Oligonucleotides containing 2'-deoxy-2'-fluoro-ribonucleotides (2'-F RNA) have found numerous beneficial applications in ribozyme,[1] antisense,[2,3] siRNA,[4,5] miRNA,[6] and aptamer[7] based nucleic acid therapeutics. Moreover, antisense oligonucleotides bearing 2'-F ribonucleotides were recently found to exhibit favorable properties for modulating splicing relative to other 2'-modifications.[8] At the ribose 2'-position fluorine preorganizes the sugar for a C3'-endo conformation that matches the preferred structure of RNA duplexes. We found that siRNAs extensively modified with 2'-F modified pyrimidines showed increased nuclease stability, reduced immune stimulation and in some cases exhibited favorable activity in vitro and in vivo relative to unmodified control RNA.[5] The stabilizing effect of 2'-F modification as measured by thermal melting of RNA duplexes amounts to ca. 1.8°C per nucleotide.
Comparison of crystal structures of all-RNA, all-2'-F RNA and mixed RNA/2'-F RNA octamer duplexes revealed that substitution of 2'-OH by 2'-F has very little effect on the local and overall helix geometry.[9] Owing to the atomic resolution of the diffraction data and in combination with osmotic stress data, our study also established differences in the hydration patterns of 2'-F RNA and RNA. Thus, 2'-F is a poor H-bond acceptor in the minor groove,[9] whereas 2'-OH is extensively hydrated and serves as a bridge head for water molecules linking strands across that groove.[5,9,10] Unexpectedly, given the poor hydration of 2'-F and the general assumption that fluorine preorganizes the backbone for the RNA target, thermodynamic data indicated that the higher stability of 2'-F RNA is entirely based on favorable enthalpy and not entropy.[9]
Stacking and H-bonding are the main contributors to the enthalpic change as a result of duplex formation. However, it is not immediately clear if only one of these factors accounts for the stability increase or whether both contribute. In order to gain a better understanding of the source(s) of the favorable enthalpy of duplex formation displayed by 2'-F RNA, we conducted a series of NMR and thermodynamic experiments.
H-bond lengths can be empirically determined by measuring one-bond scalar coupling (1JNH)[11] of Watson-Crick C:G and A:U base pairs and by tracing proton chemical shifts δ(1H) of imino protons.[12,13] Grzesiek and coworkers showed that with decreasing H-bond distance (increasing strength), δ(1H) increases whereas 1JNH becomes less negative in DNA.[14] LiWang and coworkers measured 1JNH coupling constants at natural abundance 15N to demonstrate that N1⋯H-N3 hydrogen bonds in RNA A:U base pairs are stronger than those in DNA A:T base pairs.[15] The comparison of the H-bonding strengths in DNA and RNA duplexes may be complicated by the presence of the 5-methyl group in T compared to U[16] as well as the different stacking types (intra-strand in DNA and inter-strand in RNA).[17] By comparison, the virtually indentical conformations of RNA and 2'-F RNA duplexes[9] pose no problems in this respect.
We used two oligonucleotides, 2'-F RNA 5'-f(CGAAUUCG)-3' and RNA 5'-r(CGAAUUCG)-3' at natural abundance 15N to assess a potential effect on 1JNH for 15N-1H imino groups in C:G and A:U base pairs as a result of replacing the 2'-hydroxyl group with fluorine (Figure 1). Individual proton resonances were assigned using a combination of NOESY, DQF-COSY and TOCSY spectra and conventional assignment strategies for double stranded RNA (Supporting Information, Figure S2). The 1H NMR spectra in 10% D2O/H2O showed all four imino resonances, confirming the double helical structures (Figure 2). 1JNH coupling constants for imino groups were measured following published methods and by adapting the two-dimensional in phase anti phase (IPAP) technique to an 15N-filtered, 1D proton detected, one-dimensional NMR measurement.[15] The 1JNH coupling values for imino groups from the three internal base pairs are depicted in Figure 1. As expected we could not measure the 1JNH values for terminal C:G base pairs due to rapid exchange with solvent.
