Abstract
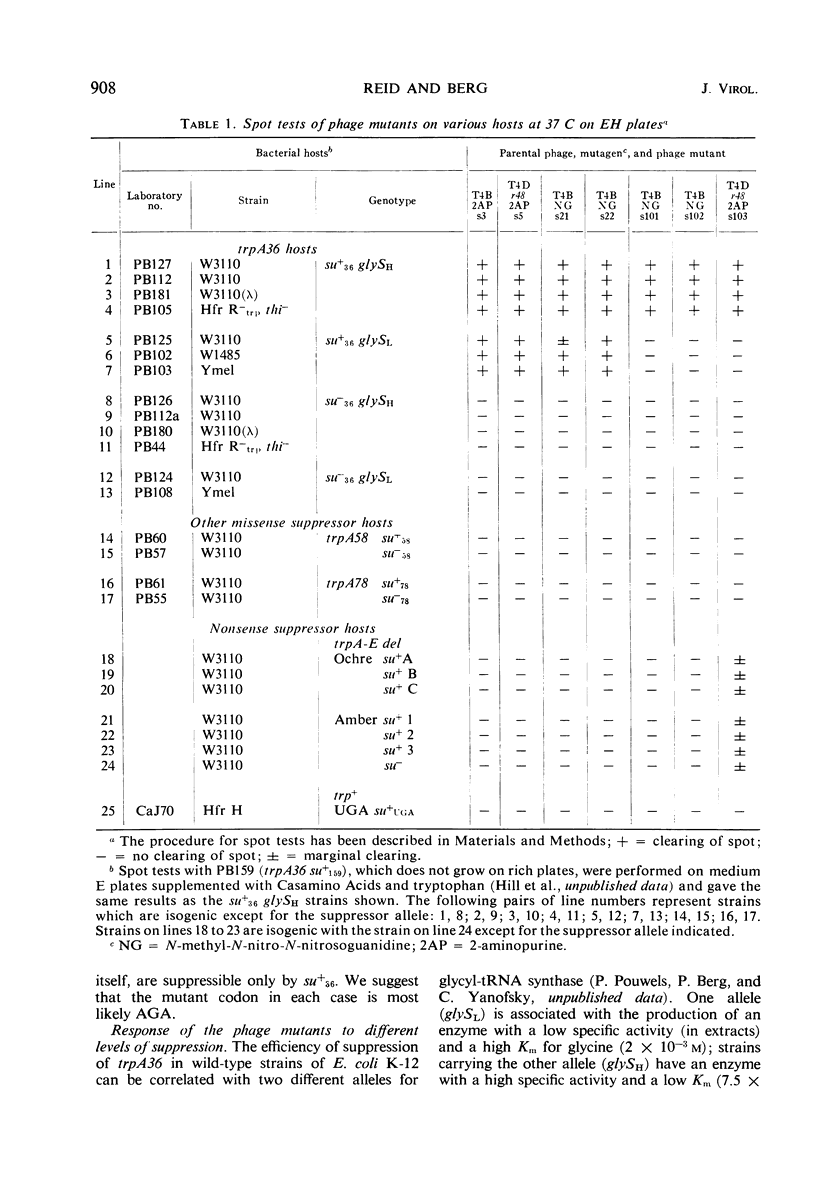
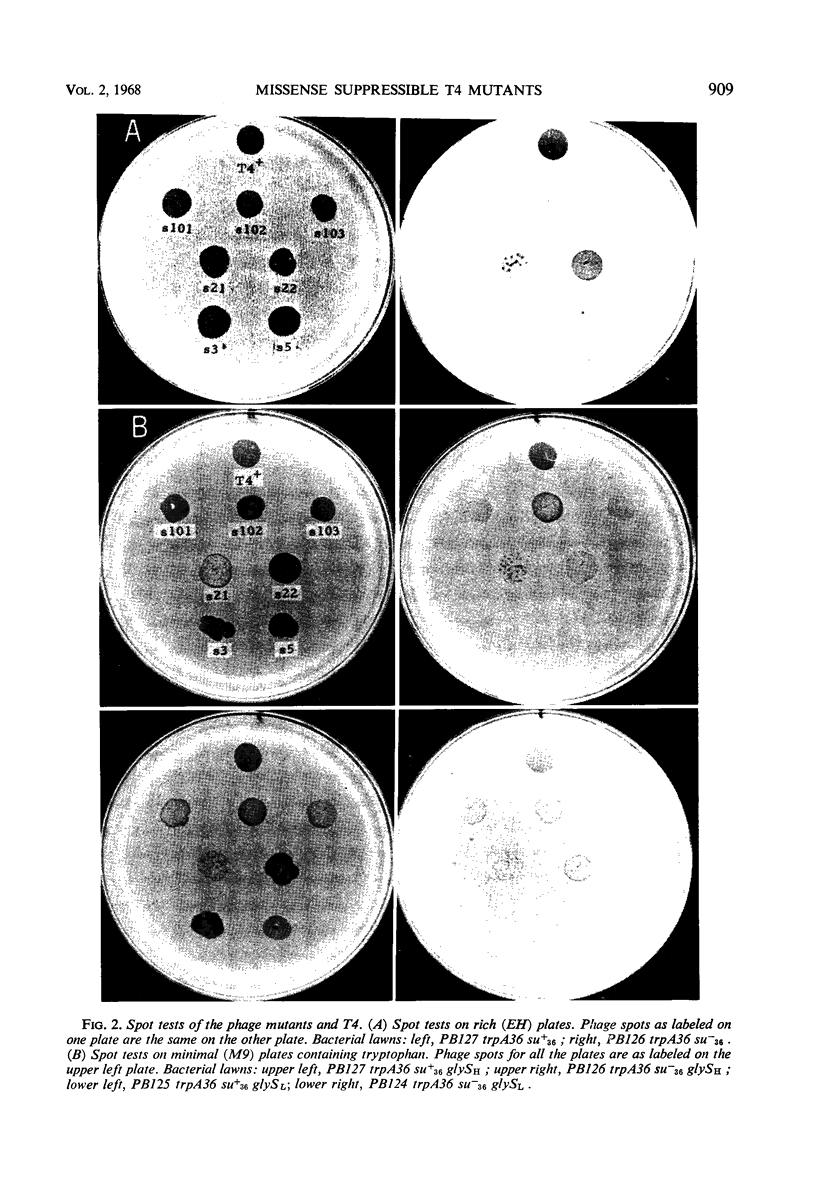
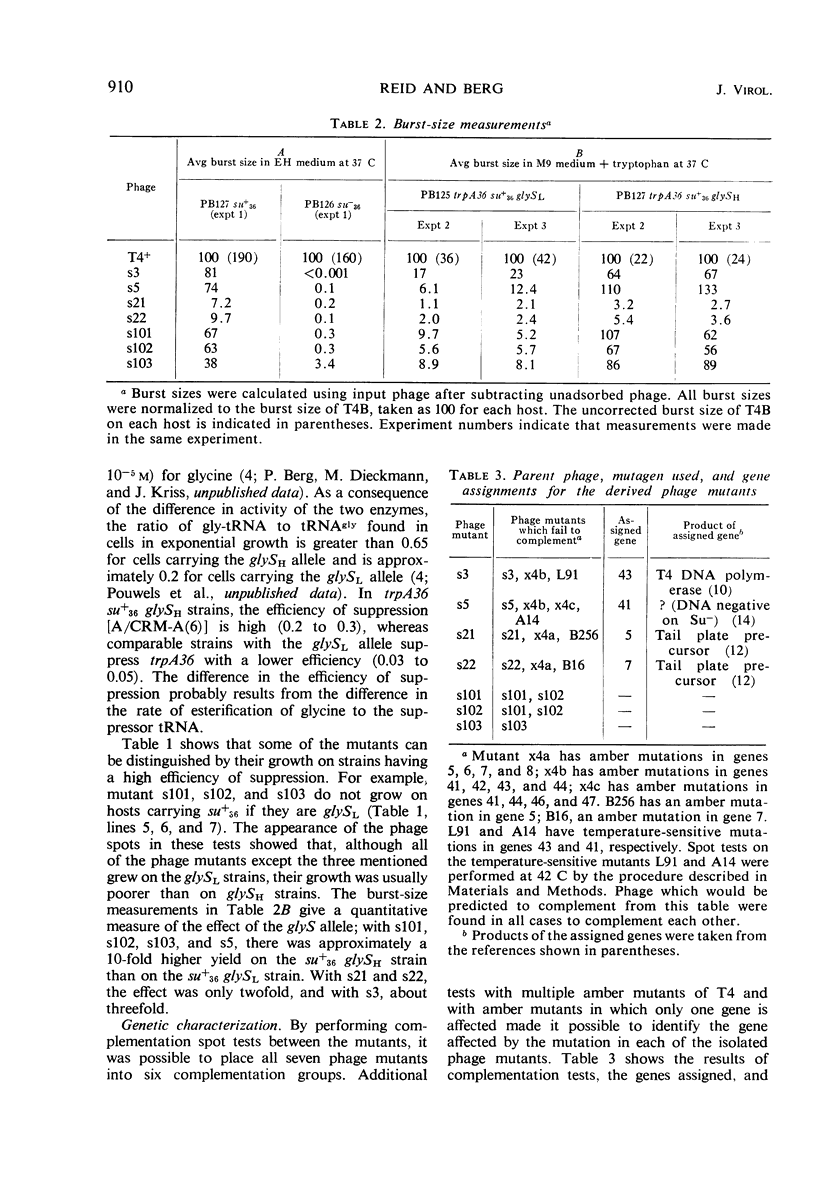
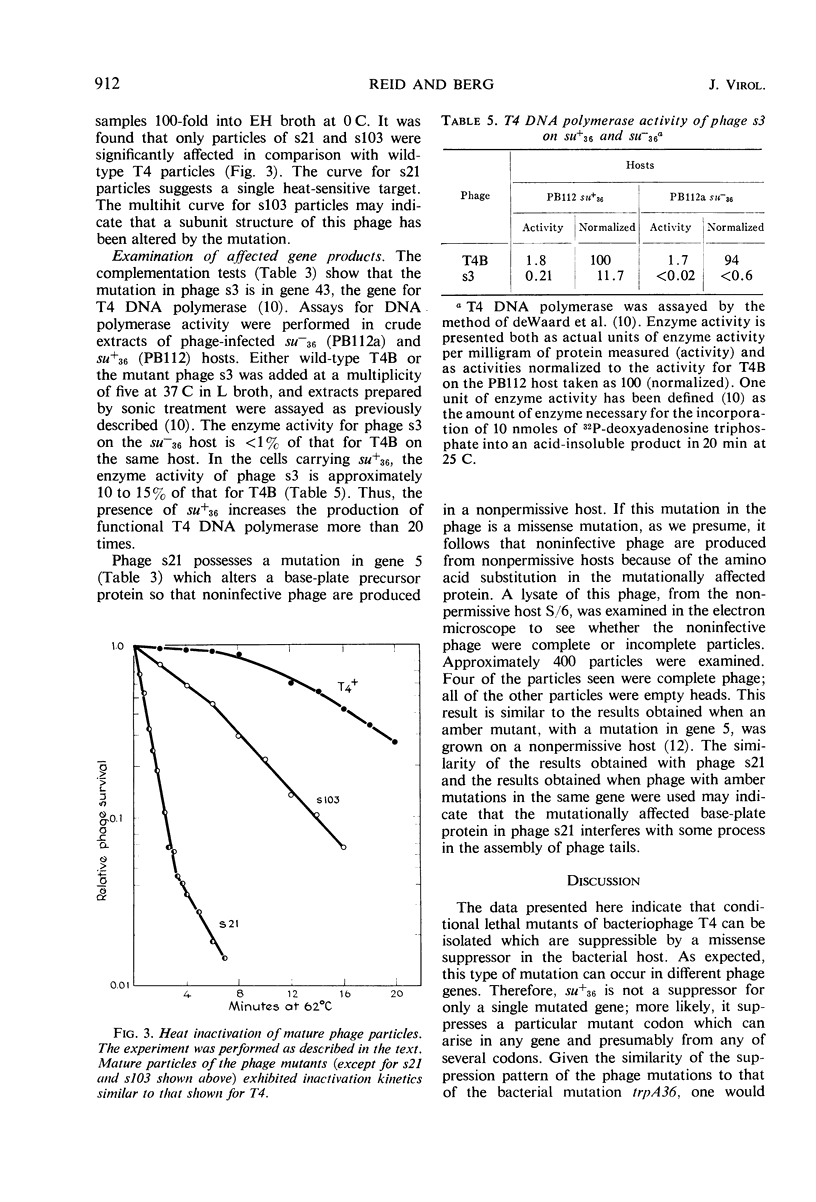
Phage mutants of T4 have been isolated which can multiply only on Escherichia coli strains which contain a missense suppressor which is known to cause the substitution of glycine for arginine in response to the AGA codon. Mutations producing the suppressible phenotype were mapped and shown to occur in six different phage cistrons. Two of the cistrons were concerned with deoxyribonucleic acid synthesis, two were concerned with phage structural components, and two were concerned with functions required for growth in E. coli K-12 but not in E. coli B. The burst size of the different phage mutants grown on strains carrying the same suppressor was dependent upon the efficiency of