Abstract
Herbal based remedies are used worldwide to treat psychiatric disorders. The aim of this study was to analyse the essential oil composition of Achillea Wilhemsii C. Koch (Asteraceae) and to evaluate its anxiolytic effects in the elevated plus maze (EPM) model of anxiety in rat. Gas chromatography/mass spectrometry (GC/MS) analysis of the essential oil showed that the main compounds of the oil were p-ocimen (23%), 1, 8-cineole (20.8%) and carvone (19.13%). The EPM results showed that 1 mg/kg (i.p.) of the oil significantly (P<0.05) increased the percentage of the time spent and the number of entries in the open arms of the maze while it did not change the total number of entries in the maze arms. These effects were not reversed with 2 mg/kg flumazenil and 5 mg/kg naloxone. We concluded that a minimum dose of 1 mg/kg of the oil has anxiolytic effects which are not probably mediated through GABA and opioid receptors.
Keywords: Achillea Wilhelmsii C. Koch, Anxiolytic, Essential oil, GC/MS, Elevated plus maze
INTRODUCTION
Anxiety disorders are among the most common psychiatric disorders that have debilitating effects on the quality of life of many people around the world. Pharmaco-therapy has shown good results in many cases but prevalence of side effects such as physical dependence to the main group of used drugs, benzodiazepines, has drawn attention towards developing new drugs or using alternative medicine and especially plant-derived product as substitutes(1). Some of the medicinal plants that are used as sedative or anxiolytic are Matricaria recutita(2), Salvia guaranitica(3), Valeriana officinalis(4), Passiflora caerulea(5) and Stachys lavandulifolia(6).
Achillea (Asteraceae) is a perennial herb that has around 100 species worldwide from which about 19 species are found in Iran. Different species of Achillea are used in folk medicine as sedative, anti-inflammatory, analgesic, anthelmintic and to relieve symptoms in premenstrual syndrome (PMS)(7). Studies show that different Achillea spp have a wide range of pharmacologic effects such as antioxidant, antimicrobial, anxiolytic and cytotoxic activities(8–10). Achillea Wilhelmsii C. Koch (A. Wilhelmsii) is one of the widespread species of Achillea in Iran. Numerous studies have been conducted to evaluate the pharmacologic effects of the essential oil as well as different extracts of this herb. It is shown that this plant has antimicrobial(11), antitumoral(12), immuno-modulating(13), antihypertensive(14) and vagolitic(15) properties.
Although A. Wilhelmsii is used as an anxiolytic plant in folk medicine, however studies to support anxiolytic properties of the volatile oil have not yet been reported. The aim of the present study was therefore to determine the essential oil components of A. Wilhelmsii and to evaluate its anxiolytic effects in a rat model. In this study the volatile oil constituents of A. Wilhelmsii from Iran and its anxiolytic effects on the elevated plus maze (EPM) model of anxiety in rats were investigated.
MATERIALS AND METHODS
Plant
Aerial parts of A. wilhelmsii were collected in Kermanshah (West of Iran) in July 2012 and the voucher specimens were deposited at the herbarium of School of Pharmacy (012 C) at Kermanshah University of Medical Sciences,, of Iran.
The air dried aerial parts of the plant were powdered and subjected to hydrodistillation using a Clevenger-type apparatus for 4 h. Anhydrous sodium sulphate was used to dehydrate the essential oil. The oil was stored at -20˚C until the use.
Gas chromatography/mass spectrometry (GC/MS) analyses of the volatile oil
An HP 6890N GC system, coupled with an HP MSD5973N quadruple mass spectrometer was used. The extracted compounds were separated on an HP-5MS capillary column (30 m length, 0.25 mm internal diameter, 0.25 mm film thickness). Split injection was employed for distillation of the samples with a ratio of 50:1. The column oven temperature was programmed to rise from an initial temperature of 40 °C to 150 °C at 4 °C /min, and then to 240 °C at 10 °C /min. The injection temperature and ion source temperature were 240 °C. Helium was used as the carrier gas with a flow rate of 1.2 ml/min. The ionizing energy was 70 eV. All data were obtained by collecting the full-scan mass spectra within the scan range 50-550 amu. Compounds were identified using the Wiley 7n.L Mass Spectral Library (Wiley, New York, NY, USA).