Figure 1.
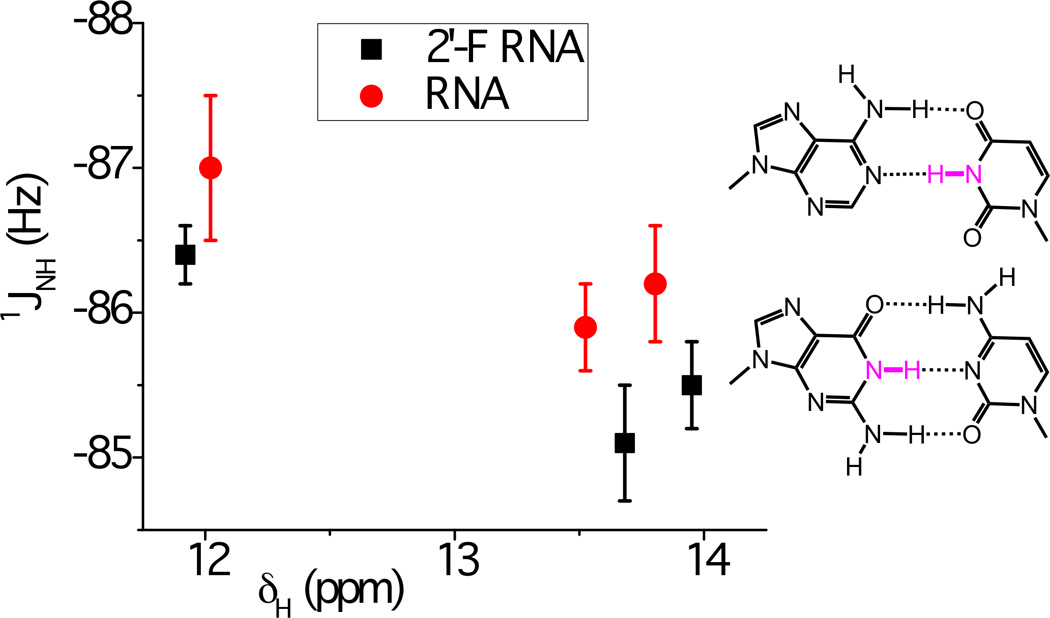
Chemical shifts of imino protons δH (highlighted in magenta in the A:U and G:C base-pair diagrams) versus one-bond scalar coupling constants 1JNH, for 2'-F RNA (black squares) and RNA (red dots) of sequence C1G2A3A4U5U6C7G8. The graph shows average values with standard deviations (vertical bars) based on six independent one-dimensional 15N-coupled 1H IPAP spectra of the imino region in 10% D2O/H2O at 5°C (note the reversed scale on the y-axis). Entries around 12 ppm are for the G2 base, and those at 13.5 and 14 ppm are for the U6 and U5 bases, respectively. The average difference in 1JNH between 2'-F RNA and RNA amounts to 0.8 ± 0.3.
Figure 2.
