Abstract
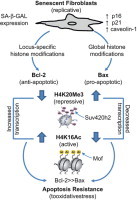
Aging and age-related diseases are associated with cellular senescence that results in variable apoptosis susceptibility to oxidative stress. Although fibroblast senescence has been associated with apoptosis resistance, mechanisms for this have not been well defined. In this report, we studied epigenetic mechanisms involving histone modifications that confer apoptosis resistance to senescent human diploid fibroblasts (HDFs). HDFs that undergo replicative senescence display typical morphological features, express senescence-associated β-galactosidase, and increased levels of the tumor suppressor genes, p16, p21, and caveolin-1. Senescent HDFs are more resistant to oxidative stress (exogenous H2O2)-induced apoptosis in comparison to non-senescent (control) HDFs; this is associated with constitutively high levels of the anti-apoptotic gene, Bcl-2, and low expression of the pro-apoptotic gene, Bax. Cellular senescence is characterized by global increases in H4K20 trimethylation and decreases in H4K16 acetylation in association with increased activity of Suv420h2 histone methyltransferase (which targets H4K20), decreased activity of the histone acetyltransferase, Mof (which targets H4K16), as well as decreased total histone acetyltransferase activity. In contrast to Bax gene, chromatin immunoprecipitation studies demonstrate marked enrichment of the Bcl-2 gene with H4K16Ac, and depletion with H4K20Me3, predicting active transcription of this gene in senescent HDFs. These data indicate that both global and locus-specific histone modifications of chromatin regulate altered Bcl-2:Bax gene expression in senescent fibroblasts, contributing to its apoptosis-resistant phenotype.
Keywords: Epigenetics, Histone modifications, Aging, Apoptosis, Fibrosis
Graphical abstract
Highlights
► Fibroblasts that undergo replicative senescence (Fb-S) are resistant to apoptosis. ► Fb-S display altered expression of Bcl-2 family genes (Bcl-2>Bax). ► Decreased Bax expression is related to global histone modifications in Fb-S. ► Increased Bcl-2 expression is associated with chromatin locus-specific alterations. ► This study elucidates epigenetic mechanisms underlying apoptosis resistance in Fb-S.
Introduction
Cellular senescence is defined as a state of irreversible growth arrest, which is protective against the development of cancer, but may also contribute to age-related diseases [1]. Senescence of cells may be triggered by a number of mechanisms, both intrinsic (e.g. replicative) and extrinsic (e.g. stress-induced). While senescence has classically been considered as tumor-suppressive mechanism that promotes apoptosis of epithelial cells, the activation of a senescence program in certain cell types, such as fibroblasts [2] and T-cells [3], may result in resistance to apoptosis. The accumulation of such cell types in mitotically active, post-natal tissues may contribute to the pathogenesis of certain age-related diseases, including fibrotic disorders [4,5].
Senescent fibroblasts have been reported to resist apoptosis by the up-regulation of a number of anti-apoptotic genes such as survivin [6], c-myb [7], major vault protein [8], and Bcl-2 [9,10]. However, whether epigenetic mechanisms control the expression of pro- and anti-apoptotic genes in senescent fibroblasts have not been defined. Cellular senescence, itself, is thought to have major epigenetic underpinnings [11]. It has become increasingly clear that chromatin-associated histone modifications are important determinants of gene expression profiles that define senescent phenotypes [12]. For example, histone deacetylase inhibitors have been shown to induce a senescence-like state [13], suggesting that alteration of histone acetylation is an important step leading to senescence.
In this study, we investigated the response of human diploid fibroblasts (HDFs) in low (non-senescent) and high (senescent) passage cells that undergo replicative senescence to oxidative stress (induced by exogenous hydrogen peroxide, H2O2). We assessed their apoptosis susceptibility and explored epigenetic mechanisms for the acquisition of an apoptosis-resistance in senescent HDFs. Our data indicate an imbalance of the constitutive expression Bcl-2 family proteins (Bcl-2 >Bax) that is related to both global and chromatin-specific histone modifications. Senescent HDFs are associated with globally increased repressive histone modification (H4K20Me3), which is also enriched in association with Bax; in contrast, although the active histone modification (H4K16Ac) is globally decreased in senescent HDFs, it is enriched in association with Bcl-2, consistent with a high Bcl-2:Bax ratio in senescent HDFs. This study provides novel epigenetic mechanisms that involve both global and chromatin-specific histone modifications that lead to an imbalance in the expression of Bcl-2 family proteins and apoptosis resistance in senescent fibroblasts.
Materials and methods
Cell Culture and H2O2 Exposures
Human diploid fibroblasts (HDFs; IMR-90 lung fibroblasts) were purchased from Coriell Institute for Medical Research (Camden, NJ) at the population doubling level (PDL) of 17. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS, Life Technologies) with 5% CO2 at 37 °C. Cells with PDL <30 were categorized as low PDL (LPDL; non-senescent), while PDL between 47 and 55 as high PDL (HPDL; senescent) [14]. PDL was calculated at each time of passage with formula, PDL=3.32(log (total viable cells at harvest/total viable cells at seed)). Cells were seeded in 100 mm dishes at a density of 2×106 cells/dish for 24 h, and then treated with 100 or 200 μM H2O2 for 2 hours; fresh cell culture medium was added and cells analyzed at 24 h, unless otherwise specified [15].
Annexin V-FITC Apoptosis Assay
HDFs were assayed for apoptosis using an AnnexinV-FITC apoptosis kit (MBLI, Woburn, WA), as previously described [16]. Briefly, after the cell treatment as above, at the end of 24 h incubation, the cells are collected, resuspended in 500 μl binding buffer from the kit. Annexin V-FITC and propidium iodide were added (5 μl of each), followed by incubabtion at room temperature in dark and quantification by flow cytometry.