suppression, which in turn is known to be dependent upon the glycyl-transfer ribonucleic acid synthetase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODY S., YANOFSKY C. Suppressor gene alteration of protein primary structure. Proc Natl Acad Sci U S A. 1963 Jul;50:9–16. doi: 10.1073/pnas.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S., Champe S. P. AMBIVALENT rII MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1961 Jul;47(7):1025–1038. doi: 10.1073/pnas.47.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Yanofsky C. Mechanism studies of suppressor-gene action. J Bacteriol. 1965 Sep;90(3):687–695. doi: 10.1128/jb.90.3.687-695.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Location of the structural gene for glycyl ribonucleic acid synthetase by means of a strain of Escherichia coli possessing an unusual enzyme. Z Vererbungsl. 1966;98(3):187–192. doi: 10.1007/BF00888946. [DOI] [PubMed] [Google Scholar]
- Carbon J., Berg P., Yanofsky C. Missense suppression due to a genetically altered tRNA. Cold Spring Harb Symp Quant Biol. 1966;31:487–497. doi: 10.1101/sqb.1966.031.01.063. [DOI] [PubMed] [Google Scholar]
- Carbon J., Berg P., Yanofsky C. Studies of missense suppression of the tryptophan synthetase A-protein mutant A36. Proc Natl Acad Sci U S A. 1966 Aug;56(2):764–771. doi: 10.1073/pnas.56.2.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbrück M. Interference Between Bacterial Viruses: III. The Mutual Exclusion Effect and the Depressor Effect. J Bacteriol. 1945 Aug;50(2):151–170. [PMC free article] [PubMed] [Google Scholar]
- Doermann A H, Hill M B. Genetic Structure of Bacteriophage T4 as Described by Recombination Studies of Factors Influencing Plaque Morphology. Genetics. 1953 Jan;38(1):79–90. doi: 10.1093/genetics/38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- HELINSKI D. R., YANOFSKY C. Correspondence between genetic data and the position of amino acid alteration in a proein. Proc Natl Acad Sci U S A. 1962 Feb;48:173–183. doi: 10.1073/pnas.48.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. F., Fan D. P., Brenner S. A strong suppressor specific for UGA. Nature. 1967 Apr 29;214(5087):452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanofsky C., Drapeau G. R., Guest J. R., Carlton B. C. THE COMPLETE AMINO ACID SEQUENCE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN (alpha SUBUNIT) AND ITS COLINEAR RELATIONSHIP WITH THE GENETIC MAP OF THE A GENE. Proc Natl Acad Sci U S A. 1967 Feb;57(2):296–298. doi: 10.1073/pnas.57.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]