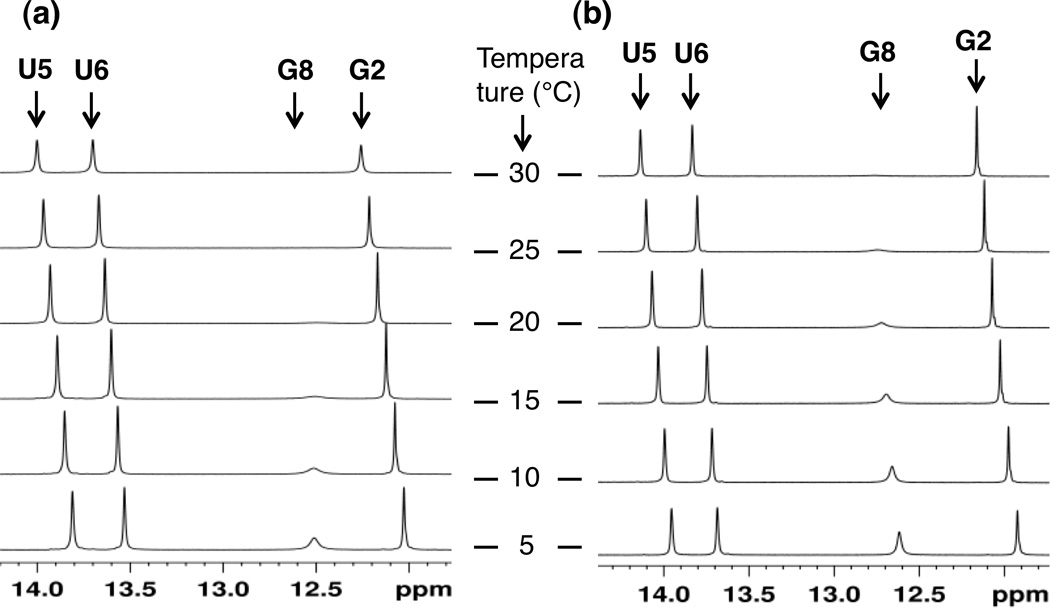
Overlay of one-dimensional proton spectra of the imino region for (a) 5'-r(CGAAUUCG)-3' and (b) 5'-f(CGAAUUCG)-3' in 10% D2O/H2O. The spectra were recorded at varying temperatures, from 5°C (bottom spectrum) to 30°C (top spectrum), with increments of 5°C. Protons shown are assigned as U5, U6, G8 and G2 (from left) in the two duplexes.
The plot of chemical shifts for RNA and 2'-F RNA versus the corresponding 1JNH couplings shows that 2'-F RNA 1JNH values are less negative than those in RNA by an average of 0.8 ± 0.3. The chemical shifts of three of the four imino protons in 2'-F RNA increases compared to RNA (Figure 2 and Supporting information), thus indicating increased H-bonding strength. In line with this interpretation, we found that the terminal C:G base pairs in 2'-F RNA tend to be less frail than in native RNA, arguably due to stronger H-bonding, as measured by line broadening of the G1 imino proton at specific temperatures (Figure 2). The direction and magnitude of the changes in the 1JNH couplings and/or chemical shifts δ(1H) in 2'-F RNA relative to RNA are also consistent with the previously established changes in these parameters between RNA and DNA.[15] The stronger electronegativity of the 2'-fluoro substituent compared to 2'-OH can reasonably be expected to further polarize nucleobase functions.
In order to corroborate the observed differences in H-bonding between 2'-F RNA and RNA based on 1JNH coupling and temperature-dependent line widths, we measured the deuterium isotope effect (DIE) on adenine C2 in A:U base pairs (see inset in Figure 1) as a result of H/D exchange at N3 of uracil.[18,19] As a result of the slow N3(U) imino hydrogen exchange with solvent, the 13C2 resonances from adenine split into doublets, whereby the magnitude of the splitting provides a measure of the strength of the H-bond. Accordingly, larger absolute values in the difference in chemical shift (2hΔ13C2 in parts per billion, ppb) are indicative of increased H-bonding strength. To assess the DIE we acquired 1H, 13C HMQC spectra for the 2'-F RNA and RNA octamers (Figure 3). Consistent with earlier measurements for RNA[18] we found an average value for 2hΔ13C2 of −56.7 ppb for A3 and A4 in r(CGAAUUCG). By comparison, the average value for 2hΔ13C2 for the corresponding residues in f(CGAAUUCG) was −63.2 ppb (see Table S1 in the Supporting Information for individual values). Thus, the DIE data support the above observations of increased Watson-Crick H-bonding strength in 2'-F RNA relative to RNA.
Figure 3.