Casapse-3 Activity Assay
Caspase-3 activity assays were measured using an established protocol and kit, according to manufacturer's instructions (MBLI, Woburn, WA). Briefly, the cells (1×106/ml) were induced apoptosis by H2O2 at 100 μM for 2 h, then incubated with cell culture medium for 24 h prior to adding chilled 50 μl of lysis buffer on ice for 10 min. Cell lysates were then centrifuged and the supernatant collected and protein concentrations measured; 50 μg of protein was added in 50 μl of cell lysis buffer, 5 μl of substrate were added and incubated at 37 °C for 60 min. Samples were read at 400-nm in a microtiter plate reader.
Senescence β-galactosidase Staining
Cellular senescence was assessed by using the Senescence β-galactosidase staining kit (Cell Signaling, Beverly, MA). Senescence-associated β-galactosidase (SA-β-gal)-positive cells were counted at five random locations and three times in per field using a Zeiss axiovert 200 M fluorescence/phase microscope (Carl Zeiss International, Germany).
Quantitative Real-Time RT-PCR
Total RNA was extracted by RNeasy kit (Qiagen, Valencia, CA) and transcribed to cDNA by reverse transcription using a cDNA synthesis kit (Clontech, Mountain View, CA). All real-time RT-PCR were performed in triplicate and normalized to 18S, as previously described [17]. Primers used are listed in Table 1.
Table 1.
Primer sequences for real-time PCR.
| Name | Sequence | |
|---|---|---|
| Bcl-2 (ENSG00000171791) | RT-PCR and | F: 5′-GAGTGGGATGCGGGAGATGTG-3′ |
| ChIP-PCR | R: 5′-CGGGATGCGGCTGTATGGG-3′ | |
| Bax (ENSG00000087088) | RT-PCR | F: 5′-TCAGGATGCGTCCACCAAGAA-3′ |
| R: 5′-TCTGCAGCTCCATGTTACTGTCCA-3′ | ||
| ChIP-PCR | F: 5′-GCACTTGCTAATTCCTTCTGCGCT-3′ | |
| R: 5′-ATGAGCATCTCCCGATAAGTGCCA-3′ | ||
| 18S | RT-PCR | F: 5′-GTCTGCCCTATCAACTTTCG-3′ |
| R:5′-ATGTGGTAGCCGTTTCTCA-3′ | ||
Antibodies and Immunoblotting
Antibodies against Bax (#5023), Bcl-2 (#2870), p21 (#2947), Caveolin-1 (Cav-1, #3238), β-tubulin (#2128) and Lamin B1 (#9087) were from Cell Signaling, Beverly, MA. Antibody p16 (ab51243) was from Abcam, Cambridge, MA. Anti-bodies to Mof (sc-271691) and Suv420h2 (sc-131078) were from Santa Cruz Biotech. Antibodies against H4K16Ac and H4K20Me3 and used for ChIP assays were from Active Motif, Carlsbad, CA.
Proteins were extracted by Allprep Kit (Qiagen), nuclear proteins were extracted by using the EpiQuick Nuclear extraction kit (Epigentek) as before [18]. Western blots were analyzed as previously described [19]. Normalization was ensured by stripping membranes after probing for the protein of interest, and then re-probing with β-tubulin as control. Total histone H4 or Lamin B1 were used as loading controls for nuclear extracts.
Chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation (ChIP) assays were performed as per manufacturer's protocol (Epigentek, Brooklyn, NY), with minor modifications [18]. ChIP-DNA was amplified by real-time PCR with primers as noted in Table 1. Results are normalized to input DNA.
Statistical Analysis
Data are expressed as mean±standard deviation (SD). Student's t test and one-way ANOVA statistical analyses were performed using SigmaStat 3.5. A p value of less than 0.05 was considered statistically significant.
RESULTS
Senescent HDFs are resistant to apoptosis induced by H2O2
Replication-induced senescence was studied in a cell culture model of human diploid fibroblasts (HDFs) that has been previously described [20,21], with minor modifications as described in “Materials and Methods”. The senescent phenotype of HDFs at high population doubling level (HPDL) was compared to low population doubling level (LPDL), using morphological and biochemical approaches. HPDL cells demonstrated larger cell size with flattened morphology and a number of filamentous cytoplasmic extensions in comparison to LPDL cells. HPDL-HDFs cells were also slower growing and a high percentage of cells stained positively for senescence-associated β-galactosidase (SA-β-gal) activity (Fig. 1A and B). The expression of tumor suppressor proteins associated with cellular senescence, p16, p21 and caveolin-1 (Cav-1), were increased in the HPDL-HDFs (Fig. 1C and D), confirming senescence-associated characteristics of these cells in contrast to LPDL-HDFs cells.
Fig. 1.
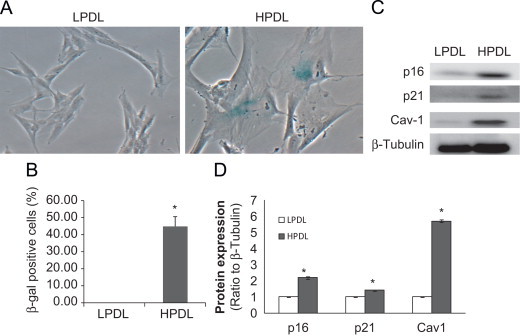
Morphology and expression of senescence markers in young and senescent cells.
