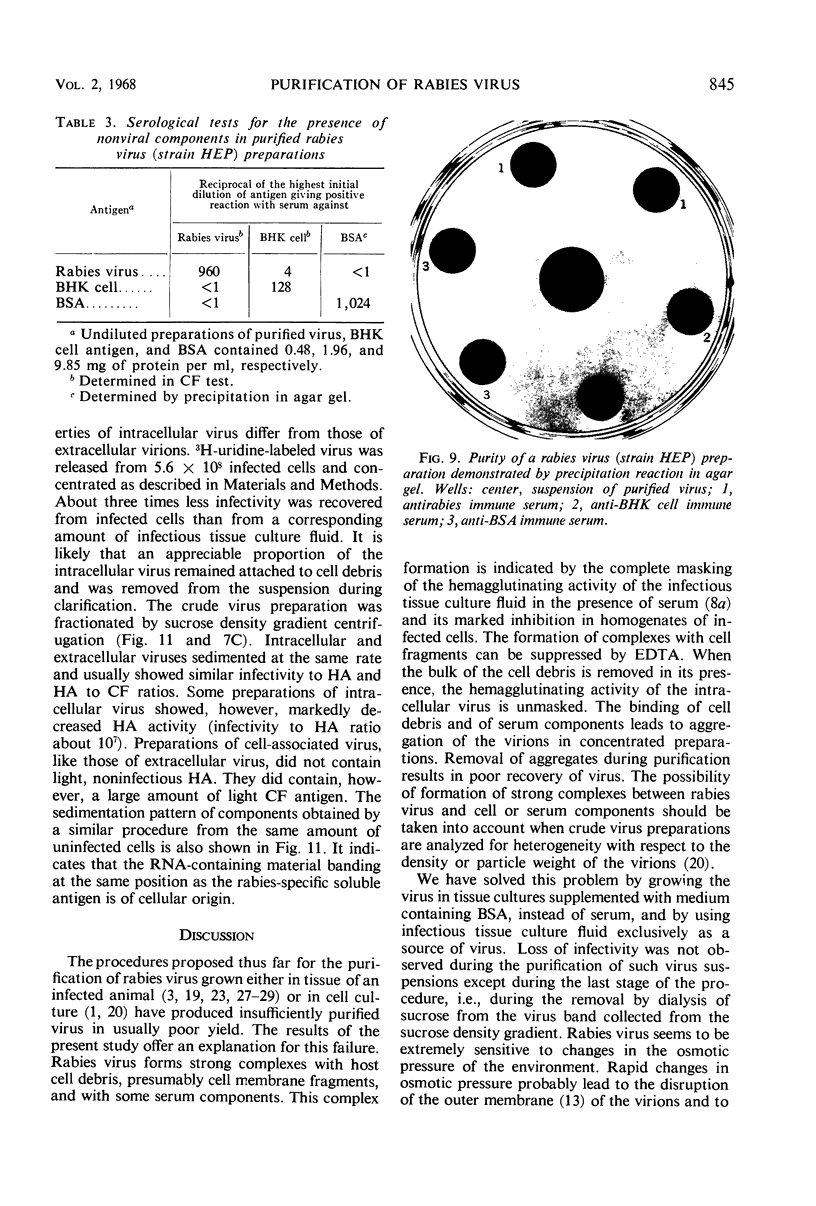
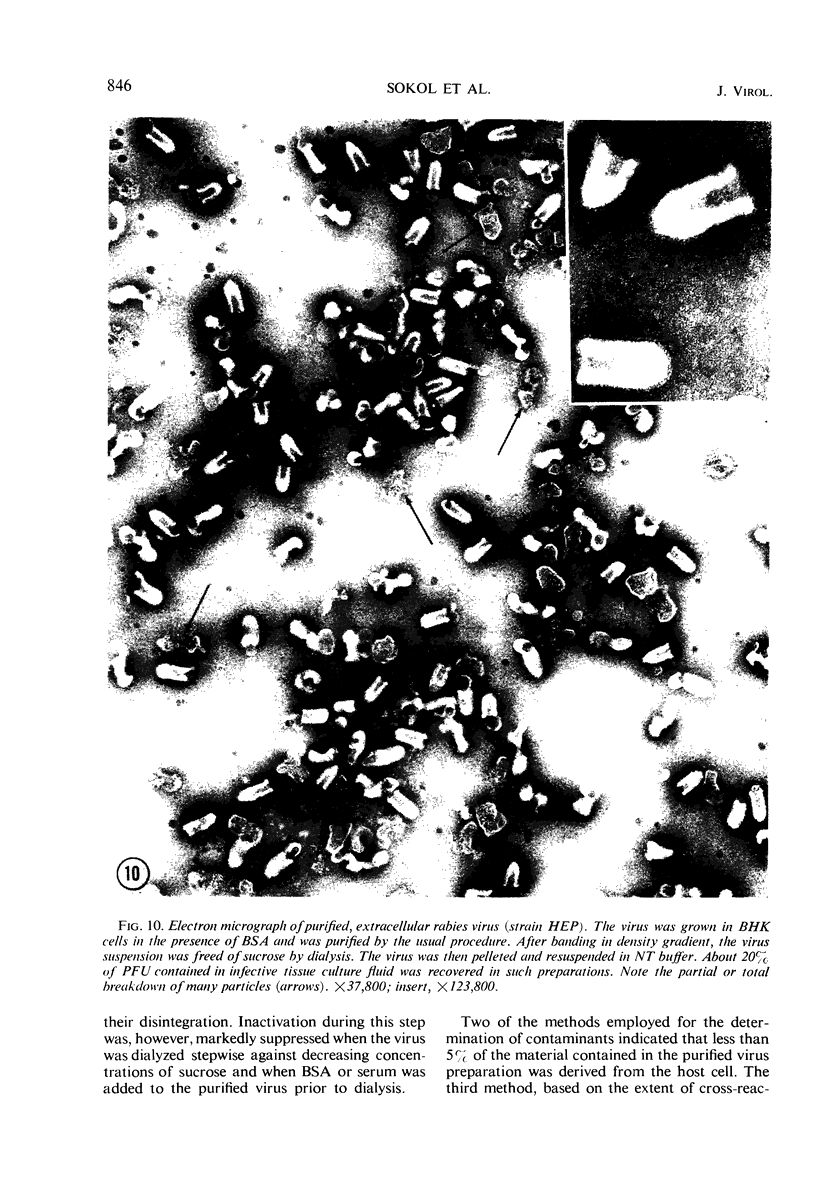
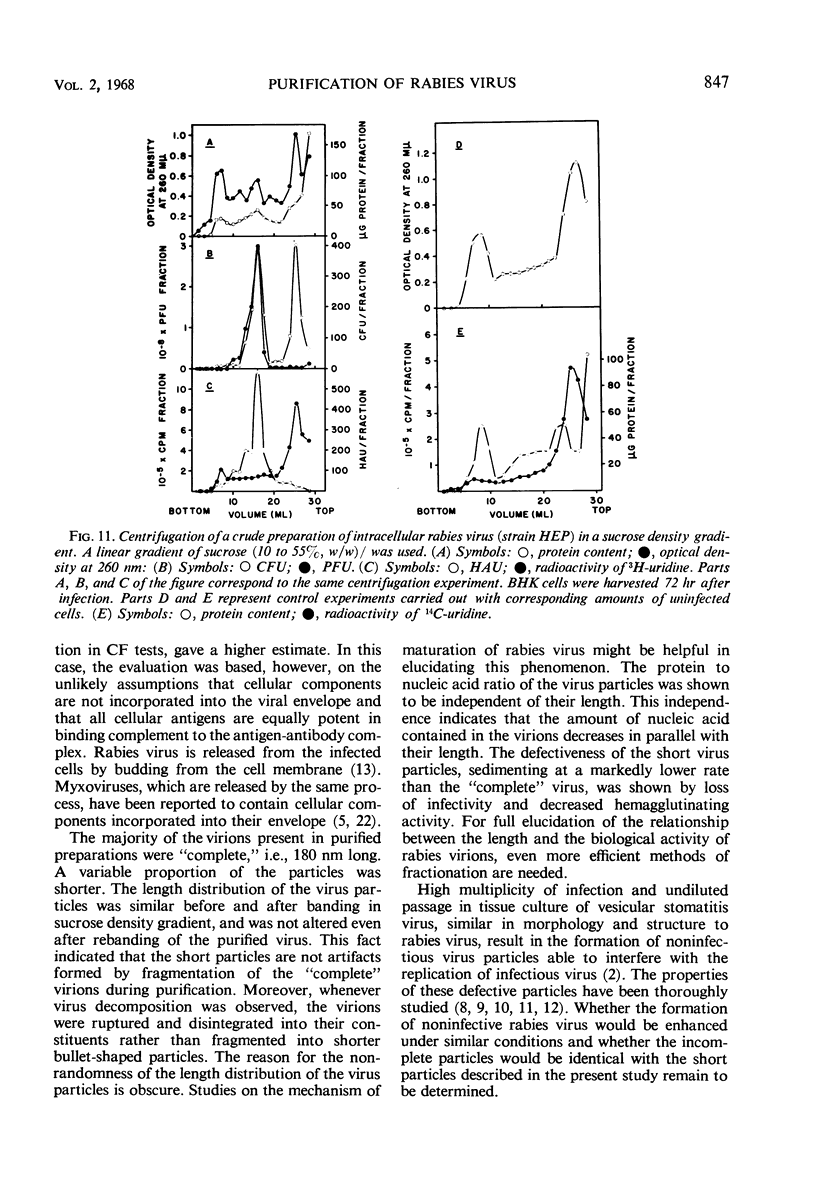
Abstract
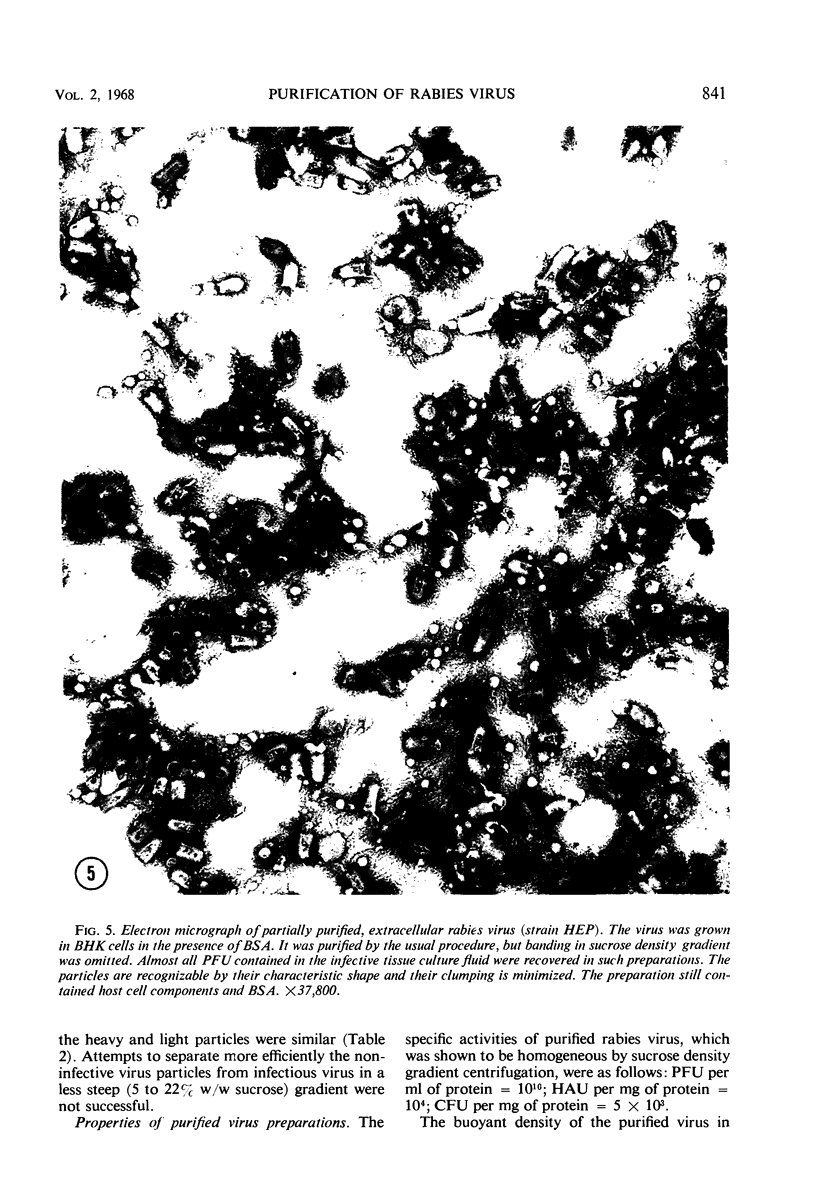
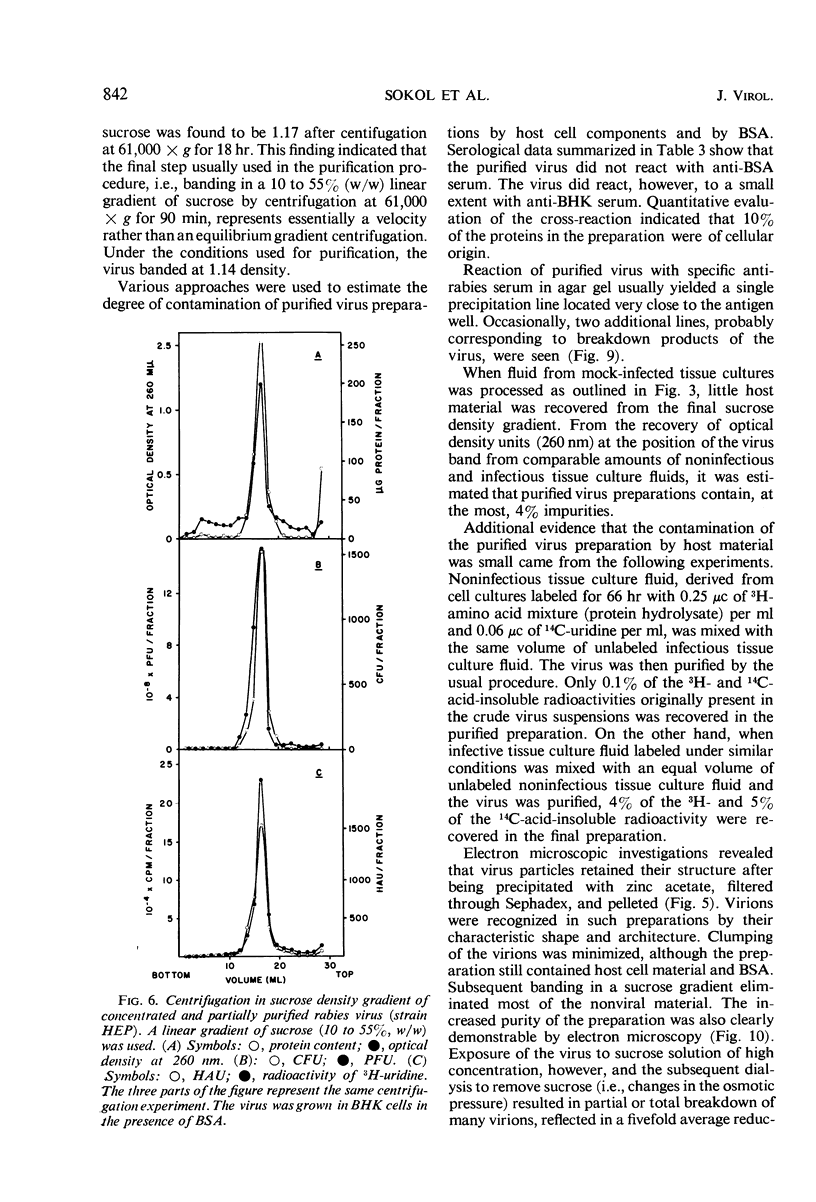
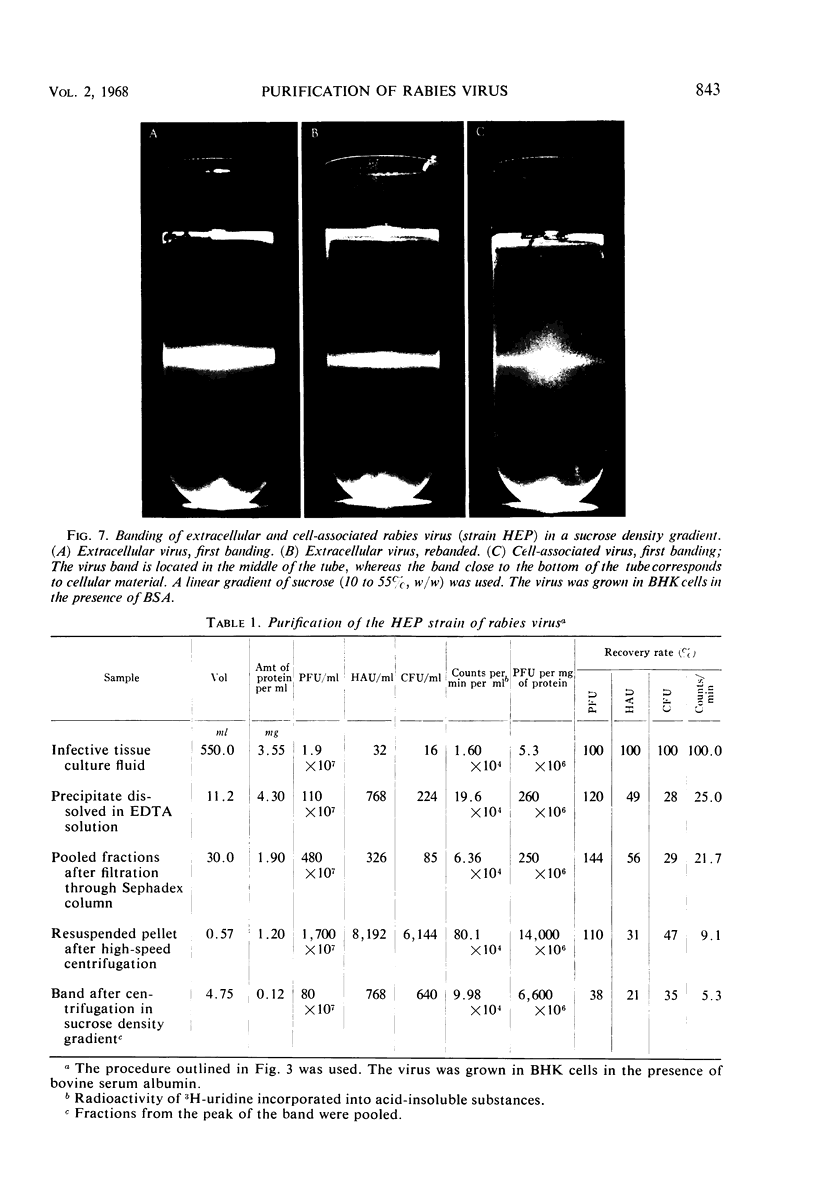
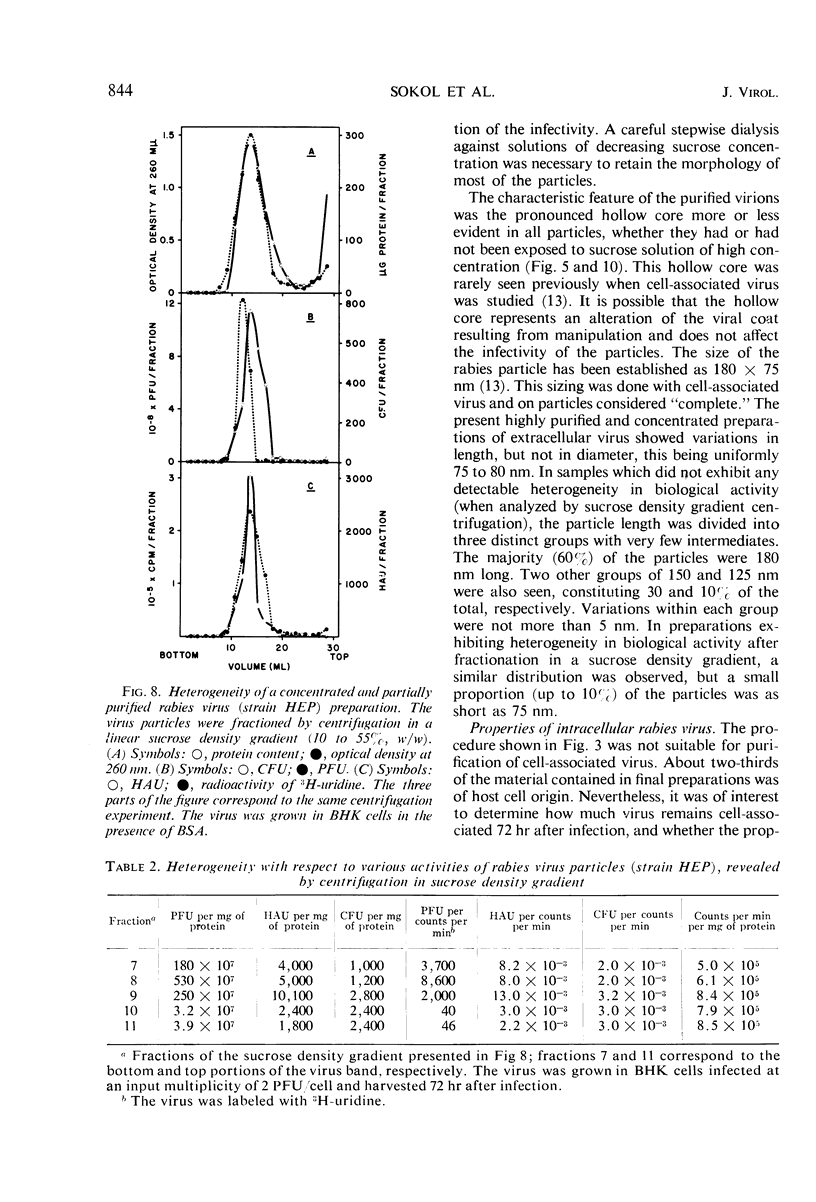
Extracellular rabies virus, grown in monolayer cultures of BHK21 cells in the presence of medium supplemented with bovine serum albumin, was purified by the following procedure. Virus was precipitated from infectious tissue culture fluid by zinc acetate and was resuspended in a solution of ethylenediaminetetraacetate. The suspension was filtered through a Sephadex column and was treated with ribonuclease and deoxyribonuclease. The virions were then pelleted by centrifugation