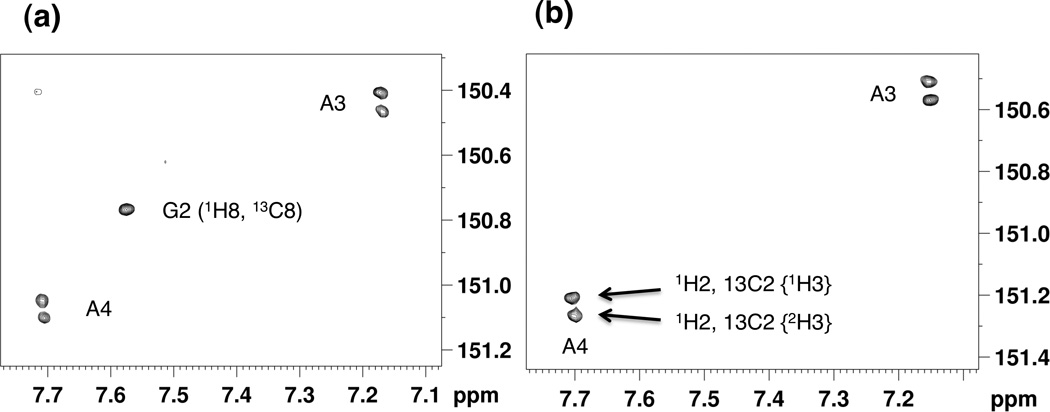
Region of 1H, 13C HMQC spectra of (a) 5'-r(CGAAUUCG)-3' and (b) 5'-f(CGAAUUCG)-3', showing the isotope effect at 13C2 of adenosine residues due to deuterium/proton substitution at the imino H3 site. The individual spectra were recorded at natural abundance 13C in 50% D2O/H2O at 25°C at 14.1 T spectrometer (600 MHz 1H frequency). The x- and y-axes refer to the 1H and 13C nuclei, respectively. The DIE measurements (2hΔ13C2 = δ13C2{1H3} − δ13C2{2H3}) show that 2'-F RNA exhibits a larger isotope effect than RNA (see Table S1 in the Supporting Information).
To assess a potential contribution of base stacking to the stabilization of 2'-F RNA relative to RNA, we studied the thermodynamics of short hairpins,[20] either with blunt ends or 3'-overhanging nucleotides (Figure 4a). Since the overhanging nucleotide cannot form interstrand hydrogen bonds, this is a well-established model system to measure differences in base stacking.[21–23] Because of the negative inclination of the RNA backbone relative to the base-pair axes, a 3'-dangling purine stacking onto the 5'-terminal purine from the opposite strand typically results in a considerably higher stabilization than a 5'-dangling residue or either 3'- or 5'-dangling ends in duplex DNA.[21,24] Indeed, addition of a single adenosine at the 3'-end of 5'-GCGUUUUCGC hairpins led to significant increases in the thermodynamic stability (Table 1, sequences 1 and 2 and 4 and 5). The melting temperature of the hairpin was increased by almost 17°C by addition of a 2'-fluoro-A (terminal fC:fG pair) compared to an increase of 11.6°C by addition of a 2'-ribo-A (terminal rC:rG pair). Van’t Hoff analysis of the UV melting curves (Table 1, columns 4–6) showed that the stabilization was driven by an enhanced binding enthalpy and was larger for 2'-F RNA (ΔΔG = 2.2, ΔΔH = 8.7 kcal/mol) than for RNA (ΔΔG = 1.4, ΔΔH = 4.3 kcal/mol). The larger ΔΔH for the 2'-F RNA relative to RNA overhangs indicates that a least a part of the enthalpic stabilization of 2'-F RNA is due to enhanced stacking. The effect of the addition of a second overhanging adenosine was relatively small by comparison and the difference between 2'-F RNA and RNA within the error limits of the data. The enthalpy calculated using differentiated melting curves[25] (see Supporting Information for experimental details) showed a similar trend (Table 1, column 3).
Figure 4.