Animal studies
Male Wistar rats (KUMS breeding house, Kermanshah) weighing 200 ± 20 g were kept in 12:12 hr light/dark cycle and controlled room temperature of 23-26 °C. The animals had access to tap water and food ad libitum. Experiments were done on 8 groups of rats (n=8). Test groups were as follows; Volatile oil (0.5 and 1 mg/kg), Diazepam (1 mg/kg), Vehicle (saline with tween 80 0.1% V/V), combination of diazepam (1mg/kg) and naloxone (5 mg/kg) or flumazenil (2 mg/kg), combination of volatile oil (1 mg/kg) and naloxone (5 mg/kg) or flumazenil (2 mg/kg). All procedures were approved by the Ethical Committee of the Kermanshah University of Medical Sciences. All drugs were obtained from Sigma-Aldrich Corporation and dissolved in saline (with tween 80, 0.1% V/V).
Elevated plus maze (EPM) test
EPM model of anxiety which is a standard model for testing the anxiolytic drugs(16) was used to evaluate the effect of A. wilhelmsii essential oil on anxiety and locomotor activity of the rats. The plus shaped apparatus consisted of two open (10 × 50 cm) and two closed arms (10 × 50 × 40 cm) that extended from a common central platform (10 × 10 cm). The maze was elevated to a height of 50 cm above the floor. Testing sessions started 30 min after the intraperitoneal (i.p.) injection of the drugs. Each test session was 5 min. long. The rats were first placed on the open arm of the maze and variables such as the number of entries to open and closed arms and the percentage of time spent in open and closed arms were measured during the test period.
Data analysis
SPSS (version 11.5) was used for the statistical analysis of the data. One way analysis of variance (ANOVA) with the Tukey post test was used to analyze the differences between groups. The significant level was set at P <0.05.
RESULTS
EPM results
The results of the EPM showed that diazepam (1 mg/kg), as confirmed in other studies (17,18), has anxiolytic activity. To determine the minimum effective dose of the oil, doses were started from 0.5 mg/kg with 0.5 mg/kg increments and it was found that 1 mg/kg of the oil significantly (P <0.05) increased the percentage of time spent and the number of entries in the open arms of the maze compared to the vehicle treated group. This dose of the drug did not change the total number of entries in the maze arms. The effect of diazepam on open arm entry and time is expectedly abated by GABAAreceptor antagonist; flumazenil (2 mg/kg, i.p.) (Figs. 1 and 2). Total number of entries was decreased significantly in diazepam treated group (P <0.05). Neither naloxone (5 mg/kg) nor flumazenil (2 mg/kg) could significantly decrease the number of open arm entries, total number of entries or the percentage of open arm time in the oil treated group (Figs. 1 and 2).
Fig. 1.
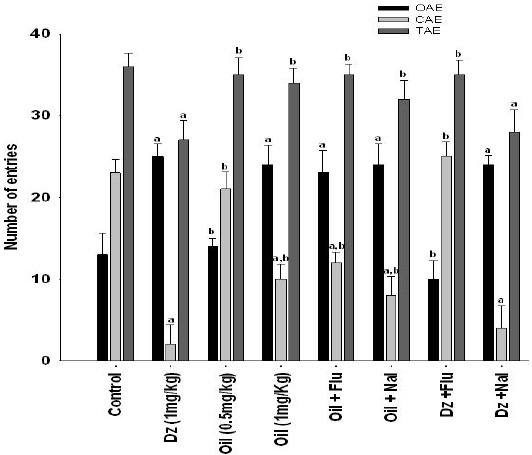
Effects of intraperitoneal injectio2 mg/kg, Dz 1n of essential oil; Oil 0.5 and 1mg/kg, diazepam; Dz 1mg/kg, flumazenil/oil; Flu 2 mg/kg, Oil 1mg/kg, naloxone/oil; Nal 5 mg/kg, Oil 1mg/kg, flumazenil/diazepam; Flu mg/kg, naloxone/diazepam; Nal 5 mg/kg, Dz 1mg/kg, and vehicle control were assessed on the number of entries in open arms OAE, number of entries in close arms CAE and total number of entries in open and close arms TNE of the EPM model of anxiety in rats. (n=8). The letter “a” means a significant difference p<0.05) when compared with control. The letter “b” means a significant difference p<0.05) when compared with diazepam. Data are presented as mean ±SEM.
