Abstract
PBSH, a defective phage of Bacillus subtilis strain 168, is described. Conditions are given for optimal induction of the prophage with mitomycin C. After a latent period of 90 min, cells were lysed and phage-like particles were released with a burst size of approximately 100 to 400 phage per bacterium. Since no known host supports phage replication after infection, burst size was determined by electron microscope count. Purification procedures and criteria for purity are described. The molecular weight of deoxyribonucleic acid (DNA) extracted from PBSH was estimated by length measurement and sedimentation. No circular DNA molecules were found by either technique. PBSH DNA molecules are linear, double-stranded, and of homogeneous molecular weight, about 12 × 106 daltons. There is no evidence for single-strand breaks. The majority of PBSH DNA molecules show a sedimentation behavior dependent on ionic strength. It is inferred that most of the DNA molecules are less hydrodynamically rigid than native DNA having a similar average base composition and molecular weight. Possible reasons for the sedimentation behavior are discussed.
Full text
PDF





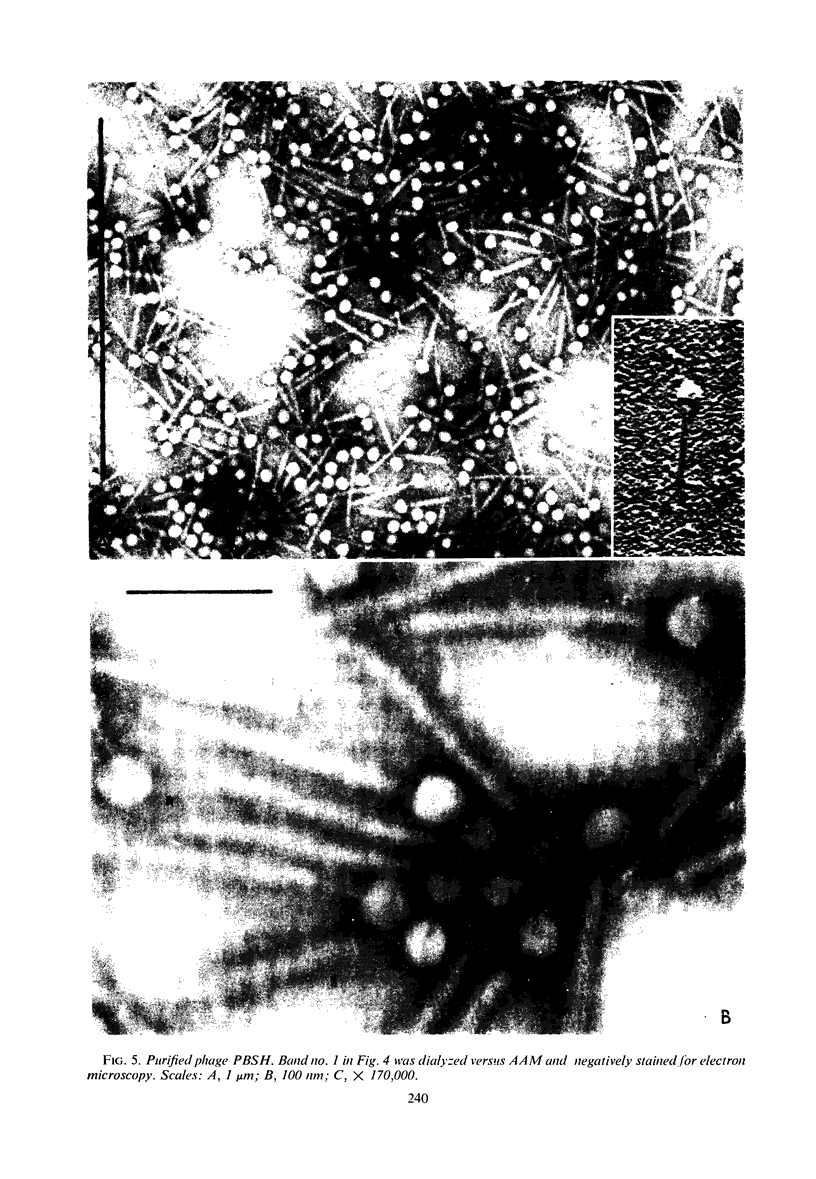

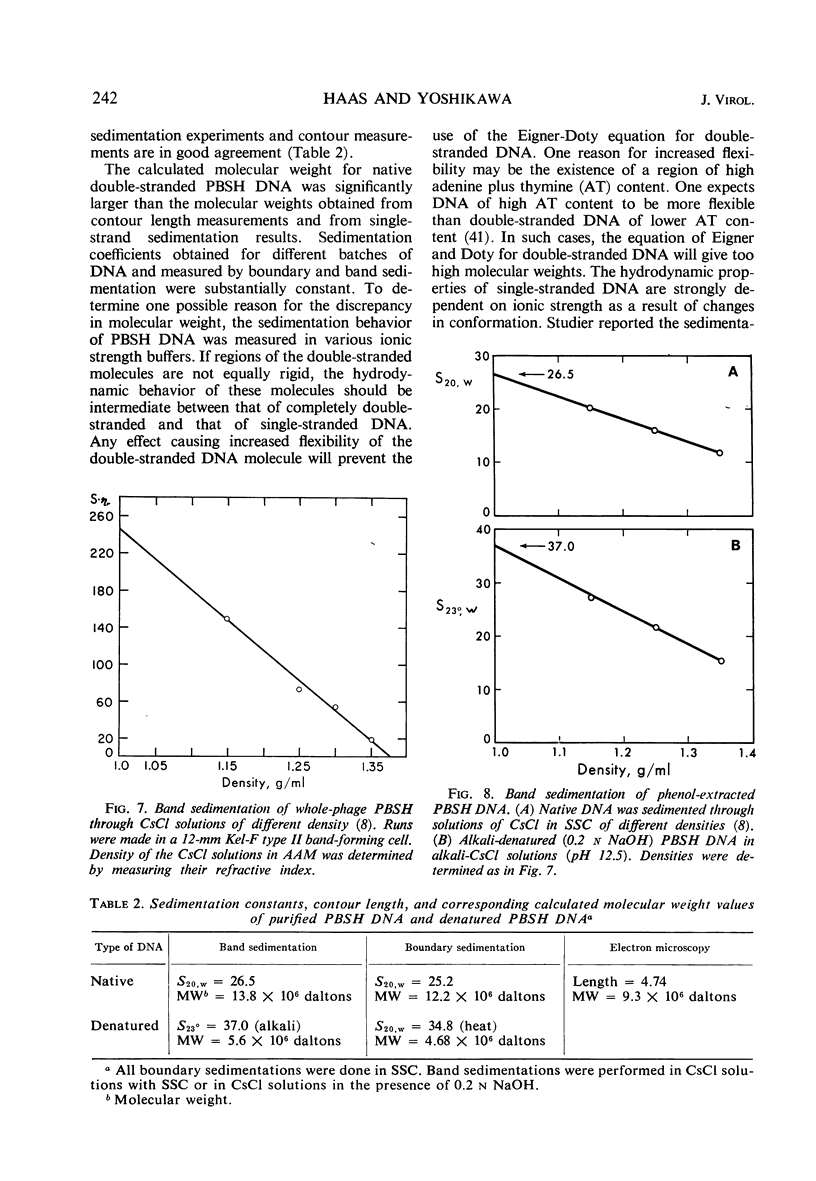




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- ALTENBERN R. A., STULL H. B. INDUCIBLE LYTIC SYSTEMS IN THE GENUS BACILLUS. J Gen Microbiol. 1965 Apr;39:53–62. doi: 10.1099/00221287-39-1-53. [DOI] [PubMed] [Google Scholar]
