Abstract
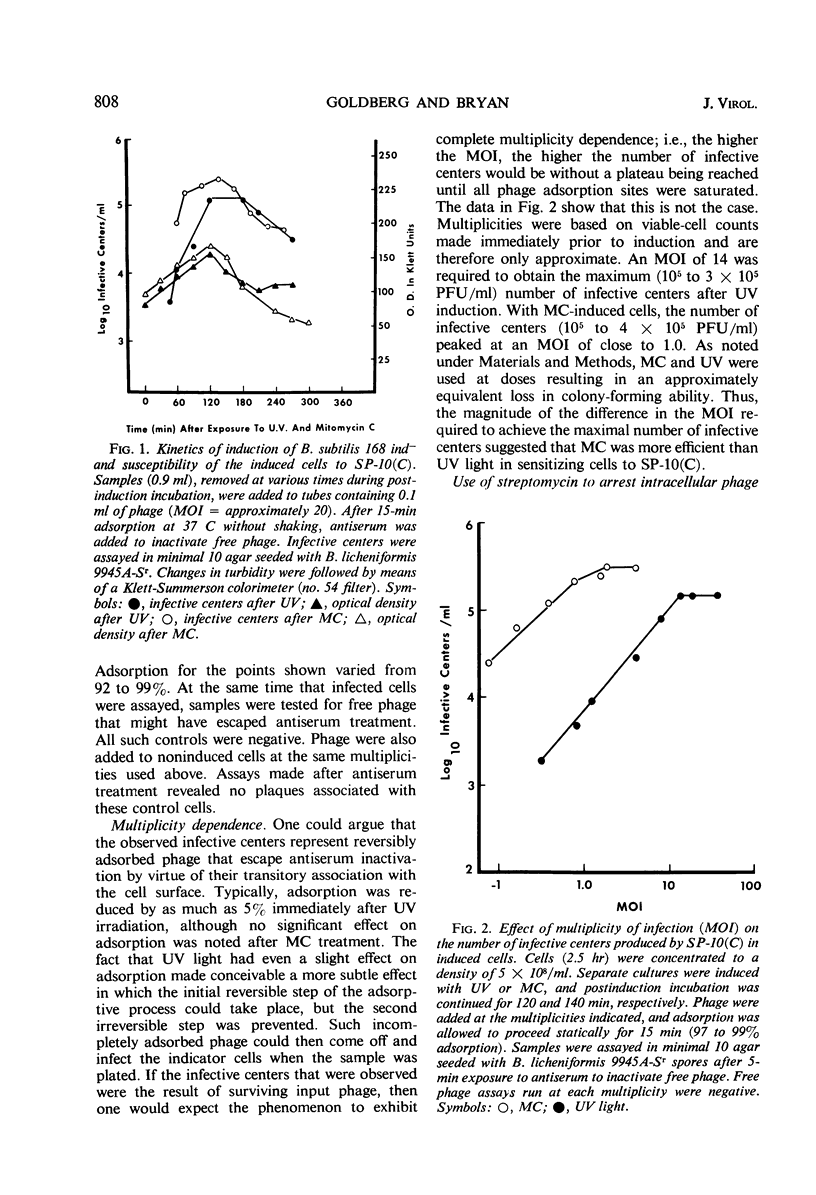
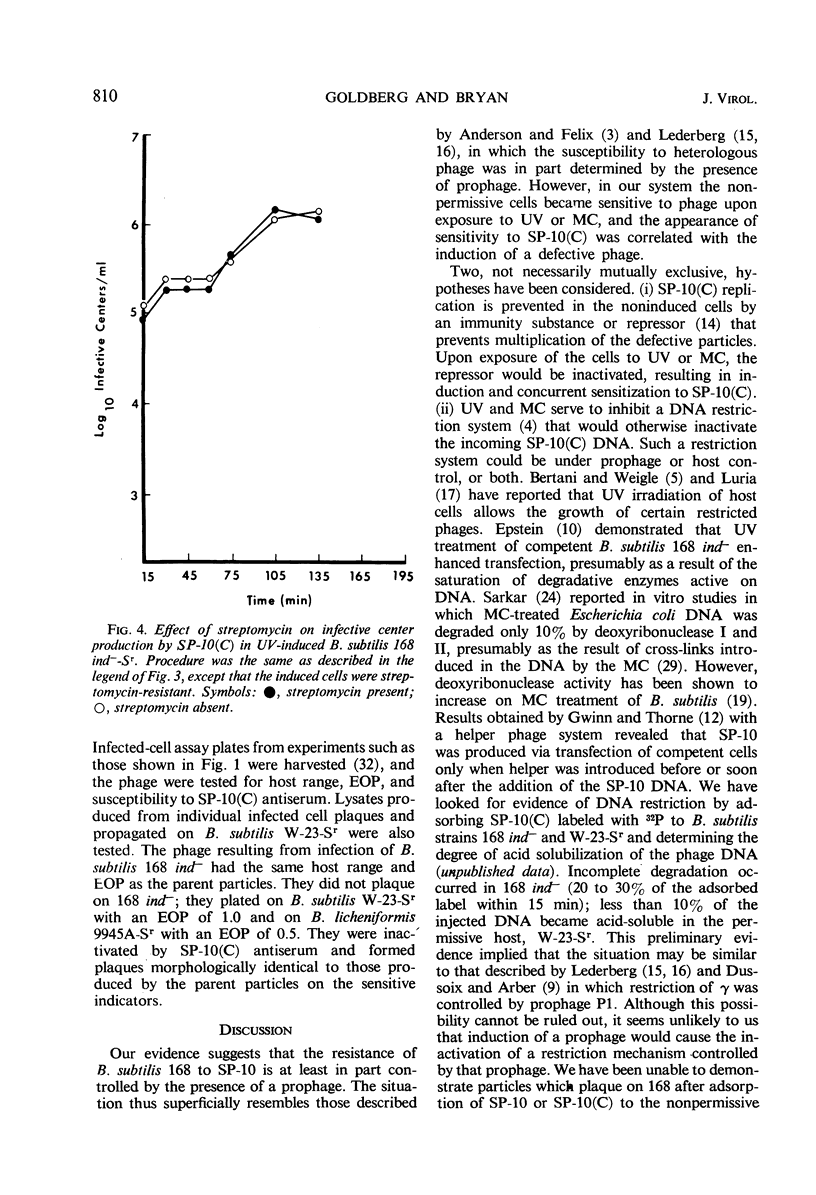
Strains of Bacillus that harbor defective phage PBSX were found to be insensitive to SP-10(C), although the phage adsorbed to these insensitive strains. Strains that did not carry the phage were sensitive to SP-10(C). B. subtilis 168 ind−, which can be tranduced by SP-10(C) but is nonpermissive for the phage, was rendered phage-sensitive after treatment with ultraviolet (UV) light or mitomycin C. After induction with UV light, maximal sensitivity to SP-10(C) was obtained at a multiplicity of infection (MOI) of approximately 14; with mitomycin C induction, an MOI of approximately 1.0 was required. Phage maturation in sensitized cells was followed by plating infected streptomycin-sensitive cells in the presence of streptomycin at various stages during phase development. The latent period was estimated at 60 to 75 min. We suggest that the resistance of B. subtilis 168 to SP-10 is controlled, at least in part, by the presence of a defective prophage.
Full text
PDF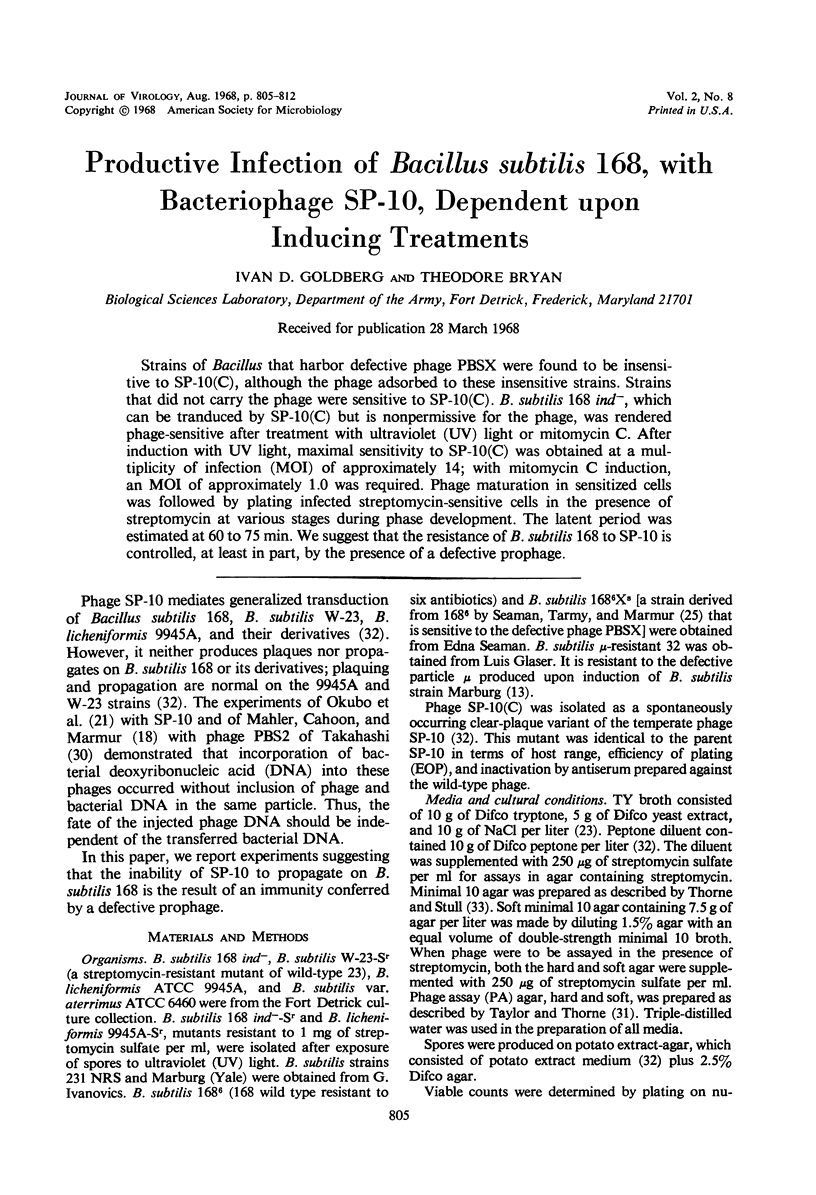





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., FELIX A. The Vi type-determining phages carried by Salmonella typhi. J Gen Microbiol. 1953 Aug;9(1):65–88. doi: 10.1099/00221287-9-1-65. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- BROCK T. D. ACTION OF STREPTOMYCIN AND RELATED ANTIBIOTICS. Fed Proc. 1964 Sep-Oct;23:965–975. [PubMed] [Google Scholar]