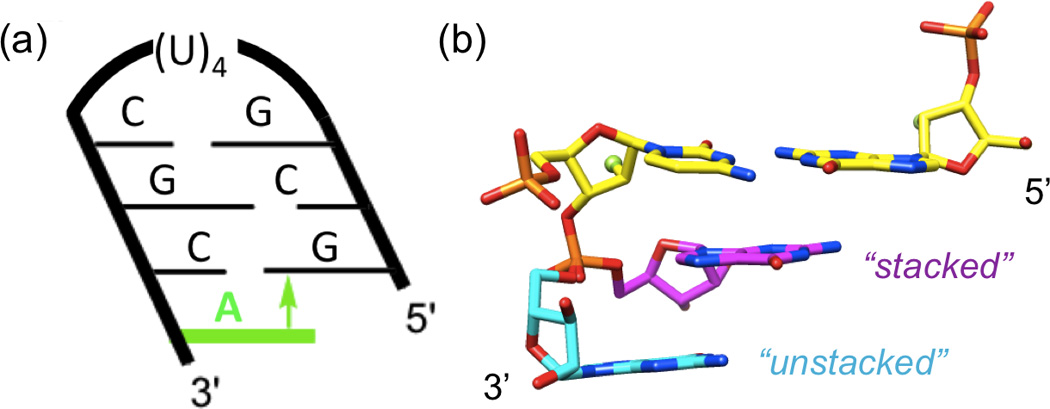
(a) Hairpin construct used to assess the contribution of base stacking to the relative stabilities of 2'-F RNA and RNA. The negative inclination between backbone and base pairs in 2'-F RNA and RNA results in considerable cross-strand stacking[23,24] and a stabilizing π-π interaction (arrow) between the 3'-overhanging A (green) and the 5'-terminal G. (b) Dual conformations of a 3'-terminal G in the crystal structure of a mixed 2'-F/2'-OH RNA duplex (PDB ID 3P4C),[9] unstacked (cyan carbons) and stacked (magenta carbons) onto the terminal C:G pair (yellow carbons). F2'/O2' atoms of the terminal pair are highlighted as green spheres. Neither conformation of G features an intra-nucleoside H-bond involving the 2'-OH moiety.
Table 1.
Thermodynamic Data for RNA and 2'-F Modified RNA Hairpins with or without 3'-Terminal A or 2'-F A Overhangs.a
| Hairpin sequenceb |
Tm [°C] |
ΔTmc | −ΔH δα/δTm [kcal/mol] |
ΔΔHc | −ΔH Van't Hoff [kcal/mol] |
ΔΔHc | −ΔS Van't Hoff [cal/molK] |
−ΔG, (at 37°C) [kcal/mol] |
ΔΔGc | Δnw ethylene glycol |
ΔΔnwc | Δnw acetamide |
ΔΔnwc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. GCGUUUUCGC | 51.9±0.6 | 27.8±0.6 | 30.9±0.8 | 95.0±2.6 | 1.5±0.1 | 9 ± 3 | 16 ± 2 | ||||||
| 2. GCGUUUUCGCA | 63.5±0.4 | 11.6 | 34.2±1.3 | 6.4 | 35.2±1.6 | 4.3 | 104.3±4.6 | 2.9±0.2 | 1.4 | 23 ± 3 | 14 | 29 ± 3 | 13 |
| 3. GCGUUUUCGCAA | 67.9±0.4 | 4.4 | 35.7±1.7 | 1.5 | 38.2±2.7 | 3.0 | 112.1±7.7 | 3.5±0.3 | 0.6 | 20 ± 2 | −3 | 34 ± 3 | 5 |
| 4. GfCGUUUUCGCf | 57.4±0.3 | 26.5±1.4 | 29.5±1.4 | 89.0±4.5 | 1.9±0.1 | 8 ± 2 | 15 ± 2 | ||||||
| 5. GfCGUUUUCGCfAf | 74.3±0.5 | 16.9 | 36.0±3.0 | 9.5 | 38.2±1.2 | 8.7 | 110.1±3.6 | 4.1±0.1 | 2.2 | 18 ± 4 | 10 | 27 ± 4 | 12 |
| 6. GfCGUUUUCGCfAfAf | 77.1±0.3 | 2.8 | 36.3±1.0 | 0.3 | 39.2±1.3 | 1.0 | 111.8±3.8 | 4.5±0.2 | 0.4 | 21 ± 3 | 3 | 28 ± 3 | 1 |
Melting of each oligonucleotide hairpin (16 µM) was performed in 10 mM sodium cacodylate (pH = 7.4), 0.1 mM EDTA, and 300 mM NaCl.