LPDL or HPDL human diploid fibroblasts cells (HDFs) were seeded at an equal density (2×106 cells/100 mm dish) for 24 h. A, Cells are subjected to β-galactosidase (β-gal) staining, and representative phase-contrast images were photographed at 40x magnification. B, The number of β-gal positive cells is expressed as the percentage of total cells counted. Results are mean±SD of at least three independent experiments; ⁎p<0.001, HPDL vs. LPDL. C, Cell lysates in RIPA buffer were collected and subjected to SDS-PAGE and immunoblotted for p16, p21 and Cav-1, and β-tubulin. D, Densitometric analyses of the western blots in “C”. Results are mean±SD of at least three independent experiments; ⁎p<0.05, comparing HPDL vs. LPDL.
Oxidative stress has been associated with fibrotic diseases, including IPF [22,23]. We studied the apoptosis susceptibility of control and senescent HDFs in response to exogenous hydrogen peroxide (H2O2) at two different concentrations for 24 h, at which no cytotoxicity was observed (data not shown). Flow cytometry analyses with Annexin V-FITC demonstrated resistance to apoptosis in senescent HPDL-HDFs; while LPDL-HDFs (non-senescent) cells demonstrated significant induction of apoptosis (∼2-fold and 3-fold increase in the % of cells staining for Annexin V in response to 100 μM and 200 μM H2O2, respectively), there was no significant induction in response to low-dose H2O2, and only about 1.2-fold induction in response to the higher dose of H2O2 in HPDL-HDFs (Fig. 2A). We also assessed the activation of caspase-3 in control and senescent HDFs in response to H2O2. The activity of caspase-3 doubled in LPDL-HDFs, while there was no significant increase noted in senescent HPDL-HDFs in response to H2O2 (Fig. 2B). These data support the concept that senescent fibroblasts acquire an apoptosis-resistant phenotype.
Fig. 2.
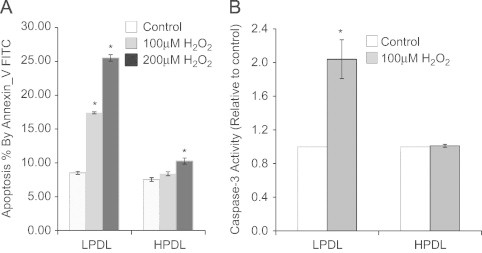
Differential responses of LPDL (non-senescent) and HPDL (senescent) HDFs to H2O2 induced apoptosis.
A, H2O2 at 100 μM or 200 μM was used to induce apoptosis in LPDL or HPDL. LPDL and HPDL fibroblasts were subjected to Annexin-V FITC assay 24 h after 2 h treatment of H2O2. Percentage (mean±SD) of fibroblasts apoptosis are measured by flow cytometry, results are average of at least three independent experiments. B, Caspase-3 activity assay. LPDL and HPDL HDFs were subjected to caspsae-3 activity assay 24 h after induced apoptosis with or without H2O2 at 100 μM for 2 h. Results are average± SD of caspse-3 activity of at least three independent experiments. ⁎p<0.05: 24 h after 2 h treatment of 100 μm or 200 μM H2O2vs. its own group untreated control.
Differential expression of apoptosis-regulating Bcl-2 family genes in senescent HDFs
Mechanisms for the acquisition of apoptosis resistance in senescent HDFs are incompletely understood [9,10]. We explored the potential differential expression and regulation of Bcl-2 family genes in the resistance of senescent HDFs to H2O2-induced apoptosis. The main location of pro-apoptotic gene Bax is in the cytoplasm [24]. In the cytoplasmic fraction, Bax levels were higher at baseline in LPDL-HDFs in comparison to HPDL-HDF; in response to H2O2, apoptosis-susceptible control LPDL cells further increased Bax expression, while senescent cells did not (Fig. 3A, B). We also examined the expression of Bax mRNA under the same conditions. The pattern for constitutive expression of Bax mRNA was similar to that observed for protein levels (Fig. 3C), supporting the likelihood that the major regulatory mechanism for this difference in Bax expression was at level of the gene expression. We did not detect a significant increase in Bax mRNA in response to H2O2 exposure, suggesting potential post-transcriptional mechanisms regulating H2O2-induced Bax protein expression in response to oxidative stress.
Fig. 3.
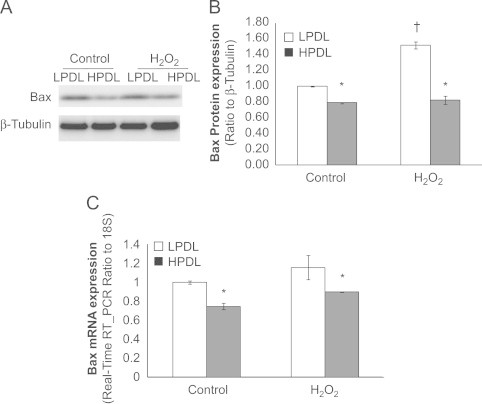
Differential expression of the pro-apoptotic Bax gene/protein in LPDL and HPDL HDFs.
Cells were treated with or without H2O2 at 100 μM for 2 h then changed into fresh full medium for 24 h before collecting the protein lysates for western blots or RNA for RT-PCR (see text for detailed information). A, Representative immunoblot of Bax protein expression in LPDL and HPDL. Protein lystates from cytoplasm of LPDL and HPDL cells are subjected to immunoblots. Cytoplasm β-tubulin was used as a loading control. B, Densitometric analyses of the western blots in “A”. Results are mean±SD of at least three independent experiments; ⁎p<0.05, comparing HPDL vs. LPDL in its own group, †p<0.05, H2O2 treated LPDL vs. control LPDL. C, Real-time RT-PCR of Bax. LPDL and HPDL fibroblasts mRNA levels were measured by real-time RT-PCR and normalized to 18S at baseline level or 24 h after 2 h 100 μM H2O2 treatment. Results are averages of at least three independent experiments. Bar graphs indicate mean±SD. ⁎p <0.05, HPDL vs. LPDL within each group.