at high speed and were resuspended in buffer solution. Banding of the virus by centrifugation in a sucrose density gradient was the final step in the purification procedure. Purified preparations contained bullet-shaped virus particles of variable length and little (up to 5%) contaminating host-cell material. Most of the virions were “complete”, i.e., 180 nm long, but some virus particles were shorter. The length distribution of the virions was nonrandom. Shorter virions seemed to be noninfectious and showed markedly decreased hemagglutinating activity. The complement-fixing activity and the ribonucleic acid to protein ratio of the virions were not related to the length of the virus particles. Although the properties of extracellular and intracellular viruses were similar, the procedure was not suitable for purification of intracellular rabies virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Immunofluorescent studies of the potentiation of an adenovirus-associated virus by adenovirus 7. J Exp Med. 1967 May 1;125(5):755–765. doi: 10.1084/jem.125.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Isolation of adenovirus-associated viruses from man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1410–1415. doi: 10.1073/pnas.58.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968 Feb;40(2):319–327. [PubMed] [Google Scholar]
- COOPER P. D., BELLETT A. J. A transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:485–497. doi: 10.1099/00221287-21-3-485. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. Two spontaneously transformed cell lines derived from the same hamster embryo culture. Int J Cancer. 1967 Mar 15;2(2):143–152. doi: 10.1002/ijc.2910020209. [DOI] [PubMed] [Google Scholar]
- Dimayorca G. A., Eddy B. E., Stewart S. E., Hunter W. S., Friend C., Bendich A. ISOLATION OF INFECTIOUS DEOXYRIBONUCLEIC ACID FROM SE POLYOMA-INFECTED TISSUE CULTURES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1805–1808. doi: 10.1073/pnas.45.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Noninfectious forms of Newcastle disease and influenza viruses; studies on noninfectious virus occurring within cells that are producing fully infectious virus. Virology. 1955 Dec;1(5):516–532. doi: 10.1016/0042-6822(55)90040-4. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Schaffer F. L., Madin S. H. The separation of infectious and autointerfering particles in vesicular stomatitis virus preparations. Virology. 1967 Jan;31(1):114–119. doi: 10.1016/0042-6822(67)90014-1. [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Murphy F. A., Fields B. N., Reese D. R. Hemagglutinin of rabies and some other bullet-shaped viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1037–1042. doi: 10.3181/00379727-127-32864. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITOAKA M., NISHIMURA C. Noninfectious hemagglutinin and complement-fixing antigen of Japanese B encephalitis. Virology. 1963 Feb;19:238–239. doi: 10.1016/0042-6822(63)90014-x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. III. Identification of two different hemagglutinins. Virology. 1963 Feb;19:147–157. doi: 10.1016/0042-6822(63)90004-7. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Wiktor T. J., Koprowski H. Density gradient centrifugation studies on rabies virus. J Bacteriol. 1966 Jul;92(1):102–106. doi: 10.1128/jb.92.1.102-106.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- SOKOL F. HETEROGENEITY OF NEWCASTLE DISEASE VIRUS (NDV) PARTICLES WITH RESPECT TO THEIR HEMOLYTIC ACTIVITY. Virology. 1963 Sep;21:126–128. doi: 10.1016/0042-6822(63)90311-8. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D., Thiel J. F. Properties of a small virus associated with adenovirus type 4. J Immunol. 1966 Dec;97(6):754–766. [PubMed] [Google Scholar]
- TAGAYA I., OZAWA Y., KONDO A. Studies on the purification of rabies virus. I. Application of methanol precipitation and two other methods. Yokohama Med Bull. 1953 Apr;4(2):78–86. [PubMed] [Google Scholar]
- THOMAS J. B., RICKER A. S., BAER G. M., SIKES R. K. PURIFICATION OF FIXED RABIES VIRUS. Virology. 1965 Feb;25:271–275. doi: 10.1016/0042-6822(65)90205-9. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Kaplan C. Some properties of fixed rabies virus. J Gen Virol. 1967 Oct;1(4):537–551. doi: 10.1099/0022-1317-1-4-537. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Zgorniak-Nowosielska I., Sedwick W. D., Hummeler K., Koprowski H. New assay procedure for separation of mycoplasmas from virus pools and tissue culture systems. J Virol. 1967 Dec;1(6):1227–1237. doi: 10.1128/jvi.1.6.1227-1237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]