- Abram D., Vatter A. E., Koffler H. Attachment and structural features of flagella of certain bacilli. J Bacteriol. 1966 May;91(5):2045–2068. doi: 10.1128/jb.91.5.2045-2068.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield V., Van Holde K. E., Dalton W. O. Frictional coefficients of multisubunit structures. II. Application to proteins and viruses. Biopolymers. 1967 Feb;5(2):149–159. doi: 10.1002/bip.1967.360050203. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The isolation and morphology of some new bacteriophages specific for Bacillus and Acetobacter species. J Gen Microbiol. 1965 Nov;41(2):233–241. doi: 10.1099/00221287-41-2-233. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Davison P. F., Freifelder D. Lability of single-stranded deoxyribonucleic acid to hydrodynamic shear. J Mol Biol. 1966 Apr;16(2):490–502. doi: 10.1016/s0022-2836(66)80187-0. [DOI] [PubMed] [Google Scholar]
- ENDO H., AYABE K., AMAKO K., TAKEYA K. INDUCIBLE PHAGE OF ESCHERICHIA COLI 15. Virology. 1965 Mar;25:469–471. doi: 10.1016/0042-6822(65)90067-x. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- FRAMPTON E. W., BRINKLEY B. R. EVIDENCE OF LYSOGENY IN DERIVATIVES OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:446–452. doi: 10.1128/jb.90.2.446-452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIFELDER D. A NOVEL METHOD FOR THE RELEASE OF BACTERIOPHAGE DNA. Biochem Biophys Res Commun. 1965 Jan 4;18:141–144. doi: 10.1016/0006-291x(65)90897-1. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D., DAVISON P. F. Physicochemical studies on the reaction between formaldehyde and DNA. Biophys J. 1963 Jan;3:49–63. doi: 10.1016/s0006-3495(63)86803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. II. Intracellular development of the induced prophage. J Virol. 1969 Feb;3(2):248–260. doi: 10.1128/jvi.3.2.248-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IONESCO H., RYTER A., SCHAEFFER P. SUR UN BACT'ERIOPHAGE H'EBERG'E PAR LA SOUCHE MARBURG DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Dec;107:764–776. [PubMed] [Google Scholar]