Fig. 2.
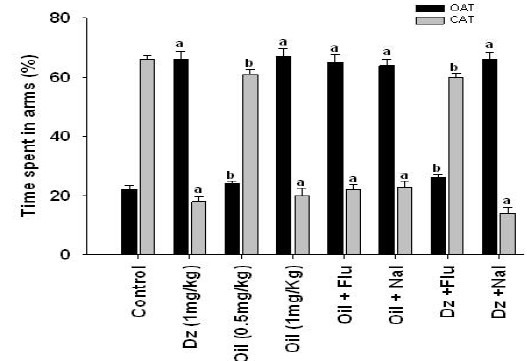
Effects of intraperitoneal injection of essential oil; Oil 0.5 and 1mg/kg, diazepam; Dz 1 mg/kg, flumazenil/oil; Flu 2 mg/kg, Oil 1 mg/kg, naloxone/oil; Nal 5 mg/kg, Oil 1mg/kg, flumazenil/diazepam; Flu 2 mg/kg, Dz 1mg/kg, naloxone/diazepam; Nal 5 mg/kg, Dz 1mg/kg, and vehicle control were assessed on the percentage of time spent in the open arms OAT and percentage of time spent in the close arms CAT of the EPM model of anxiety in rats n=8. The letter “a” means a significant difference p<0.05) when compared with control. The letter “b” means a significant difference p<0.05) when compared with diazepam. Data are presented as mean ±SEM
The oil composition of A. wilhelmsii
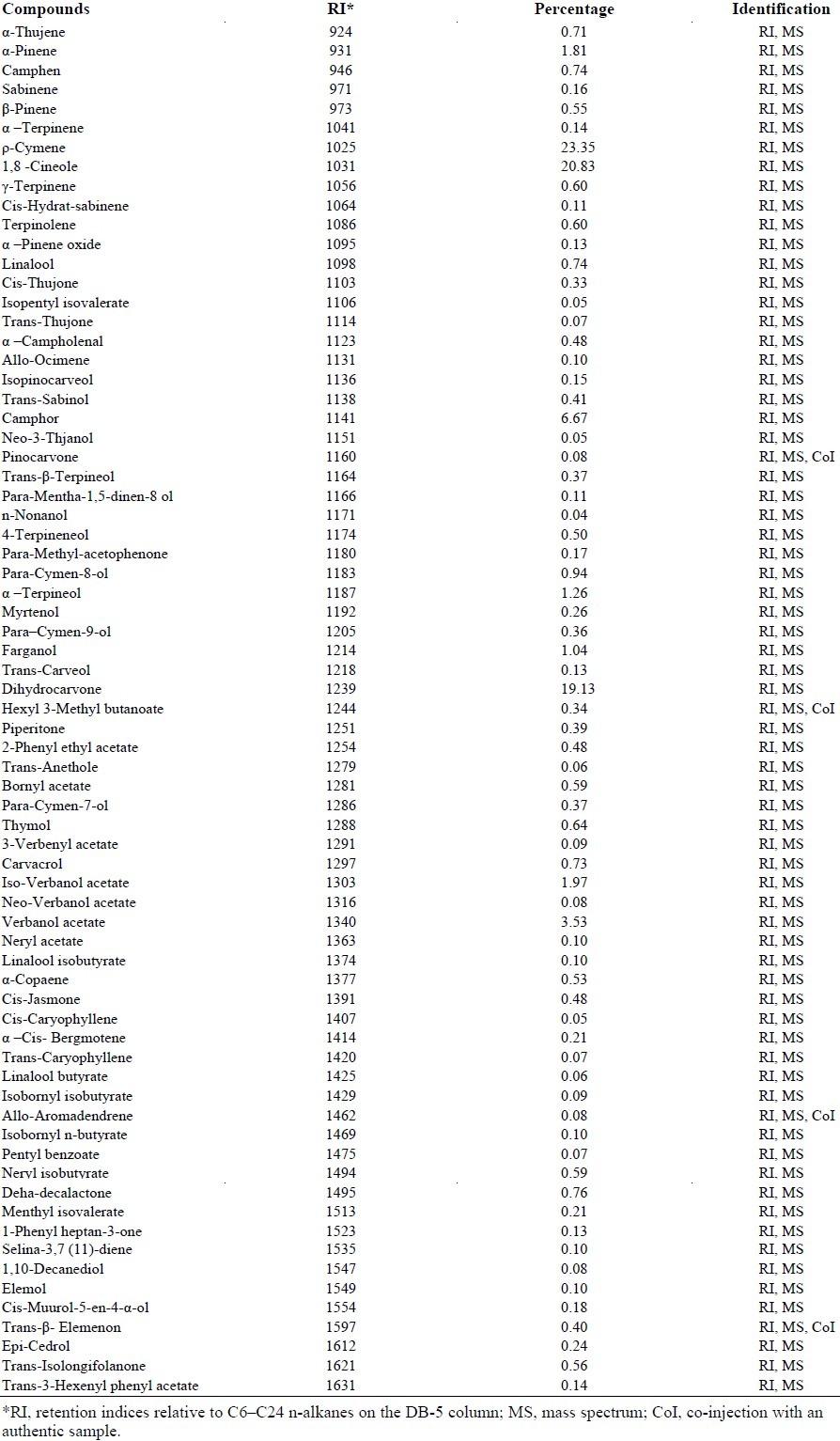
Mass spectra and IR were used to determine the essential oil composition of A. wilhelmsii. Fifty five compounds were identified which represented 98% of the oil constituents. The main compounds of the oil were p-ocimen (23%), 1,8-cineole (20.8%), carvone (19.13%), camphor (6.67%), and verbanol acetate (3.53%). Composition of the essential oil of A. wilhelmsii is shown in Table 1.
Table 1.
Volatile oil components of Achillea wilhelmsii C. Koch from the west of Iran
DISCUSSION
The aim of the present study was to analyze the composition of the volatile oil of A. Wilhelmsii and also to evaluate its effects on anxiety state in EPM model of anxiety.
Javidnia and coworkers have previously shown that the main components of the volatile oil of A. Wilhelmsii are carvacrol (25.1%), linalool (11.5%) and 1,8 cineol (10.3%)(19) while in our study we detected the main three components as p-ocimen (23%), 1,8-cineole (20.8%) and carvone (19.13%). Another study which has been done by Brunke EJ and coworkers on essential oils of A. Wilhelmsii collected from Egypt and Turkey showed that 1, 8 cineol is the main component(20). Studies on other Achillea spp have shown camphor, 1,8 cineol, linalool and α-terpineol as the main oil constituents(21–23). The difference in composition and percentage of the constituents of the oil may be attributed to their geographic and bioclimatic distributions(24–27).
The elevated plus maze is an animal model of anxiety which was first introduced by Montgomery in 1955 and is based upon two opposing instincts of exploring a novel environment and fearing of an open environment(28). The results of the volatile oil on EPM test showed that it had anxiolytic effects at the minimum concentration of 1mg/kg. Total number of entries was not changed in oil treated group while it was decreased with diazepam treatment. Total number of entries can be used as an indicator of spontaneous motor activity in EPM(29) so the anxiolytic effect of the oil did not interfere with locomotor activity of the rats. Diazepam, as expected, decreased their locomotor activity.