In contrast to Bax, the anti-apoptotic Bcl-2 protein was found to be increased at baseline (control) in senescent vs. non-senescent control LPDL-HDFs, without significant changes in response to exogenous H2O2 at 24 h (Fig. 4A, B). Additionally, we detected a further increase in Bcl-2 mRNA expression in HPDL in response of H2O2 by real-time RT-PCR (Fig. 4C), suggesting an earlier transcriptional response (relative to protein expression) or independent post-transcriptional effects of H2O2 on protein expression. Together, these data demonstrate both constitutive and H2O2-inducible changes in Bax and Bcl-2 expression that support an apoptosis-resistant phenotype of senescent HDFs.
Fig. 4.
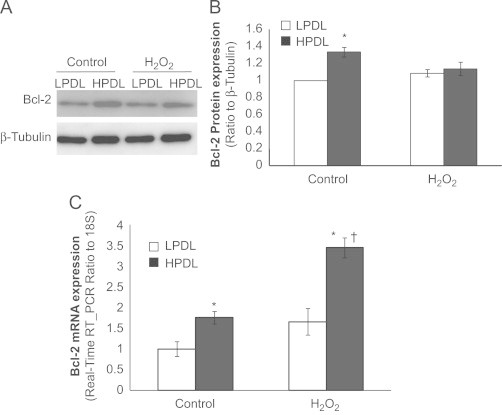
Differential expression of anti-apoptotic gene Bcl-2 gene/protein in LPDL and HPDL HDFs.
Cells were treated with or without H2O2 at 100 μM for 2 h then changed into fresh full medium for 24 h before collecting the protein lysates for western blots or RNA for RT-PCR (see text for detailed information). A, Representative immunoblot of Bcl-2 protein expression in LPDL and HPDL. Whole cell lystates of LPDL and HPDL cells are subjected to immunoblots. β-tubulin was used as a loading control. B, Densitometric analyses of the western blots in “A” and normalized to β-tubulin. Results are averages of at least three independent experiments, bars indicate the mean±SD. C, Real-time RT-PCR of Bcl-2. LPDL and HPDL fibroblasts mRNA levels were measured by real-time RT-PCR and normalized to 18S at baseline level or 24 h after 2 h 100 μM H2O2 treatment. Results are averages of at least three independent experiments, bars indicate mean±SD. ⁎p <0.05, HPDL vs. LPDL within each group; †p<0.05, H2O2 treated HPDL vs. control HPDL.
Differential regulation of histone modifications in senescent HDFs
We hypothesized that epigenetic mechanisms involving histone modifications account for the observed baseline expression and/or inducible regulation of Bcl-2 family genes in senescent HDFs. Changes in chromatin structure have been implicated in mechanisms of cellular senescence and aging [11]. In particular, histone H4 lysine 20 trimethylation (a repressive histone mark) and histone H4 lysine 16 acetylation (an active histone mark) are associated with aging [11,25,26]. First, we examined the global expression of these histone modifications. Expression of H4K20Me3 was increased, while that of H4K16Ac was markedly reduced in senescent HDFs when compared to the young HDFs (Fig. 5). Exogenous H2O2 treatment for 24 h decreased the expression of both histone marks, which was greater for the H4K16Ac mark; however, the magnitude (and direction) of change was not different comparing non-senescent to senescent cells (Fig. 5).
Fig. 5.
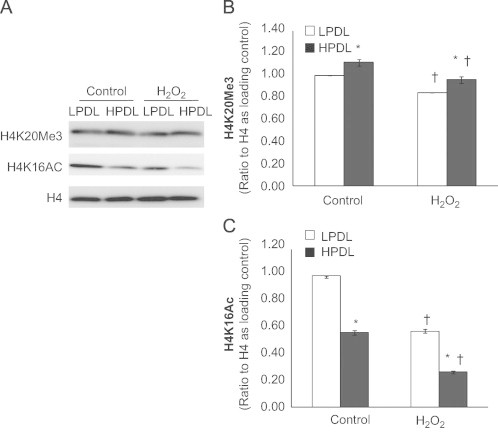
Expression of the histone modification markers, H4K20Me3 and H4K16Ac, in LPDL and HPDL HDFs.
Cells were treated with or without H2O2 at 100 μM for 2 h then changed into fresh full medium for 24 h before collecting nuclear extracts for western blots (see text for detailed information). A, Representative immunoblot of H4K20Me3 and H4K16Ac in LPDL and HPDL. Total H4 was used as loading control. B and C, Densitometric analyses of the western blots in “A”, H4K20Me3 (B) or H4K16Ac (C) after normalization to H4. Results are averages of at least three independent experiments, bar graphs indicate mean±SD; ⁎p <0.05, HPDL vs. LPDL within each group; †p<0.05, H2O2 treated LPDL or HPDL vs. untreated LPDL or HPDL.
Next, we assessed if the expression of histone-associated enzymes correlate with these modifications. Suv4–20h2 is a histone methyltransferase that has been reported to mediate methylation of H4K20 [27]. In nuclear extracts, expression of this enzyme was constitutively higher in senescent cells (Fig. 6A, B), while the response to H2O2, LPDL was not significantly changed, while HPDL was decreased. This increased histone methyltransferase expression corresponds with the constitutively increased trimethylation of H4K20 in senescent HDFs.
