Abstract
Objective
Knowledge of the accuracy of continuous glucose monitoring (CGM) devices is important for its use as a management tool for individuals with diabetes and for its use to assess outcomes in clinical studies. Using data from several inpatient studies, we compared the accuracy of two sensors, the Medtronic Enlite™ using MiniMed Paradigm® Veo™ calibration and the Sof-Sensor® glucose sensor using Guardian® REAL-Time CGM calibration (all from Medtronic Diabetes, Northridge, CA).
Subjects and Methods
Nocturnal data were analyzed from eight inpatient studies in which both CGM and reference glucose measurements were available. The analyses included 1,666 CGM–reference paired glucose values for the Enlite in 54 participants over 69 nights and 3,627 paired values for the Sof-Sensor in 66 participants over 91 nights.
Results
The Enlite sensor tended to report glucose levels lower than the reference over the entire range of glucose values, whereas the Sof-Sensor values tended to be higher than reference values in the hypoglycemic range and lower than reference values in the hyperglycemic range. The overall median sensor–reference difference was −15 mg/dL for the Enlite and −1 mg/dL for the Sof-Sensor (P<0.001). The median relative absolute difference was 15% for the Enlite versus 12% for the Sof-Sensor (P=0.06); 66% of Enlite values and 73% of Sof-Sensor values met International Organization for Standardization criteria.
Conclusions
We found that the Enlite tended to be biased low over the entire glucose range, whereas the Sof-Sensor showed the more typical sensor pattern of being biased high in the hypoglycemic range and biased low in the hyperglycemic range.
Introduction
Continuous glucose monitoring (CGM) devices have been shown to improve management for individuals with diabetes1–3 and can be an effective tool for the assessment of outcomes in clinical studies.4 CGM accuracy is a critical factor in the development of a commercially viable artificial pancreas device system.
The Medtronic Enlite™ subcutaneous glucose sensor (referred to as “Enlite”) has a smaller needle and is easier to insert than the Sof-Sensor® sensor (referred to as “Sof-Sensor”) (both from Medtronic Diabetes, Northridge, CA). The MiniMed Paradigm® Veo™ (referred to as “Veo”) calibration algorithm used with the Enlite sensor was designed to be more accurate than the previous Sof-Sensor and Guardian® REAL-Time (referred to as “Guardian”) calibration algorithm (both from Medtronic Diabetes), particularly in the hypoglycemic and near-hypoglycemic range.5 The Enlite and Veo are currently available in Europe and are awaiting approval in the United States. Differences in accuracy are important factors for clinicians and patients to consider when transitioning to the new devices. Using data from eight inpatient studies, we compared the accuracy of the two Medtronic sensors.
Research Design and Methods
Data collected in eight studies (both published6–10 and unpublished) were used to assess the accuracy of the Sof-Sensor and Enlite sensors in comparison with reference plasma glucose measurements (Table 1). Subjects had type 1 diabetes and were studied in an inpatient clinical research center setting. Three protocols (71 nights from 31 patients) included periodic dosing of glucagon. Reference venous blood glucose measurements were obtained by sending blood samples to a central laboratory (Study B) or using a YSI model 2300 glucose analyzer (YSI Inc., Yellow Springs, OH) (Studies A and E– G), GlucoScout® blood glucose monitor (International Biomedical, Inc., Austin, TX) (Studies C–F and H), or HemoCue 201+® glucose analyzer (Hemocue, Inc., Angelholm, Sweden) (Studies E and F). CGM calibration was performed at manufacturer-recommended intervals using capillary home glucose meter values (Studies B and E–G) or venous reference values (Studies A, C, D, and H). Veo calibration was used for all Enlite glucose sensors, and Guardian calibration was used for all Sof-Sensor glucose sensors.
Table 1.
