
A431 cells were grown to ∼60% confluency on pLys-coated coverslips for immunostaining or on normal cell culture plates for immunoblotting. Cells were treated with growth medium without serum supplemented with 4 mM EGTA for 3 h, followed by several washing steps and addition of normal growth medium for the indicated time points. For the analysis of perinuclear accumulation of E-cadherin, the proportion of cells containing E-cadherin–labeled perinuclear clusters of ≥6 μm2 was counted from LSM pictures using AxioVision 4.8 software (Carl Zeiss). When indicated, 10 μM of cycloheximide was added 1.5 h before fixation or lysis. The dynamin-2 blocker MiTMAB (Calbiochem) was added at 20 μM during Ca2+ chelation and recovery. Quantification of immunostainings was done from 100–200 cells per condition (three or four independent experiments), and blots were measured from three independent experiments (paired t test for statistical analysis).
Live cell imaging
HeLa and A431 cells were transfected for 48 h on pLys-coated coverslips and recorded with the Colibri imaging system (470 and 555 nm) and the α-Plan Fluar 100×/1.45 objective at the Axiovert 200M equipped with an AxioCamHRm (Carl Zeiss). Cells were maintained in MEM and DMEM, respectively, at 37°C, 5% CO2, and controlled humidity. Images were acquired with 100% light-emitting diode power every 100 or 1000 ms for 1–5 min and analyzed using AxioVision 4.8.
Electron microscopy analyses
HeLa cells after R1-EGFP transfection and normal PC12 cells were processed for postembedding immuno–electron microscopy gold labeling, avoiding antigen redistribution, as previously described (Langhorst et al., 2008). Reggie-1 was labeled with the mAb ESA and Rab11a with a pAb. Antibody labeling was visualized by protein A (pA)–gold conjugates with a 5-nm gold tag (pA-Au5nm) and mAb labeling with goat anti-mouse F(ab)2–gold conjugates with 10-nm gold (F[ab]2-Au10nm). This combination excludes cross-reactive gold labeling. Specifically pA–gold binds only to the Fc region of pAb but cannot bind to F(ab)2. Conversely, F(ab)2–gold conjugate cannot bind to the pAb used or to pA.
Supplementary Material
Abbreviations used:
- Ab
antibody
- PM
plasma membrane
- shRNA
short hairpin RNA
- TfR
transferrin receptor
- Tf-rhod
rhodamine-coupled transferrin
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-12-0854) on July 3, 2013.
*These authors contributed equally to this work.
†Present address: Department of Systemic Cell Biology, Max-Planck-Institute of Molecular Physiology, 44227 Dortmund, Germany.
The authors declare no conflict of interest.
REFERENCES
- Abdesselem H, Shypitsyna A, Solis GP, Bodrikov V, Stuermer CA. No Nogo66- and NgR-mediated inhibition of regenerating axons in the zebrafish optic nerve. J Neurosci. 2009;29:15489–15498. doi: 10.1523/JNEUROSCI.3561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning A, Ockenga W, Finger F, Siebrasse P, Tikkanen R. Transcriptional regulation of flotillins by the extracellularly regulated kinases and retinoid x receptor complexes. PLoS One. 2012;7:e45514. doi: 10.1371/journal.pone.0045514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- Berger T, et al. Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene. 2012 doi: 10.1038/onc.2012.499. doi: 10.1038/onc.2012.499. [DOI] [PubMed] [Google Scholar]
- Bodrikov V, Solis GP, Stuermer CA. Prion protein promotes growth cone development through reggie/flotillin-dependent N-cadherin trafficking. J Neurosci. 2011;31:18013–18025. doi: 10.1523/JNEUROSCI.4729-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- Chang L, Chiang SH, Saltiel AR. TC10alpha is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology. 2007;148:27–33. doi: 10.1210/en.2006-1167. [DOI] [PubMed] [Google Scholar]
- Chen XW, Inoue M, Hsu SC, Saltiel AR. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem. 2006;281:38609–38616. doi: 10.1074/jbc.M512847200. [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J Cell Biol. 1998;142:837–846. doi: 10.1083/jcb.142.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona ML, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- Cullen PJ. Phosphoinositides and the regulation of tubular-based endosomal sorting. Biochem Soc Trans. 2011;39:839–850. doi: 10.1042/BST0390839. [DOI] [PubMed] [Google Scholar]
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J Cell Sci. 2006;119:4758–4769. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006;20:705–707. doi: 10.1096/fj.05-4661fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. A molecular crosstalk between E-cadherin and EGFR signaling networks. In: Haley JD, Gullick WJ, editors. Cancer Drug Discovery and Development: EGFR Signaling Networks in Cancer Therapy. New York: Humana Press; 2008. pp. 139–154. [Google Scholar]
- Ge L, Qi W, Wang LJ, Miao HH, Qu YX, Li BL, Song BL. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc Natl Acad Sci USA. 2011;108:551–556. doi: 10.1073/pnas.1014434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehne M, de Couet HG, Stuermer CA, Fischbach KF. Loss- and gain-of-function analysis of the lipid raft proteins Reggie/Flotillin in Drosophila: they are posttranslationally regulated, and misexpression interferes with wing and eye development. Mol Cell Neurosci. 2005;30:326–338. doi: 10.1016/j.mcn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hong S, Troyanovsky RB, Troyanovsky SM. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc Natl Acad Sci USA. 2010;107:3528–3533. doi: 10.1073/pnas.0911027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M, Kieken F, Naslavsky N, Sorgen PL, Caplan S. Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling. Mol Biol Cell. 2009;20:2731–2743. doi: 10.1091/mbc.E08-11-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J. 2008;27:509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase K, Nakamura T, Takaya A, Aoki K, Namikawa K, Kiyama H, Inagaki S, Takemoto H, Saltiel AR, Matsuda M. GTP hydrolysis by the Rho family GTPase TC10 promotes exocytic vesicle fusion. Dev Cell. 2006;11:411–421. doi: 10.1016/j.devcel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kioka N, Ueda K, Amachi T. Vinexin, CAP/ponsin, ArgBP2: a novel adaptor protein family regulating cytoskeletal organization and signal transduction. Cell Struct Funct. 2002;27:1–7. doi: 10.1247/csf.27.1. [DOI] [PubMed] [Google Scholar]