- Inman R. B. Some factors affecting electron microscopic length of deoxyribonucleic acid. J Mol Biol. 1967 Apr 28;25(2):209–216. doi: 10.1016/0022-2836(67)90138-6. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- LWOFF A., GUTMANN A. Recherches sur un Bacillus megatherium lysogène. Ann Inst Pasteur (Paris) 1950 Jun;78(6):711–739. [PubMed] [Google Scholar]
- LWOFF A., SIMINOVITCH L., KJELDGAARD N. Induction de la production de bacteriophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950 Dec;79(6):815–859. [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., SHATKIN A. J., TATUM E. L. Bacteriocidal action of mitomycin C. Biochim Biophys Acta. 1961 Oct 14;53:132–149. doi: 10.1016/0006-3002(61)90800-9. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- STICKLER D. J., TUCKER R. G., KAY D. BACTERIOPHAGE-LIKE PARTICLES RELEASED FROM BACILLUS SUBTILIS AFTER INDUCTION WITH HYDROGEN PEROXIDE. Virology. 1965 May;26:142–145. doi: 10.1016/0042-6822(65)90035-8. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The arrangement of information in DNA molecules. J Gen Physiol. 1966 Jul;49(6):143–169. doi: 10.1085/jgp.49.6.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Effect of ultraviolet irradiation on bacteriophage lambda immunity. J Mol Biol. 1967 Jan 28;23(2):247–263. doi: 10.1016/s0022-2836(67)80031-7. [DOI] [PubMed] [Google Scholar]
- Wingert L., Von Hippel P. H. The conformation dependent hydrolysis of DNA by micrococcal nuclease. Biochim Biophys Acta. 1968 Mar 18;157(1):114–126. doi: 10.1016/0005-2787(68)90270-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]