Studies Providing Data for the Analyses (n=148 Nights)
| Study | Number of subjects | Age range (years) | A1c range (%) | On CLa | Reference method | Reference frequency | Number of sensors | CGM frequency |
|---|---|---|---|---|---|---|---|---|
| A9 | 12 | 5–17 | (6.5%, 13.3%) | No | YSI | 15 min | 1 Sof-Sensor | 1 min |
| B7 | 26 | 5–17 | (6.1%, 9.4%) | No | Laboratory | 30 min | 2 Sof-Sensors | 5 min |
| C8 | 10 | 19–71 | (6.2%, 8.5%) | Yes | GlucoScout | 5 min | 1 Sof-Sensor | 5 min |
| D10 | 6 | 33–72 | (6.4%, 8.3%) | Yes | GlucoScout | 15 min | 1 Sof-Sensor | 5 min |
| E (unpublished)b | 12 | 17–42 | (5.8%, 8.6%) | Yes | YSI, GlucoScout, HemoCue | 30 min | 1 Sof-Sensor, 1 Enlite | 5 min |
| F6 | 15 | 18–42 | (6.1%, 8.2%) | Yes | YSI, GlucoScout, HemoCue | 30 min | 1 Enlite | 5 min |
| G (unpublished)b | 12 | 12–18 | (8%, 12%) | No | YSI | 30 min | 1 Enlite | 5 min |
| H (unpublished)b | 15 | 12–61 | (6.3%, 8.5%) | Yes | GlucoScout | 15 min | 1 Enlite | 5 min |
Nighttime was defined as 10 p.m.–6 a.m.
Indicates whether patients were connected to a closed-loop (CL) device during the study. All continuous glucose monitoring (CGM) measurements driving the controller were excluded.
Study E was coordinated by the Jaeb Center; data were acquired by the Stanford University and Barbara Davis Center clinical teams and are on file at the Jaeb Center. Study G was run by R. Hovorka at Cambridge University, and a data subset was provided to the Jaeb Center; data are on file at both Cambridge University and the Jaeb Center. Study H was run by E. Damiano at Boston University, and a data subset was provided to the Jaeb Center; data are on file at both Boston University and the Jaeb Center.
Glucose data were restricted to nighttime measurements (10 p.m.–6 a.m.) to make the studies more similar. CGM devices driving the controller tend to shift glucose toward the target range, thereby changing the distribution of glucose values and the bias.11 Therefore, this analysis did not include data from CGM devices driving the controller. The reference glucose measurements were paired to the closest CGM measurement within ±5 min. When reference values were simultaneously available from more than one source, YSI values were used instead of those from the GlucoScout or HemoCue. For studies in which patients wore two passive CGM devices (Studies B and E), the reference glucose measurements were paired to each device. Across studies, there were 1,666 CGM–reference paired glucose values for the Enlite sensor in 54 participants over 69 nights and 3,627 paired values for the Sof-Sensor in 66 participants over 91 nights. For the Enlite sensor, 19% of reference values were obtained from a YSI analyzer, 66% using a GlucoScout monitor, and 15% using a HemoCue analyzer. For the Sof-Sensor pairs, the reference source was 18% central lab, 14% YSI, 65% GlucoScout, and 3% HemoCue.
The difference (CGM minus reference value) and relative absolute difference (RAD) (absolute difference divided by the reference value, expressed as a percentage) were computed for each pair. The RAD evaluates the magnitude of the errors, whereas the difference evaluates whether there is any bias for the sensor to read systematically high or low. Each pair also was evaluated for adherence to the International Organization for Standardization criteria (ISO): for reference values ≤75 mg/dL, CGM value within ±15 mg/dL, and for reference values >75 mg/dL, CGM value within±20%. Summary statistics were calculated by pooling all paired values for each device. The bootstrap technique was performed to test the differences in accuracy and bias between the two CGM sensor devices controlling for reference glucose and accounting for correlated data within the same subject. One study showed a particularly inaccurate Enlite sensor, and a sensitivity analysis was performed to compare the accuracy and bias between the two devices after this study was excluded. Analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