Fig. 6.
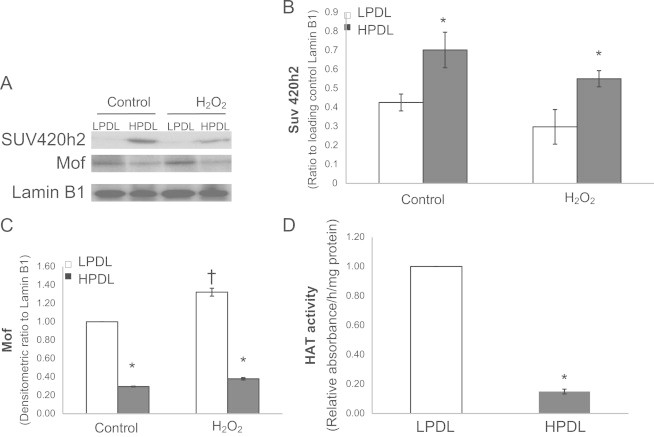
Expression/activity of related histone-modifying enzymes in LPDL and HPDL HDFs.
LPDL or HPDL were treated with or without (control) H2O2 at 100 μM for 2 h then changed into fresh full medium for 24 h before collecting the nuclear extracts for western blots (see text for detailed information). A, Representative immunoblot of histone methyltransferase Suv4–20h2 and histone acetyltransferases Mof. Lamin B was used as a loading control. B and C, densitometric analyses of the western blots in “A”, Suv420h2 (B) or Mof (C), normalized to Lamin B1. D, Decreased HAT activity in HPDL. Nuclear extracts from untreated (baseline) LPDL and HPDL fibroblasts were subjected to HAT activity assay. All results are averages of at least three independent experiments, histogram indicated the mean±SD; ⁎p <0.05, HPDL vs. LPDL within each group; †p<0.05, H2O2 treated LPDL or HPDL vs. control LPDL or HPDL.
Histone acetylation is controlled by the acetylation activity of histone acetyltransferases (HATs) and deacetylation by histone deacetylases (HDACs) [19]. Mof (males absent on the first), also called MYST1, is an H4K16 acetyltransferase [28]. The expression of Mof was found to be lower in HPDL than in LPDL; with H2O2 exposure, Mof expression is slightly increased in in LPDL, while it is not changed in HPDL-HDFs (Fig. 6A and C). We also measured total HAT activity in these cells at baseline condition; senescent HDFs manifest constitutively lower total HAT activity when compared to control HDFs (Fig. 6D).
In addition to HATS, the acetylation of histones is regulated by HDACs. There are several HDACs that are reported to be involved in the deacetylatione of H4K16Ac, including class III HDAC sirutin 1 [29] and other classes of HDACs [30]; however, a specific HDAC that controls H4K16Ac has not been described. Nevertheless, our finding of decreased Mof expression in HPDL is consistent with the observed constitutive decrease in H4K16 acetylation in senescent HDFs.
Histone modifications associated with differential expression of Bax and Bcl-2 in senescent cells
Finally, we determined the relationships between the observed differential regulation of Bax and Bcl-2 genes and their association with H4K16 acetylation and H4K20 trimethylation in non-senescent control and senescent cells. Using ChIP assays, the repressive histone mark, H4K20Me3 associated with closed chromatin structure, is enriched with Bax in senescent cells; in contrast, it is significantly depleted in association with the Bcl-2 gene in the same senescent cells (Fig. 7A). In contrast, we observed the reverse pattern in association with H4K16Ac, demonstrating marked enrichment with the Bcl-2 gene of this “active” histone mark in senescent HDFs (Fig. 7B). Thus, although the H4K16Ac histone mark is globally reduced in senescent cells (Fig. 5A), this finding predicts increased transcriptional activation of Bcl-2. Together, these results suggest that histone marks associated with open chromatin structure is associated with the transcriptionally active anti-apoptosis gene, Bcl-2, in senescent HDFs, which is constitutively expressed at a high level in senescent HDFs; while the pro-apoptosis gene Bax is associated with a marker of closed chromatin structure that predicts decreased transcriptional activity in senescent HDFs.
Fig. 7.
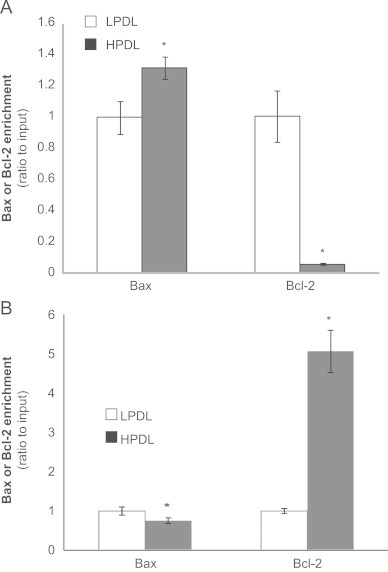
Histone modifications associated with Bax and Bcl-2 by chromatin immunoprecipitation (ChIP) assays.
A, H4K20Me3 (repressive mark), and B, H4K16Ac (active mark), histone modifications associated with the Bax or Bcl-2 gene. The quantitative ChIP assays were performed to analyze the association of Bax and Bcl-2 with the histone modifications, H4K20Me3 or H4K16Ac. Bars represent the relative levels of PCR product of the Bax or Bcl-2 region associated with this histone modification state at baseline (untreated). DNA cross-linked with the immunoprecipitated proteins using with specific antibodies were used in the PCR reaction. Quantitative PCR data were analyzed using the 2−ΔΔCt method, and results normalized to input DNA were expressed as fold changes relative to untreated LPDL cells. Bar graphs represent mean±SD from the average of at least three independent experiments; ⁎p<0.05 HPDL vs. LPDL.