Loop residues are underlined, Cf, Gf and Af indicate 2'-F modified C, G and A, respectively, and 3'-overhanging A or Af nucleotides are highlighted in bold font.
Differences in Tm, ΔH, ΔG or Δnw between blunt end hairpin and hairpin with a single overhang (first value in bold), or hairpin with a single and two overhangs (second value in bold).
Using osmotic stress experiments[26–28] (Table 1, columns 7 and 8), we determined that the original hairpins (sequences 1 and 4) were poorly hydrated, presumably because the short stem and the relatively flexible uridine tetraloop did not provide good enough anchors for a stable hydration network. The NMR solution structure of an RNA hairpin with a U4 loop revealed absence of hydrogen bonding and stacking interactions among uracils[29,30] (Supporting Information, Figure S11). These conformational properties are consistent with the reduced thermodynamic stability of the U4 loop compared with the more common UUCG and GNRA (N=any nucleotide; R=purine) RNA tetraloops that both feature intricate interactions among loop residues.[31] Addition of one adenosine caused significant increase in hydration of the hairpins. Consistent with our previous findings that the 2'-F modification caused dehydration of duplex RNA,[9] the increase of hydration upon addition of one adenosine appeared to be somewhat smaller for 2'-F RNA than for RNA, although the differences were within the limits of experimental errors. Therefore, the differential stabilizations afforded by 3'-dangling 2'-F and 2'-OH adenosines cannot be attributed to hydration. Similarly, it is unlikely that conformational differences between the 2'-F and 2'-OH adenosines (i.e. due to an intra-nucleoside O2'-H⋯N3 H-bond that is absent in the 2'-F nucleoside) affect the respective gains in stacking enthalpy in a crucial way. Thus, we did not observe formation of such a H-bond in the unstacked configuration of a 3'-terminal G with dual occupancy in the crystal structure of a mixed 2'-F/2'-OH RNA duplex (Figure 4b).[9]
The combined NMR and thermodynamic data provide evidence that electronic effects of the 2'-fluorine substituent in the axial configuration boost the RNA affinity of the modified strand by favorably affecting both W-C H-bonding and stacking. The former gains are consistent with less negative 1JNH couplings (N3H of both Us and N1H of G2), increased chemical shifts (imino protons of both Us and G8) and reduced line widths, in particular for N1H of terminal G8, in 2'-F RNA relative to RNA. Our observations are also in-line with those by others who had used a similar strategy based on measurements of 1JNH coupling constants to establish the increased strength of [A]N1⋯H-N3[U/T] H-bonds in duplex RNA relative to duplex DNA.[15] Thus, it appears that the more electronegative fluorine polarizes imino moieties more strongly than the RNA 2'-hydroxyl group, thereby tightening W-C H-bonds and contributing to the previously established, favorable ΔH term underlying the higher RNA affinity of 2'-F RNA relative to RNA.[9]
Whereas an investigation of the role of the 2'-hydroxyl group in potentially strengthening base stacking interactions in A-RNA relative to B-DNA duplexes is complicated by different stacking types in the two species,[17,23,24] the A-form conformation of 2'-F RNA and RNA[9] allows a direct comparison. The thermodynamic data gathered for native and 2'-F modified RNA hairpins with A overhangs support a favorable effect of fluorine on stacking. Given the long reach of fluorine in terms of the aforementioned polarization of imino protons, the effect on the entire aromatic system is not surprising. Fluorine directly attached to the base (i.e. in the T analog 2,4-difluorotoluene) resulted in increased stacking interactions in DNA duplexes as assessed by dangling ends.[22] Moreover NMR experiments provided evidence that W-C H-bonding and stacking interactions are coupled in DNA.[32]
In summary, the higher, enthalpy-based stability of 2'-F RNA relative to RNA is the result of a strengthening of both the H-bonding and stacking interactions in the modified duplex. Our findings provide evidence that fluorine’s electron-withdrawing power is propagated through the entire nucleobase moiety and that such effects dominate a potential role of fluorine in the higher rigidity of the 2'-F RNA backbone compared with RNA. The results described here are directly relevant in terms of the origins of the higher stabilities of mimics of 2'-F RNA, 3'-fluoro hexitol nucleic acid (F-HNA)[3] and 3'-fluoro cyclohexenyl nucleic acid (F-CeNA),[33] relative to HNA and CeNA, respectively.