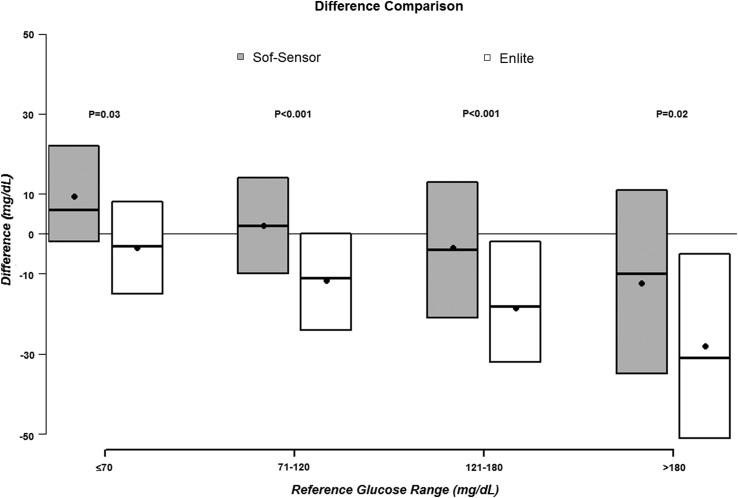
As seen in Figure 1, the Enlite sensor tended to report glucose levels lower than the reference over the entire range of glucose values, whereas the Sof-Sensor values tended to be higher than reference values in the hypoglycemic range and lower than reference levels in the hyperglycemic range as is more typically seen when sensor glucose values are compared with a reference. Overall, the median sensor–reference difference was −1 mg/dL for the Sof-Sensor and −15 mg/dL for the Enlite (P<0.001), the median RAD was 12% versus 15%, respectively (P=0.06), and the mean RAD was 16% versus 18%, respectively; 73% of Sof-Sensor values and 66% of Enlite values met ISO criteria (Table 2). Results for each of the eight studies are shown in Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertpub.com/dia).
FIG. 1.
Sof-Sensor and Enlite sensor bias based on reference glucose level. Black dots denote the mean difference, and boxes denote the median (25th, 75th percentiles) difference.
Table 2.
Comparison of Overnight Sof-Sensor with Paradigm Pump and Enlite Continuous Glucose Monitoring with Veo Pump Point Accuracy
| |
Number of pairs |
RADa |
ISOb |
|||||
|---|---|---|---|---|---|---|---|---|
| Sof-Sensor | Enlite | Sof-Sensor | Enlite | P value | Sof-Sensor | Enlite | P value | |
| Overall | 3,627 | 1,666 | 12% (6%, 21%) | 15% (7%, 25%) | 0.06 | 73% | 66% | 0.11 |
| Reference glucose (mg/dL) | ||||||||
| ≤70 | 210 | 122 | 14% (7%, 38%) | 19% (9%, 27%) | 0.91 | 68% | 75% | 0.66 |
| 71–120 | 1,713 | 691 | 13% (6%, 21%) | 14% (6%, 27%) | 0.46 | 72% | 62% | 0.16 |
| 121–180 | 1,086 | 559 | 12% (6%, 21%) | 15% (7%, 23%) | 0.20 | 73% | 68% | 0.44 |
| >180 | 618 | 294 | 10% (5%, 19%) | 15% (9%, 24%) | 0.01 | 78% | 64% | 0.06 |
Relative absolute difference (RAD)=absolute difference/reference. Data are median values (25th, 75th percentiles).
International Organization for Standardization (ISO) criteria are continuous glucose monitoring measurements within±15 mg/dL for reference glucose values ≤75 mg/dL and within ±20% for reference glucose values >75 mg/dL.
One study (Study F6) showed greater inaccuracy with the Enlite compared with the other studies (median RAD of 27% compared with 15%, 14%, and 13% for three other studies; Supplementary Table S2). However, excluding this study only decreased the overall median RAD by 1%. Median sensor–reference difference and RAD tended to fluctuate when adjusting for reference source and studies with capillary and venous calibration but gave similar results between sensors (Supplementary Table S3).
Discussion
We found that the Enlite sensor tended to be biased low compared with reference glucose values across the reference range, whereas the Sof-Sensor sensor followed the more typical sensor pattern of being biased high in the hypoglycemic range and biased low in the hyperglycemic range. These findings were fairly consistent across studies. Sensor bias could be affected by delayed changes in subcutaneous interstitial fluid glucose with respect to blood glucose and offsets in current estimates.12 The pronounced bias with the Enlite sensor may reflect changes in the Paradigm Veo's internal calibration factors.5 This calibration seemingly increases sensitivity for detection of hypoglycemia but at the expense of an increased false-positive rate for hypoglycemia.