Discussion
Cellular senescence represents a stress and tumor suppressive response [31]. Senescent cells may accumulate in aging tissues, contributing to diseases of aging if not to the intrinsic aging process itself [32,33]. Fibrosis and cancer represent diseases in which senescent fibroblasts within the tissue microenvironment acquire resistance to apoptosis [5,34,35], although mechanisms that link senescence to apoptosis resistance are not well defined. In this study, we established a cell culture model of replicative senescence that recapitulates the apoptosis-resistant phenotype of senescent fibroblasts, as reported by others [6–10,36]. We demonstrate altered constitutive expression of the Bcl-2 family genes, Bcl-2 and Bax, favoring an apoptosis-resistant phenotype. This pattern of gene expression is explained by histone modifications that are globally associated with replicative senescence (reduced transcription of Bax), and by gene loci-specific chromatin remodeling that predicts increased transcription of Bcl-2. This is the first study to elucidate epigenetic mechanisms for senescence-associated apoptosis resistance in lung fibroblasts.
With increasing evidence showing that epigenetic mechanisms are key contributors to the aging and age-related diseases [37], we explored the epigenetic basis for altered phenotype gene expression patterns in senescent cells. Chromatin structure is important in regulating gene expression; it is under dynamic changes throughout life span [11]. One major mechanism for epigenetic regulation is histone modification. Methylation and acetylation of lysine residues on the tail of nucleosome core histones has a critical role in regulating gene expression [38]. Histone 4 lysine 20 trimethylation has been reported to increase with advancing age [25,39]; in contrast, it is commonly reduced in cancer cells [39,40]. We observed increased H4K20Me3 in senescent HDFs compared to non-senescent HDFs. The mechanisms for constitutively increased H4K20Me3 with aging are not fully understood. The methyltransferase, Suv4–20h2, has been reported to be relatively specific for histone H4 lysine 20 methylation [27]. Studies in cancer cells indicate loss of expression of this enzyme contributes to reduced H4K20 trimethylation [38]. In our studies of senescent HDFs, we detected higher expression of Suv4–20h2 that correlates with increased trimethylation of H4K20. However, steady-state levels of methylation are dependent on the relative activities of methyltransferases and demethylases. We are not aware of specific H4K20 trimethylation demethylase at this time [41]. Further studies on the activities of these regulatory enzymes with loss/gain of function approaches will provide additional insights into mechanisms that determine the status of H4K20 trimethylation.
Other histone modifications may also be relevant to the process of aging, including acetylation of histone. Reduced H4K16 acetylation is strongly correlated with cellular senescence [42]. Our data demonstrates decreased levels of H4K16 acetylation in senescent HDFs. This observation is consistent with studies in mice that show hypoacetylation of H4K16 with increased age [42]. There may be some species-dependent differences, as increased acetylation levels of H4K16, which depends on SIRT2, has been reported in yeast [26]; thus, this highlights important differences between yeast and mammalian aging. Histone acetylation is associated with active transcription; it is balanced by the activities of HATs and HDACs [38]. A number of families of HATs and HDACs have been identified [43]. The roles of specific enzymes that control acetylation of H4K16 are not well defined. The histone acetyltransferase, MOF, has been reported to be responsible for the acetylation status of H4K16 [44]. In our studies, we demonstrated the lower expression of Mof in senescent cells when compared to the non-senescent cells, we also examined total HAT activity in senescent vs. non-senescent cells, and detected significantly lower total histone acetyltransferases activity in senescent HDFs. However, with exposure to H2O2, increased Mof expression was noticed in LPDL HDFs (Fig. 6), but the H4K16Ac is lower (Fig. 5). Though low Mof and total HAT activity may account for the observed reduced acetylation of H4K16 in senescent cells, the status of acetylated H4K16 may also related to higher activity of HDACs. There are four classes and total about 18 different HDACs [45]. A few HDACs reported involved in the deacetylation activity of H4K16 [29,30], but none specific HDACs have been clarified to have a major role. Besides other HDACs, the class III HDAC, SIRT1, [29], has been reported to participate in the deacetylation of H4K16 [46]. We noticed increased SIRT1 expression in the nuclear extract of senescent cells, while in the whole cell lysates, its expression is decreased in senescent cells (data not shown), which indicating nucleocytoplasmic shuttling [47,48] and increased activation in senescent cells. Since SIRT1 has a very complex and controversial role in aging [49,50], also it deacetylates several transcription factors [47,48] in addition to histones, thus the role of SIRT1 did not explore further in this study.
Histone acetylation usually enhances gene transcription, while histone methylation may determine transcriptional repression or activation, depending on the particular lysine residue that is methylated. H4K20Me3 is associated with transcriptional repression [40], and H4K16 acetylation with active transcription [42]. We examined if the baseline difference in gene expression of the apoptosis-regulating genes, Bcl-2 and Bax, may be determined by the status of trimethylation and acetylation of H4K20 and H4K16, respectively. Despite the finding that senescent HDFs are globally reduced in H4K16 acetylation, which predicts overall decreased gene expression, ChIP analysis demonstrated that the anti-apoptotic gene, Bcl-2, is significantly enriched in association with this active mark, corresponding with its high expression in senescent cells. In contrast, the association of the pro-apoptotic gene, Bax, with H4K16Ac (active mark) is reduced, while it is enriched for the repressive mark, H4K20Me3; this corresponds with its lower expression in senescent HDFs. These studies provide the first evidence for epigenetic mechanisms for the dysregulated expression of apoptosis-regulating genes and apoptosis resistance in senescent HDFs.