- Koch JC, Solis GP, Bodrikov V, Michel U, Haralampieva D, Shypitsyna A, Tonges L, Bahr M, Lingor P, Stuermer CA. Upregulation of reggie-1/flotillin-2 promotes axon regeneration in the rat optic nerve in vivo and neurite growth in vitro. Neurobiol Dis. 2012;51:168–176. doi: 10.1016/j.nbd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Reuter A, Jaeger FA, Wippich FM, Luxenhofer G, Plattner H, Stuermer CA. Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur J Cell Biol. 2008;87:211–226. doi: 10.1016/j.ejcb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Langhorst MF, Solis GP, Hannbeck S, Plattner H, Stuermer CA. Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 2007;581:4697–4703. doi: 10.1016/j.febslet.2007.08.074. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem. 2005;280:16125–16134. doi: 10.1074/jbc.M500940200. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191:771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, Stuermer CA. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow IC, Parton RG. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Munderloh C, Solis GP, Bodrikov V, Jaeger FA, Wiechers M, Málaga-Trillo E, Stuermer CA. Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J Neurosci. 2009;29:6607–6615. doi: 10.1523/JNEUROSCI.0870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122–131. doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Giesen C, Falkenbach B, Beicht P, Claasen S, Luers G, Stuermer CA, Herzog V, Tikkanen R. Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J. 2004;378:509–518. doi: 10.1042/BJ20031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto GP, Nichols BJ. The roles of flotillin microdomains—endocytosis and beyond. J Cell Sci. 2011;124:3933–3940. doi: 10.1242/jcs.092015. [DOI] [PubMed] [Google Scholar]
- Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Milla E, Stuermer CA, Málaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63:343–357. doi: 10.1007/s00018-005-5434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitbak T, Surviladze Z, Tikkanen R, Wandinger-Ness A. A polycystin multiprotein complex constitutes a cholesterol-containing signalling microdomain in human kidney epithelia. Biochem J. 2005;392:29–38. doi: 10.1042/BJ20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, Hell SW, Simons M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28:2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock Y, Solis GP, Stuermer CA. Regulation of focal adhesion formation and filopodia extension by the cellular prion protein (PrPC) FEBS Lett. 2009;583:389–393. doi: 10.1016/j.febslet.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Shao Y, Akmentin W, Toledo-Aral JJ, Rosenbaum J, Valdez G, Cabot JB, Hilbush BS, Halegoua S. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol. 2002;157:679–691. doi: 10.1083/jcb.200201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ. Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J Biol Chem. 2008;283:5127–5137. doi: 10.1074/jbc.M703300200. [DOI] [PubMed] [Google Scholar]
- Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, Stuermer CA. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis GP, Luchtenborg AM, Katanaev VL. Wnt secretion and gradient formation. Int J Mol Sci. 2013;14:5130–5145. doi: 10.3390/ijms14035130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis GP, Malaga-Trillo E, Plattner H, Stuermer CA. Cellular roles of the prion protein in association with reggie/flotillin microdomains. Front Biosci. 2010;15:1075–1085. doi: 10.2741/3662. [DOI] [PubMed] [Google Scholar]
- Solis GP, Schrock Y, Huelsbusch N, Wiechers M, Plattner H, Stuermer CA. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol Biol Cell. 2012;23:1812–1825. doi: 10.1091/mbc.E11-12-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stuermer CA. The reggie/flotillin connection to growth. Trends Cell Biol. 2010;20:6–13. doi: 10.1016/j.tcb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Stuermer CA, Lang DM, Kirsch F, Wiechers M, Deininger SO, Plattner H. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol Biol Cell. 2001;12:3031–3045. doi: 10.1091/mbc.12.10.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuermer CA, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Shapiro ME, Vicini S, Wenthold RJ. Flotillin-1 mediates neurite branching induced by synaptic adhesion-like molecule 4 in hippocampal neurons. Mol Cell Neurosci. 2010;45:213–225. doi: 10.1016/j.mcn.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Shapiro ME, Yi Z, Chang K, Wenthold RJ. NMDA receptors interact with flotillin-1 and -2, lipid raft-associated proteins. FEBS Lett. 2009;583:1226–1230. doi: 10.1016/j.febslet.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kubo K, Waguri S, Yabashi A, Shin HW, Katoh Y, Nakayama K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci. 2012;125:4049–4057. doi: 10.1242/jcs.102913. [DOI] [PubMed] [Google Scholar]
- Traer CJ, Rutherford AC, Palmer KJ, Wassmer T, Oakley J, Attar N, Carlton JG, Kremerskothen J, Stephens DJ, Cullen PJ. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol. 2007;9:1370–1380. doi: 10.1038/ncb1656. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Verkade P, Cullen PJ. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol. 2010;21:371–380. doi: 10.1016/j.semcdb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.