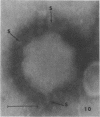
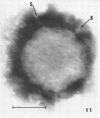
Abstract
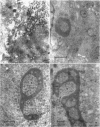
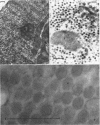
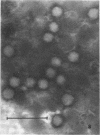
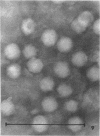
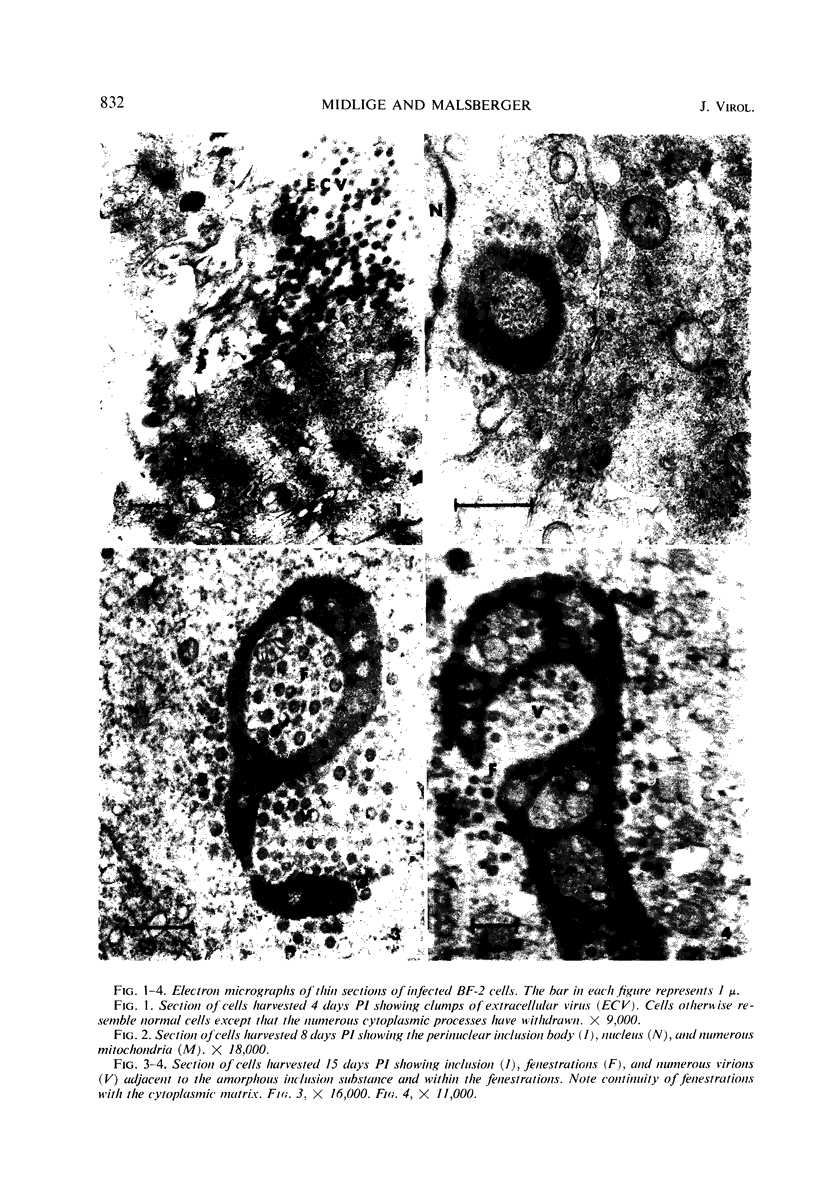
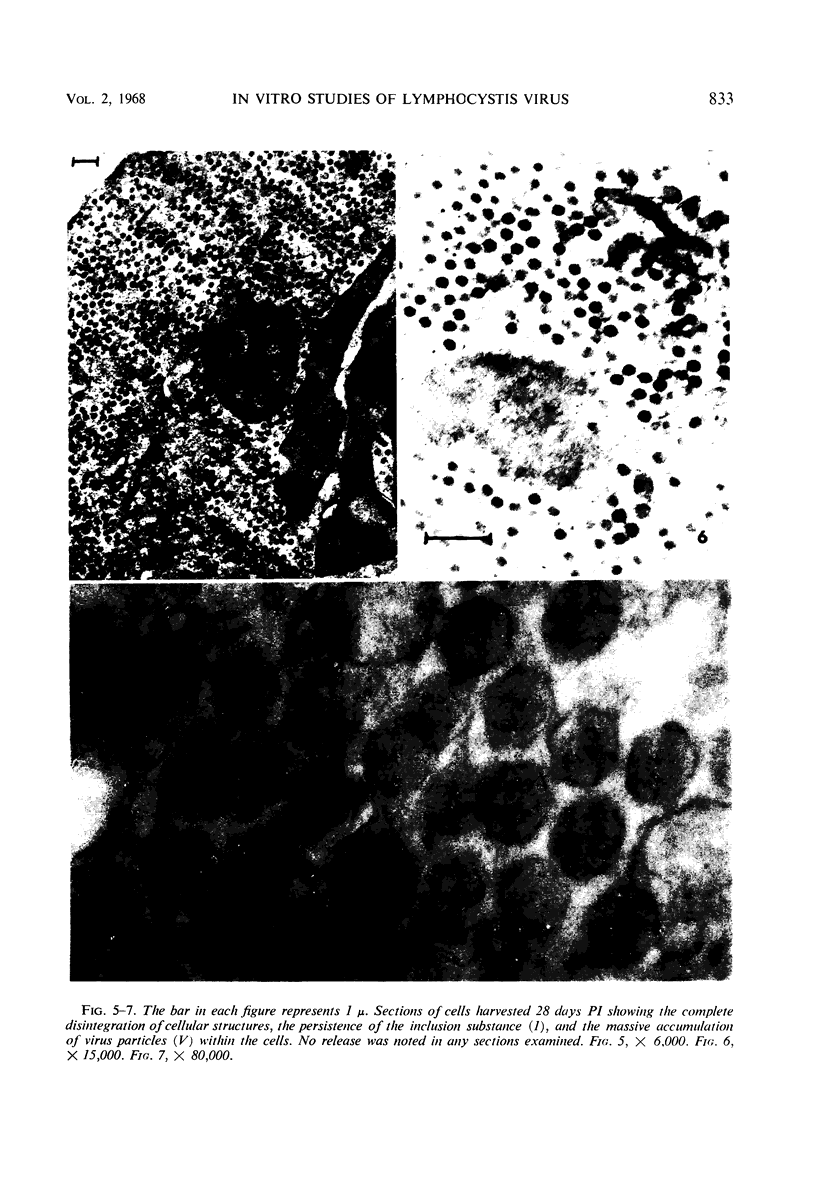
The temporal sequence of development of lymphocystis disease virus (LDV) was studied by electron microscopy of thin sections of infected tissue-culture monolayers. Neither the typical cytoplasmic inclusion nor virus was detected at 4 days postinfection (PI). Inclusions, but no viruses, were detected at 8 days PI. Inclusions and associated virions were detected at 15 days PI, and by 28 days PI the undisrupted cells were filled with the typical virions. No release mechanism was detected, and severe clumping of particles was noted. Negatively stained preparations revealed particles 200 nm in diameter with no capsomere structure and apparent spikes associated with the particle. The relationship of LDV to the well-defined deoxyribonucleic acid virus groups is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., MERCER E. H. THE MULTIPLICATION OF SERICESTHIS IRIDESCENT VIRUS IN CELL CULTURES FROM ANTHERAEA EUCALYPTI SCOTT. I. QUALITATIVE EXPERIMENTS. Virology. 1964 Dec;24:645–653. doi: 10.1016/0042-6822(64)90219-3. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Granoff A., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. II. Ultrastructural studies and sequential development of virus isolated from normal and tumor tissue. Virology. 1966 May;29(1):149–156. doi: 10.1016/0042-6822(66)90204-2. [DOI] [PubMed] [Google Scholar]
- Dunbar C. E., Wolf K. The cytological course of experimental lymphocystis in the bluegill. J Infect Dis. 1966 Oct;116(4):466–472. doi: 10.1093/infdis/116.4.466. [DOI] [PubMed] [Google Scholar]