Histone modification accounts for only one component of the “aging epigenome”. Other layers of epigenetic regulation, such as DNA methylation and microRNAs, may also contribute to mechanisms of altered gene expression in aging and age-related diseases. Cells may acquire a combination of these epigenetic modifications during cellular senescence and aging, which may affect the expression of specific genes that result in different aging phenotypes. Although we do not fully understand the environmental factors that influence this process, epigenetic modifications are more reversible than genetic alterations. Thus, interventions aimed to reverse epigenetic alterations may have greater potential to treat age-related diseases, including fibrosis and cancer.
Author Contributions
Experimental design: YYS and VJT; Performance of procedures: HL and XZ; data analysis and interpretation: YYS, HL, LH, KB, LD, GL and VJT; Manuscript preparation: YYS and VJT.
Footnotes
Supported by grants from ATS Foundation/Coalition for Pulmonary Fibrosis and the Pulmonary Fibrosis Foundation Research Program 2011 (YYS), American Heart Association grant 09SDG2260095 (YYS); and NIH grant R01HL067967 (VJT).
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Collado M., Blasco M.A., Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 2.Kim S.Y., Ryu S.J., Kang H.T., Choi H.R., Park S.C. Defective nuclear translocation of stress-activated signaling in senescent diploid human fibroblasts: a possible explanation for aging-associated apoptosis resistance. Apoptosis: an international journal on programmed cell death. 2011;16(8):795–807. doi: 10.1007/s10495-011-0612-2. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 3.Monti D., Salvioli S., Capri M., Malorni W., Straface E., Cossarizza A. Decreased susceptibility to oxidative stress-induced apoptosis of peripheral blood mononuclear cells from healthy elderly and centenarians. Mechanisms of ageing and development. 2000;121(1–3):239–250. doi: 10.1016/s0047-6374(00)00220-7. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 4.Raghu G., Weycker D., Edelsberg J., Bradford W.Z., Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 5.Beltrami A.P., Cesselli D., Beltrami C.A. Stem cell senescence and regenerative paradigms. Clinical pharmacology and therapeutics. [Review] 2012;91(1):21–29. doi: 10.1038/clpt.2011.262. Jan. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khalaf HH, Aboussekhra A. Survivin expression increases during aging and enhances the resistance of aged human fibroblasts to genotoxic stress. Age. 2012 Jan 15. [DOI] [PMC free article] [PubMed]
- 7.Lee Y.H., Lee N.H., Bhattarai G., Hwang P.H., Kim T.I., Jhee E.C. c-myb has a character of oxidative stress resistance in aged human diploid fibroblasts: regulates SAPK/JNK and Hsp60 pathway consequently. Biogerontology. 2010;11(3):267–274. doi: 10.1007/s10522-009-9244-0. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 8.Ryu S.J., An H.J., Oh Y.S., Choi H.R., Ha M.K., Park S.C. On the role of major vault protein in the resistance of senescent human diploid fibroblasts to apoptosis. Cell death and differentiation. 2008;15(11):1673–1680. doi: 10.1038/cdd.2008.96. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 9.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer research. 1995;55(11):2284–2292. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 10.Ryu S.J., Oh Y.S., Park S.C. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell death and differentiation. 2007;14(5):1020–1028. doi: 10.1038/sj.cdd.4402091. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 11.Fraga M.F., Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23(8):413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Dimauro T., David G. Chromatin modifications: the driving force of senescence and aging? Aging (Albany NY) 2009 Feb;1(2):182–190. doi: 10.18632/aging.100023. [Research Support, N.I.H., ExtramuralReview] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogryzko V.V., Hirai T.H., Russanova V.R., Barbie D.A., Howard B.H. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Molecular and cellular biology. 1996;16(9):5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E.K., Kang J.Y., Rho Y.H., Kim Y.S., Kim D.S., Bae Y.S. Silencing of the CKII alpha and CKII alpha' genes during cellular senescence is mediated by DNA methylation. Gene. 2009 Feb 15;431(1–2):55–60. doi: 10.1016/j.gene.2008.10.020. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 15.Chen Q.M., Liu J., Merrett J.B. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. The Biochemical journal. 2000;347(Pt 2):543–551. doi: 10.1042/0264-6021:3470543. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders Y.Y., Kumbla P., Hagood J.S. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. American journal of respiratory cell and molecular biology. 2007;36(2):226–235. doi: 10.1165/rcmb.2006-0178OC. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders Y.Y., Pardo A., Selman M., Nuovo G.J., Tollefsbol T.O., Siegal G.P. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2008;39(5):610–618. doi: 10.1165/rcmb.2007-0322OC. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders, Y. Y.; Tollefsbol, T. O.; Varisco, B. M.; Hagood, J. S. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. American journal of respiratory cell and molecular biology45(1):16–23; 2011. [DOI] [PMC free article] [PubMed]
- 19.Sanders, Y. Y.; Ambalavanan, N.; Halloran, B.; Zhang X.; Liu, H.; Crossman, D. K.; Bray, M.; Zhang, K.; Thannickal, V. J.; Hagood, J. S. HYPERLINK “/pubmed/22700861”Altered DNA methylation profile in idiopathic pulmonary fibrosis American journal of respiratory and critical care medicine 2012 Sep 15;186(6):525–35. 10.1164/rccm.201201-0077OC. Epub 2012 Jun 14 PMID:22700861. [DOI] [PMC free article] [PubMed]