Experimental Section
See supporting information for further details.
Supplementary Material
Footnotes
Supported by NIH grants R01 GM055237 (M.E.) and R01 GM071461 (E.R.). We thank Drs. Markus Voehler and Min-Kyu Cho for help with NMR acquisition, Raquel Rozner and Ryan Merrihue for assistance with UV melting experiments, and Prof. Nicholas Reiter for helpful discussions.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.2012 …
References
- 1.Pieken WA, Olsen DB, Benseler F, Aurup H, Eckstein F. Science. 1991;253:314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki AM, Casper MD, Freier SM, Lesnik EA, Zounes MC, Cummins LL, Gonzalez C, Dan Cook P. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- 3.Egli M, Pallan PS, Allerson C, Prakash T, Berdeja A, Yu J, Lee S, Watt A, Gaus H, Bhat B, Swayze E, Seth P. J. Am. Chem. Soc. 2011;133:16442–16449. doi: 10.1021/ja207086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, Wanders L, Griffey RH, Swayze EE, Bhat B. J. Med. Chem. 2005;48:901–904. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 5.Manoharan M, Akinc A, Pandey RK, Qin J, Hadwiger P, John M, Mills K, Charisse K, Maier MA, Nechev L, Greene EM, Pallan PS, Rozners E, Rajeev KG, Egli M. Angew. Chem. Int. Ed. 2011;50:2284–2288. doi: 10.1002/anie.201006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis S, Propp S, Freier SM, Jones LE, Serra MJ, Kinberger G, Bhat B, Swayze EE, Bennett CF, Esau C. Nucleic Acids Res. 2009;37:70–77. doi: 10.1093/nar/gkn904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Nat. Rev. Drug Discovery. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 8.Rigo F, Hua Y, Chun SJ, Prakash TP, Krainer AR, Bennett F. Nat. Chem. Biol. 2012;8:555–561. doi: 10.1038/nchembio.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallan PS, Greene EM, Jicman PA, Pandey RK, Manoharan M, Rozners E, Egli M. Nucleic Acids Res. 2011;39:3482–3495. doi: 10.1093/nar/gkq1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egli M, Portmann S, Usman N. Biochemistry. 1996;35:8489–8494. doi: 10.1021/bi9607214. [DOI] [PubMed] [Google Scholar]
- 11.Dingley AJ, Masse JE, Peterson RD, Barfield M, Feigon J, Grzesiek S. J. Am. Chem. Soc. 1999;121:6019–6027. [Google Scholar]
- 12.Wijmenga SS, Kruithof M, Hilbers CW. J. Biomol. NMR. 1997;10:337–350. doi: 10.1023/A:1018348123074. [DOI] [PubMed] [Google Scholar]
- 13.Czernek J, Fiala R, Sklenar V. J. Magn. Reson. 2000;145:142–146. doi: 10.1006/jmre.2000.2091. [DOI] [PubMed] [Google Scholar]
- 14.Barfield M, Dingley AJ, Feigon J, Grzesiek S. J. Am. Chem. Soc. 2001;123:4014–4022. doi: 10.1021/ja003781c. [DOI] [PubMed] [Google Scholar]
- 15.Manalo MN, Kong X, LiWang A. J. Am. Chem. Soc. 2005;127:17974–17975. doi: 10.1021/ja055826l. [DOI] [PubMed] [Google Scholar]