Monoterpenes are the main components of volatile oils of this plant. It is shown that these compounds have important central nervous system activities such as anticonvulsant(30), analgesic(31) anxiolytic(32–34) and antidepressant(35) effects. Gomes and coworkers showed that 1,4 cineol had anxiolytic effects in mice which was not antagonized with flumazenil(32). Anxiolytic effects of carvone and thujone were also reported in other investigations(36–37). Anxiolytic effects of A. umbellata essential oil and A.millefolium hydroalcoholic extract are also reported in other studies(8,9)
The EPM model is a valid model for those compounds which have GABAAagonistic activity like benzodiazepines,. Other compounds such as 5-HT1A agonists, selective serotonin reuptake inhibitors and tricyclic antidepressants did not show consistent results in different studies(16). The anxiolytic effect of diazepam was decreased by the GABA antagonist, flumazenil. Co-administration of the oil with flumazenil did not decrease its anxiolytic effect which suggests that GABA receptors are not involved in its mechanism of action. Since naloxone could not change open arm time and entry compared to diazepam it can be concluded that opioidergic system is also not involved in its anxiolytic effect. It is shown that other receptors such as cannabinoid (38,39), glutamate (40,41), dopamine(42), cholecycto-kinine(43) or adenosine(44) receptors are also involved in anxiety and here may have a role in anxiolytic mechanism of this oil.
CONCLUSION
This study showed that the essential oil of A. Wilhelmsii had anxiolytic effects which are probably not mediated through GABA and opioid receptors and unlike diazepam it did not change the locomotor activity of the rats. Its actions may be attributed to its main monoterpenoid compounds including p-ocimen1, 8-cineoleand carvone.
ACKNOWLEDGMENT
We gratefully aknowledge Kermanshah University of Medical Sciences Research Council for financial support of this work.
REFERENCES
- 1.Lakhan SE, Vieira KF. Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutr J. 2010;9:42–58. doi: 10.1186/1475-2891-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, et al. A component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- 3.Marder M, Viola H, Wasowski C, Wolfman C, Waterman PG, Cassels BK, et al. 6-bromoflavone, a high affinity ligand for the central benzodiazepine receptors is a member of a family of active flavonoids. Biochem Biophys Res Commun. 1996;223:384–389. doi: 10.1006/bbrc.1996.0903. [DOI] [PubMed] [Google Scholar]
- 4.Cavadas C, Araújo I, Cotrim MD, Amaral T, Cunha AP, Macedo T, et al. In vitro study on the interaction of Valeriana officinalis L. extracts and their amino acids on GABA A receptor in rat brain. Arzneimittelforschung. 1995;45:753–755. [PubMed] [Google Scholar]
- 5.Medina JH, Paladini AC, Wolfman C, Levi de Stein M, Calvo D, Diaz LE, et al. Chrysin 5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem Pharmacol. 1990;40:2227–2231. doi: 10.1016/0006-2952(90)90716-x. [DOI] [PubMed] [Google Scholar]
- 6.Rabbani M, Sajjadi SE, Zarei HR. Anxiolytic effects of Stachys lavandulifolia Vahl on the elevated plus-maze model of anxiety in mice. J Ethnopharmacol. 2003;89:271–276. doi: 10.1016/j.jep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Zargari A. Medicinal plants. 6th ed. Vol. 3. Tehran: Tehran university publications; 1996. pp. 107–117. [Google Scholar]