- 20.Nichols W.W., Murphy D.G., Cristofalo V.J., Toji L.H., Greene A.E., Dwight S.A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977;196(4285):60–63. doi: 10.1126/science.841339. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 21.Sherwood SW, Rush D, Ellsworth JL, Schimke RT. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proceedings of the National Academy of Sciences of the United States of America. [Research Support, U.S. Gov't, P.H.S.]. 1988 Dec;85(23):9086-90. [DOI] [PMC free article] [PubMed]
- 22.Kinnula V.L., Fattman C.L., Tan R.J., Oury T.D. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. American journal of respiratory and critical care medicine. 2005;172(4):417–422. doi: 10.1164/rccm.200501-017PP. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(7):854–856. doi: 10.1096/fj.04-2882fje. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 24.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proceedings of the National Academy of Sciences of the United States of America. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. 1997 Apr 15;94(8):3668-72. [DOI] [PMC free article] [PubMed]
- 25.Sarg B., Koutzamani E., Helliger W., Rundquist I., Lindner H.H. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J Biol Chem. 2002;277(42):39195–39201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- 26.Dang W., Steffen K.K., Perry R., Dorsey J.A., Johnson F.B., Shilatifard A. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459(7248):802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang L.W., Hu N., Underhill D.A. Comparative analyses of SUV420H1 isoforms and SUV420H2 reveal differences in their cellular localization and effects on myogenic differentiation. PloS one. 2010;5(12):e14447. doi: 10.1371/journal.pone.0014447. [Comparative Study Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma G.G., So S., Gupta A., Kumar R., Cayrou C., Avvakumov N. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol. 2010;30(14):3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaquero A., Sternglanz R., Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26(37):5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- 30.Miller K.M., Tjeertes J.V., Coates J., Legube G., Polo S.E., Britton S. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17(99):1144–1151. doi: 10.1038/nsmb.1899. [10.1038/nsmb.1899] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature reviews Molecular cell biology. 2007;8(9):729–740. doi: 10.1038/nrm2233. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 32.Erusalimsky J.D., Kurz D.J. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Experimental gerontology. 2005;40(8–9):634–642. doi: 10.1016/j.exger.2005.04.010. [Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 33.Price J.S., Waters J.G., Darrah C., Pennington C., Edwards D.R., Donell S.T. The role of chondrocyte senescence in osteoarthritis. Aging cell. 2002;1(1):57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 34.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proceedings of the American Thoracic Society. [Research Support, N.I.H., Extramural Review]. 2006 Jun;3(4):350-6. [DOI] [PMC free article] [PubMed]
- 35.Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Experimental gerontology. 2003;38(1–2):5–11. doi: 10.1016/s0531-5565(02)00152-3. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 36.Cristofalo V.J., Pignolo R.J. Replicative senescence of human fibroblast-like cells in culture. Physiological reviews [Review] 1993 Jul;73(3):617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalo S. Epigenetic alterations in aging. Journal of applied physiology. [Review] 2010;109(2):586–597. doi: 10.1152/japplphysiol.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 39.Biron V.L., McManus K.J., Hu N., Hendzel M.J., Underhill D.A. Distinct dynamics and distribution of histone methyl-lysine derivatives in mouse development. Developmental biology. 2004;276(2):337–351. doi: 10.1016/j.ydbio.2004.08.038. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 40.Fraga M.F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nature genetics. 2005;37(4):391–400. doi: 10.1038/ng1531. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 41.Nottke A., Colaiacovo M.P., Shi Y. Developmental roles of the histone lysine demethylases. Development [Review] 2009;136(6):879–889. doi: 10.1242/dev.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X., et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. [Research Support, Non-U.S. Gov't]. 2011 Jul 26;108(30):12325-30. [DOI] [PMC free article] [PubMed]
- 43.Clayton A.L., Hazzalin C.A., Mahadevan L.C. Enhanced histone acetylation and transcription: a dynamic perspective. Molecular cell. 2006;23(3):289–296. doi: 10.1016/j.molcel.2006.06.017. [Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 44.Taipale M., Rea S., Richter K., Vilar A., Lichter P., Imhof A. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Molecular and cellular biology. 2005;25(15):6798–6810. doi: 10.1128/MCB.25.15.6798-6810.2005. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witt O., Deubzer H.E., Milde T., Oehme I. HDAC family: What are the cancer relevant targets? Cancer letters. 2009;277(1):8–21. doi: 10.1016/j.canlet.2008.08.016. [Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 46.Hajji N., Wallenborg K., Vlachos P., Fullgrabe J., Hermanson O., Joseph B. Opposing effects of hMOF and SIRT1 on H4K16 acetylation and the sensitivity to the topoisomerase II inhibitor etoposide. Oncogene. 2010;29(15):2192–2204. doi: 10.1038/onc.2009.505. [DOI] [PubMed] [Google Scholar]
- 47.Luo J., Nikolaev A.Y., Imai S., Chen D., Su F., Shiloh A. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/s0092-8674(01)00524-4. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 48.Langley E., Pearson M., Faretta M., Bauer U.M., Frye R.A., Minucci S. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. The EMBO journal. 2002;21(10):2383–2396. doi: 10.1093/emboj/21.10.2383. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dang W. The controversial world of sirtuins. Drug Discovery Today: Technologies. 2012;(0) doi: 10.1016/j.ddtec.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng C.X. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5(2):147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]