- BROCK T. D., MOSSER J., PEACHER B. THE INHIBITION BY STREPTOMYCIN OF CERTAIN STREPTOCOCCUS BACTERIOPHAGES, USING HOST BACTERIA RESISTANT TO THE ANTIBIOTIC. J Gen Microbiol. 1963 Oct;33:9–22. doi: 10.1099/00221287-33-1-9. [DOI] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- Epstein H. T. Transfection enhancement by ultraviolet irradiation. Biochem Biophys Res Commun. 1967 Apr 20;27(2):258–262. doi: 10.1016/s0006-291x(67)80071-8. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- Gwinn D. D., Thorne C. B. Helper phage-dependent transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1966 Oct 20;25(2):260–266. doi: 10.1016/0006-291x(66)90590-0. [DOI] [PubMed] [Google Scholar]
- IONESCO H., RYTER A., SCHAEFFER P. SUR UN BACT'ERIOPHAGE H'EBERG'E PAR LA SOUCHE MARBURG DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Dec;107:764–776. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Lederberg S. Host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. Virology. 1965 Nov;27(3):378–387. doi: 10.1016/0042-6822(65)90117-0. [DOI] [PubMed] [Google Scholar]
- MAHLER I., CAHOON M., MARMUR J. BACILLUS SUBTILIS DEOXYRIBONUCLEIC ACID TRANSFER IN PBS2 TRANSDUCTION. J Bacteriol. 1964 Jun;87:1423–1428. doi: 10.1128/jb.87.6.1423-1428.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA Y., NAKATA K., SAKAMOTO Y. On the action mechanism of mitomycin C. Biochem Biophys Res Commun. 1961 Dec 20;6:339–343. doi: 10.1016/0006-291x(61)90141-3. [DOI] [PubMed] [Google Scholar]
- NESTER E. W. PENICILLIN RESISTANCE OF COMPETENT CELLS IN DEOXYRIBONUCLEIC ACID TRANSFORMATION OF BACILLUS SUBTILIS. J Bacteriol. 1964 Apr;87:867–875. doi: 10.1128/jb.87.4.867-875.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STODOLSKY M., BOTT K., STRAUSS B. SEPARATION OF THE TRANSFORMING AND VIRAL DEOXYRIBONUCLEIC ACIDS OF A TRANSDUCING BACTERIOPHAGE OF BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Oct;50:679–686. doi: 10.1073/pnas.50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITER H. THE LOCATION OF THE INHIBITORY ACTION OF KCN AND SEVERAL POLYAMINES ON BACTERIOPHAGE REPLICATION. Virology. 