- 16.Swart M, Guerra CF, Bickelhaupt FM. J. Am. Chem. Soc. 2004;126:16718–16719. doi: 10.1021/ja045276b. [DOI] [PubMed] [Google Scholar]
- 17.Egli M. In: Nucleic Acids in Chemistry and Biology. 3rd edn. Blackburn GM, Gait MJ, Loakes D, Williams DM, editors. Cambridge, UK: Royal Society of Chemistry; 2006. pp. 13–75. [Google Scholar]
- 18.Vakonakis I, LiWang AC. J. Am. Chem. Soc. 2004;126:5688–5689. doi: 10.1021/ja048981t. [DOI] [PubMed] [Google Scholar]
- 19.Vakonakis I, LiWang AC. J. Biomol. NMR. 2004;29:65–72. doi: 10.1023/B:JNMR.0000019507.95667.3e. [DOI] [PubMed] [Google Scholar]
- 20.Reif B, Wittmann V, Schwalbe H, Griesinger C, Wörner K, Jahn-Hofmann K, Engels JW, Bermel W. Helv. Chim. Acta. 1997;80:1952–1971. [Google Scholar]
- 21.Freier SM, Kierzek R, Jaeger JA, Sugimoto N, Caruthers MH, Neilson T, Turner DH. Proc. Natl. Acad. Sci. USA. 1986;83:9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guckian K, Schweitzer BA, Ren RX-F, Sheils CJ, Paris PL, Tahmassebi DC, Kool ET. J. Am. Chem. Soc. 1996;118:8182–8183. doi: 10.1021/ja961733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micura R, Bolli M, Windhab N, Eschenmoser A. Angew. Chem. Int. Ed. 1997;36:870–873. [Google Scholar]
- 24.Pallan PS, Lubini P, Bolli M, Egli M. Nucleic Acids Res. 2007;35:6611–6624. doi: 10.1093/nar/gkm612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslauer KJ. Methods Enzymol. 1995;259:221–242. doi: 10.1016/0076-6879(95)59046-3. [DOI] [PubMed] [Google Scholar]
- 26.Spink CH, Chaires JB. Biochemistry. 1999;38:496–508. doi: 10.1021/bi9820154. [DOI] [PubMed] [Google Scholar]
- 27.Rozners E, Moulder J. Nucleic Acids Res. 2004;32:248–254. doi: 10.1093/nar/gkh175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozners E. Curr. Protoc. Nucleic Acid Chem. 2010;43:7.14.1–7.4.13. doi: 10.1002/0471142700.nc0714s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanegas PL, Hudson GA, Davis AR, Kelly SC, Kirkpatrick CC, Znosko BM. Nucleic Acids Res. 2012;40:439–444. doi: 10.1093/nar/gkr943. http://cossmos.slu.edu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sashital DG, Venditti V, Angers CG, Cornilescu G, Butcher SE. RNA. 2007;13:328–338. doi: 10.1261/rna.418407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehy JP, Davis AR, Znosko BM. RNA. 2010;16:417–429. doi: 10.1261/rna.1773110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manalo MN, Pérez LM, LiWang A. J. Am. Chem. Soc. 2007;129:11298–11299. doi: 10.1021/ja0692940. [DOI] [PubMed] [Google Scholar]
- 33.Seth PP, Yu J, Jazayeri A, Pallan PS, Allerson CR, Østergaard ME, Liu F, Herdewijn P, Egli M, Swayze EE. J. Org. Chem. 2012;77:5074–5085. doi: 10.1021/jo300594b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