- LEUTENEGGER R. DEVELOPMENT OF AN ICOSAHEDRAL VIRUS IN HEMOCYTES OF GALLERIA MELLONELLA (L.). Virology. 1964 Oct;24:200–204. doi: 10.1016/0042-6822(64)90104-7. [DOI] [PubMed] [Google Scholar]
- Lunger P. D. Amphibia-related viruses. Adv Virus Res. 1966;12:1–33. doi: 10.1016/s0065-3527(08)60845-3. [DOI] [PubMed] [Google Scholar]
- Lunger P. D., Came P. E. Cytoplasmic viruses associated with Lucké tumor cells. Virology. 1966 Sep;30(1):116–126. doi: 10.1016/s0042-6822(66)81015-2. [DOI] [PubMed] [Google Scholar]
- Parsons D. F. Mitochondrial Structure: Two Types of Subunits on Negatively Stained Mitochondrial Membranes. Science. 1963 May 31;140(3570):985–987. doi: 10.1126/science.140.3570.985. [DOI] [PubMed] [Google Scholar]
- WALKER R. Fine structure of lymphocystis virus of fish. Virology. 1962 Nov;18:503–505. doi: 10.1016/0042-6822(62)90047-8. [DOI] [PubMed] [Google Scholar]
- WOLF K. Experimental propagation of lymphocystis disease of fishes. Virology. 1962 Oct;18:249–256. doi: 10.1016/0042-6822(62)90011-9. [DOI] [PubMed] [Google Scholar]
- Walker R., Weissenberg R. Conformity of light and electron microscopic studies on virus particle distribution in lymphocystis tumor cells of fish. Ann N Y Acad Sci. 1965 Aug 10;126(1):375–385. doi: 10.1111/j.1749-6632.1965.tb14287.x. [DOI] [PubMed] [Google Scholar]
- Wolf K., Carlson C. P. Multiplication of lymphocystis virus in the bluegill (Lepomis macrochirus). Ann N Y Acad Sci. 1965 Aug 10;126(1):414–419. doi: 10.1111/j.1749-6632.1965.tb14290.x. [DOI] [PubMed] [Google Scholar]
- Wolf K., Gravell M., Malsberger R. G. Lymphocystis virus: isolation and propagation in centrarchid fish cell lines. Science. 1966 Feb 25;151(3713):1004–1005. doi: 10.1126/science.151.3713.1004. [DOI] [PubMed] [Google Scholar]
- Wolf K. The fish viruses. Adv Virus Res. 1966;12:35–101. doi: 10.1016/s0065-3527(08)60846-5. [DOI] [PubMed] [Google Scholar]