Our results also showed that the Sof-Sensor was slightly more accurate than the Enlite sensor, especially when the reference glucose level was high. Other studies have reported similar median RAD values for the Sof-Sensor and Enlite CGM. Mastrototaro et al.13 reported a median RAD of 10.5% when comparing the Sof-Sensor with blood glucose meters at home in adults with type 1 diabetes. Keenan et al.14 reported a median RAD of 13.9% when comparing the Enlite using a Paradigm Veo calibration with the YSI analyzer in adults with type 1 diabetes.
Important limitations of this study are the differences in calibration methods, reference sources, and subject characteristics, which may confound these results. However, trends for the Enlite to read low, even during hypoglycemia, and for the Sof-Sensor to show comparable or better accuracy were fairly consistent across studies. The restriction to overnight values, in an attempt to minimize potentially confounding variation in subject activity levels and meals in different studies, is another limitation of this analysis. RAD values reported here may not extend to those seen in daytime or mixed studies, because of lower glucose variability overnight.
In summary, the Enlite calibration is such that sensor values tend to be lower than true glucose values, which would be expected to produce greater detection of hypoglycemia but at the expense of more false-positives.
Supplementary Material
Acknowledgments
We thank Roman Hovorka, PhD, Ed Damiano, PhD, Steven Russell, MD, PhD, the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, and the Diabetes Research in Children Network Study Group for providing the data included in this article. The Jaeb Center for Health Research received support from grant 22-2011-643 from the Juvenile Diabetes Research Foundation, Inc.
Author Disclosure Statement
C.K. reports having received consulting fees from Medtronic as part of the Veo Advisory Board. P.C., J.L., and R.W.B. declare no competing financial interests exist.
References
- 1.Deiss D. Bolinder J. Riveline JP. Battelino T. Bosi E. Tubiana-Rufi N. Kerr D. Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 2.Rodbard D. Bailey T. Jovanovic L. Zisser H. Kaplan R. Garg SK. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes Technol Ther. 2009;11:717–723. doi: 10.1089/dia.2009.0077. [DOI] [PubMed] [Google Scholar]
- 3.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 4.Beck RW. Calhoun PM. Kollman C. Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol Ther. 2012;14:877–882. doi: 10.1089/dia.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan DB. Cartaya R. Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol. 2010;4:111–118. doi: 10.1177/193229681000400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron F. Wilson DM. Buckingham BA. Arzumanyan H. Clinton P. Chase HP. Lum J. Maahs DM. Calhoun PM. Bequette BW. Inpatient studies of a Kalman-filter-based predictive pump shutoff algorithm. J Diabetes Sci Technol. 2012;6:1142–1147. doi: 10.1177/193229681200600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Research in Children Network (DirecNet) Study Group: The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther. 2008;10:266–272. doi: 10.1089/dia.2007.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Khatib FH. Russell SJ. Nathan DM. Sutherlin RG. Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2:27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovorka R. Allen JM. Elleri D. Chassin LJ. Harris J. Xing D. Kollman C. Hovorka T. Larsen AM. Nodale M. De Palma A. Wilinska ME. Acerini CL. Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 10.Russell SJ. El-Khatib FH. Nathan DM. Magyar KL. Jiang J. Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148–1255. doi: 10.2337/dc12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovorka R. Nodale M. Haidar A. Wilinska ME. Assessing performance of closed-loop insulin delivery systems by continuous glucose monitoring: drawbacks and way forward. Diabetes Technol Ther. 2013;15:4–12. doi: 10.1089/dia.2012.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebrin K. Sheppard NF., Jr Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4:1087–1098. doi: 10.1177/193229681000400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastrototaro J. Shin J. Marcus A. Sulur G. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10:385–390. doi: 10.1089/dia.2007.0291. [DOI] [PubMed] [Google Scholar]
- 14.Keenan DB. Mastrototaro JJ. Zisser H. Cooper KA. Raghavendhar G. Lee SW. Yusi J. Bailey TS. Brazg RL. Shah RV. Accuracy of the Enlite 6-day glucose sensor with Guardian and Veo calibration algorithms. Diabetes Technol Ther. 2012;14:225–231. doi: 10.1089/dia.2011.0199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.