- 8.Radulovic NS, Dekic MS, Rand-elovic JP, Stojanovic NM, Zarubica AR, Stojanovic ZZ. Toxic essential oils: Anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm. (Asteraceae) volatiles. Food Chem Toxicol. 2012;50:2016–2026. doi: 10.1016/j.fct.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Baretta IP, Felizardo RA, Bimbato VF, dos Santos MGJ, Kassuya CAL, Junior AG, et al. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J Ethnopharmacol. 2012;40:46–54. doi: 10.1016/j.jep.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Maggi F, Bramucci M, Cecchini C, Coman MM, Cresci A, Cristalli G, et al. Composition and biological activity of essential oil of Achillea ligustica All.(Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia. 2009;80:313–319. doi: 10.1016/j.fitote.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Tosun F, Kizilay CA, Sener B, Vural M, Palittapongarnpim P. Antimycobacterial screening of some Turkish plants. J Ethnopharmacol. 2004;95:273–275. doi: 10.1016/j.jep.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Ali N, Shah SW, Shah I, Ahmed G, Ghias M, Khan I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Complement Altern Med. 2011;11:106–112. doi: 10.1186/1472-6882-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. Indian J Exp Biol. 2009;47:668–671. [PubMed] [Google Scholar]
- 14.Asgary GH, Naderi N, Sarrafzadegan N, Mohammadifard, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000:2689–2693. [PubMed] [Google Scholar]
- 15.Niazmand S, Khooshnood E, Derakhshan M. Effects of Achillea wilhelmsii on rat’s gastric acid output at basal, vagotomized, and vagal-stimulated conditions. Pharmacogn Mag. 2010;6:282–285. doi: 10.4103/0973-1296.71791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 17.Dalvi A, Rodgers RJ. Anxiolytic effects of valproate and diazepam in mice are differentially sensitive to picrotoxin antagonism. Pharmacol Biochem Behav. 2001;68:23–32. doi: 10.1016/s0091-3057(00)00408-1. [DOI] [PubMed] [Google Scholar]
- 18.Kudryavtseva NN, Bondar NP. Anxiolytic and anxiogenic effects of diazepam in male mice with different experience of aggression. Bull Exp Biol Med. 2002;133:372–376. doi: 10.1023/a:1016202205966. [DOI] [PubMed] [Google Scholar]
- 19.Javidnia K, Miri R, Sadeghpour H. Composition of the Volatile Oil of Achillea Wilhelmsii C. Koch from Iran. Daru. 2004;12:63–66. [Google Scholar]
- 20.Brunke EJ, Hammerschmidt FJ, Aboutabl EA. In: Progress in Essential Oil Research. Brunke E J, editor. New York: Walter de Gruyter; 1986. pp. 85–92. [Google Scholar]
- 21.Kordali S, Cakir A, Aytas T, Akcin E, Mete A, Akcin T, et al. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan (Asteraceae) Ind Crop Prod. 2009;29:562–570. [Google Scholar]
- 22.Rahimmalek M, Tabatabaei BES, Etemadi N, Hossein Goli SA, Arzani A, Zeinali H. Essential oil variation among and within six Achillea species transferred from different ecological regions in Iran to the field conditions. Ind Crop Prod. 2009;29:348–355. [Google Scholar]
- 23.Maggi F, Bramucci M, Cecchini C, Coman MM, Cresci A, Cristalli G, et al. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia. 2009;80:313–319. doi: 10.1016/j.fitote.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Chograni H, Zaouali Y, Rajeb C, Boussaid M. Essential oil variation among natural populations of Lavandula multifida L.(Lamiaceae) Chem Biodivers. 2010;7:933–942. doi: 10.1002/cbdv.200900201. [DOI] [PubMed] [Google Scholar]
- 25.Dardioti A, Hanlidou E, Lanaras T, Kokkini S. The essential oils of the Greek endemic Satureja horvatii ssp. macrophylla in relation to bioclimate. Chem Biodivers. 2010;7:1968–1977. doi: 10.1002/cbdv.200900181. [DOI] [PubMed] [Google Scholar]
- 26.ElHadj Ali IB, Zaouali Y, Bejaoui A, Boussaid M. Variation of the chemical composition of essential oils in Tunisian populations of Thymus algeriensis Boiss. et Reut. (Lamiaceae) and implication for conservation. Chem Biodivers. 2010;7:1276–1289. doi: 10.1002/cbdv.200900248. [DOI] [PubMed] [Google Scholar]