1963 Dec;21:636–641. doi: 10.1016/0042-6822(63)90237-x. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- STICKLER D. J., TUCKER R. G., KAY D. BACTERIOPHAGE-LIKE PARTICLES RELEASED FROM BACILLUS SUBTILIS AFTER INDUCTION WITH HYDROGEN PEROXIDE. Virology. 1965 May;26:142–145. doi: 10.1016/0042-6822(65)90035-8. [DOI] [PubMed] [Google Scholar]
- SYMONDS N. Effects of ultraviolet light during the second half of the latent period on bacteria infected with phage T2. Virology. 1957 Jun;3(3):485–495. doi: 10.1016/0042-6822(57)90005-3. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., IYER V. N. CROSSLINKING OF DNA BY ENZYMATICALLY OR CHEMICALLY ACTIVATED MITOMYCINS AND PORFIROMYCINS, BIFUNCTIONALLY "ALKYLATING" ANTIBIOTICS. Fed Proc. 1964 Sep-Oct;23:946–957. [PubMed] [Google Scholar]
- Sarkar N. K. Effects of actinomycin D and mitomycin C on the degradation of deoxyribonucleic acid and polydeoxyribonucleotide by deoxyribonucleases and venom phosphodiesterase. Biochim Biophys Acta. 1967 Aug 22;145(1):174–177. doi: 10.1016/0005-2787(67)90669-7. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B., Stull H. B. Factors affecting transformation of Bacillus licheniformis. J Bacteriol. 1966 Mar;91(3):1012–1020. doi: 10.1128/jb.91.3.1012-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Stabilization of Bacillus subtilis phage with dimethylsulfoxide. Can J Microbiol. 1965 Aug;11(4):745–746. doi: 10.1139/m65-099. [DOI] [PubMed] [Google Scholar]