- 27.Lakušiæ DV, Ristiæ MS, Slavkovska VN, Sinžar-Sekuliæ JB, Lakušiæ BS. Environment-related variations of the composition of the essential oils of Rosemary Rosmarinus officinalis L.) in the Balkan Penninsula. Chem Biodivers. 2012;9:1286–1302. doi: 10.1002/cbdv.201100427. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 29.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Proto. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Sousa DP, Gonçalves JC, Quintans-Júnior L, Cruz JS, Araújo DA, de Almeida RN. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett. 2006;401:231–235. doi: 10.1016/j.neulet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Almeida RN, Navarro DS, Barbosa-Filho JM. Plants with central analgesic activity. Phytomedicine. 2001;8:310–322. doi: 10.1078/0944-7113-00050. [DOI] [PubMed] [Google Scholar]
- 32.Gomes PB, Feitosa ML, Silva MI, Noronha EC, Moura BA, Venâncio ET, et al. Anxiolytic-like effect of the monoterpene 1,4-cineole in mice. Pharmacol Biochem Behav. 2010;96:287–293. doi: 10.1016/j.pbb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Souto-Maior FN, de Carvalho FL, de Morais LC, Netto SM, de Sousa DP, de Almeida RN. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol Biochem Behav. 2011;100:259–263. doi: 10.1016/j.pbb.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Silva MI, de Aquino Neto MR, Teixeira Neto PF, Moura BA, do Amaral JF, de Sousa DP, et al. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol Biochem Behav. 2007;88:141–147. doi: 10.1016/j.pbb.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Melo FH, Moura BA, de Sousa DP, de Vasconcelos SM, Macedo DS, Fonteles MM, et al. Antidepressant-like effect of carvacrol 5-Isopropyl-2-methylphenol in mice: involvement of dopaminergic system. Fundam Clin Pharmacol. 2011;25:362–367. doi: 10.1111/j.1472-8206.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- 36.Mora S, Diaz-Veliz G, Millan R, Lungenstrass H, Quiro´s S, Coto-Morales T, Ibarrola H. Anxiolytic and antidepressant-like effects of the hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol Biochem Behav. 2005;82:373–378. doi: 10.1016/j.pbb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Hatano VY, Torricelli AS, Giassi AC, Coslope LA, Viana MB. Anxiolytic effects of repeated treatment with an essential oil from Lippia alba and (R)-(-)-carvone in the elevated T-maze. Braz J Med Biol Res. 2012;45:238–243. doi: 10.1590/S0100-879X2012007500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo FH, Moura BA, de Sousa DP, de Vasconcelos SM, Macedo DS, Fonteles MM, et al. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin. Neuroscience. 2012;204:186–192. doi: 10.1016/j.neuroscience.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 39.Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A. Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behav Pharmacol. 2008;19:716–723. doi: 10.1097/FBP.0b013e3283123c83. [DOI] [PubMed] [Google Scholar]
- 40.Harvey BH, Shahid M. Metabotropic and ionotropic glutamate receptors as neurobiological targets in anxiety and stress-related disorders: focus on pharmacology and preclinical translational models. Pharmacol Biochem Behav. 2012;100:775–800. doi: 10.1016/j.pbb.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Spooren W, Lesage A, Lavreysen H, Gasparini F, Steckler T. Metabotropic glutamate receptors: their therapeutic potential in anxiety. Curr Top Behav Neurosci. 2010;2:391–413. doi: 10.1007/7854_2010_36. [DOI] [PubMed] [Google Scholar]
- 42.Fedotova Iu O. D2-subtype of dopaminergic receptors is involved in anxiety behavior in ovariectomized rats. Patol Fiziol Eksp Ter. 2008;3:10–13. [PubMed] [Google Scholar]
- 43.Wang H, Spiess J, Wong PT, Zhu YZ. Blockade of CRF1 and CCK2 receptors attenuated the elevated anxiety-like behavior induced by immobilization stress. Pharmacol Biochem Behav. 2011;98:362–368. doi: 10.1016/j.pbb.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Correa M, Font L. Is there a major role for adenosine A2A receptors in anxiety. Front Biosci? 2008;13:4058–4070. doi: 10.2741/2994. [DOI] [PubMed] [Google Scholar]


