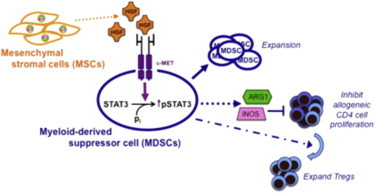
Summary
Mesenchymal stromal cells (MSCs) are multilineage progenitors with immunomodulatory properties, including expansion of immunomodulatory leukocytes such as regulatory T lymphocytes (Tregs) and tolerogenic dendritic cells. We report that human MSCs can expand CD14−CD11b+CD33+ human myeloid-derived suppressor cells (MDSCs). MSC-expanded MDSCs suppress allogeneic lymphocyte proliferation, express arginase-1 and inducible nitric oxide synthase, and increase the number of Tregs. This expansion occurs through the secretion of hepatocyte growth factor (HGF), with effects replicated by adding HGF singly and abrogated by HGF knockdown in MSCs. In wild-type mice, the liver, which secretes high levels of HGF, contains high numbers of Gr-1+CD11b+ MDSCs, and injection of HGF into mice significantly increases the number of MDSCs. Expansion of MDSCs by MSC-secreted HGF involves c-Met (its receptor) and downstream phosphorylation of STAT3, a key factor in MDSC expansion. Our data further support the strong immunomodulatory nature of MSCs and demonstrate the role of HGF, a mitogenic molecule, in the expansion of MDSCs.
Graphical Abstract
Highlights
-
•
MSCs expand myeloid-derived suppressor cells (MDSCs)
-
•
MSC-expanded MDSCs are functionally suppressive toward allogeneic lymphocytes
-
•
MSCs expand MDSC numbers through a secreted factor, hepatocyte growth factor (HGF)
-
•
MSC-secreted HGF expands MDSCs through c-Met (its receptor) and STAT3 phosphorylation
Introduction
Multipotent mesenchymal stromal cells (MSCs) are a population of multilineage progenitor cells that were first isolated from the bone marrow (Friedenstein, 1976; Pittenger et al., 1999). These somatic progenitor cells harbor the capacity to differentiate into adipocytes, osteoblasts, and chondrocytes, as well as a number of extramesodermal lineages (Prockop, 1997). Recent studies have demonstrated that MSCs exert strong immunomodulatory effects on multiple populations of leukocytes via various mechanisms, including suppression of CD4 and CD8 lymphocyte proliferation and responses, induction of T regulatory lymphocytes (Tregs; a population of immunomodulatory T cells), and secretion of immunosuppressive molecules such as transforming growth factor-β (TGF-β) and indoleamine-2,3-dioxygenase (IDO) (Uccelli et al., 2008). MSCs also strongly suppress natural killer lymphocyte cytotoxicity and affect dendritic cell (DC) maturation, e.g., by inhibiting the differentiation of monocytes to immature myeloid DCs and decreasing the effector functions of plasmacytoid DCs (Le Blanc and Mougiakakos, 2012; Uccelli et al., 2008). Many of these components are similar to the immunomodulatory armamentarium of the immune system, which is important for preventing autoimmunity and establishing tolerance (Guleria and Sayegh, 2007; Wing and Sakaguchi, 2010), with mechanisms ranging from anti-inflammatory molecules such as TGF-β, IDO, and interleukin-10 (IL-10) to leukocyte subpopulations such as Tregs and tolerogenic DCs (Mellor and Munn, 2004; Sakaguchi et al., 2006; Swiecki and Colonna, 2010).
As with many biological phenomena, immunomodulation is a double-edged sword, and many of these tolerogenic mechanisms appear to be manipulated by cancer cells to create an immunoprivileged niche to further their own growth (Rabinovich et al., 2007). One of the most prominent immunomodulatory leukocyte subpopulations in cancer consists of myeloid-derived suppressor cells (MDSCs) (Ostrand-Rosenberg and Sinha, 2009). Derived from myeloid precursors, MDSCs suppress immune response by a number of mechanisms, such as suppressing cytotoxic lymphocyte effector functions and targeting T cells by expressing the enzymes arginase 1 (ARG1) and inducible nitric oxide synthase (iNOS), both of which block the production of the T cell CD3-ζ chain by metabolizing L-arginine (Gabrilovich and Nagaraj, 2009; Gabrilovich et al., 2012). Human and mouse studies have revealed that chronic inflammation and proinflammatory mediators such granulocyte macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-6, and prostaglandin E2 (PGE2) are involved in the induction of these suppressor leukocytes (Bunt et al., 2007; Serafini et al., 2004; Sinha et al., 2007; Young and Wright, 1992). Although it is clear that the tumor microenvironment is maintained by diverse cell types, the role of secreted factors other than cytokines and proinflammatory factors in the expansion of MDSCs has largely been unexplored, with the exception of vascular endothelial growth factor (Fricke et al., 2007; Shojaei et al., 2007). We report that MDSCs can be expanded by MSC-secreted hepatocyte growth factor (HGF), a potent mitogenic growth factor.
Results
MSCs Can Expand High Numbers of Functional CD14−CD11b+CD33+ MDSCs from Peripheral Blood Leukocytes
We hypothesized that the strong immunosuppressive properties of diverse sources of MSCs extend to involve the expansion of MDSCs. We first isolated and cultured MSCs from placenta and bone marrow, and then characterized the cells for surface marker expression and multilineage differentiation potential. Both bone marrow and placental MSCs are positive for surface expression of CD73, CD105, and CD90, but negative for hematopoietic markers such as the costimulatory molecules CD80 and 86 (Figure 1A; Chang et al., 2006; Uccelli et al., 2008; Yen et al., 2005). Both populations of MSCs can differentiate into osteoblastic, chondrogenic, and adipocytic lineages, and thus meet the criteria for multipotent MSCs (Figure 1B; Dominici et al., 2006; Liu et al., 2011; Pittenger et al., 1999).
Figure 1.
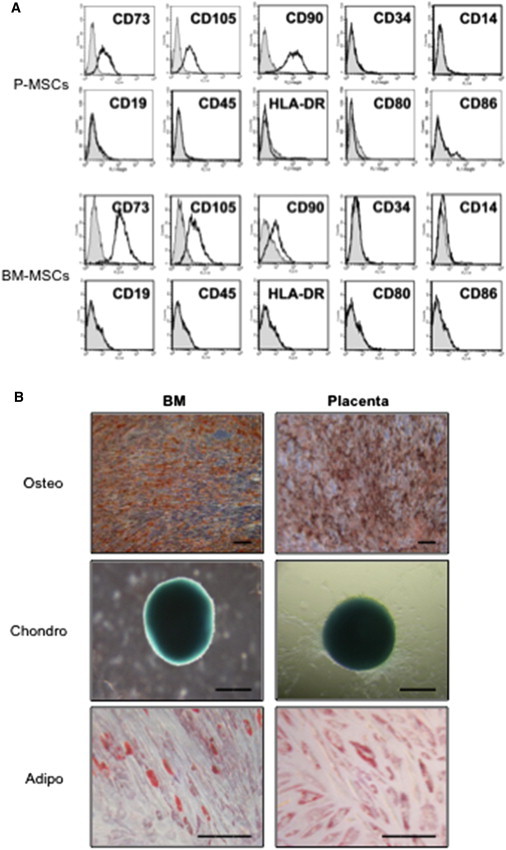
Characterization of Bone Marrow and Placental Multipotent MSCs
(A and B) Surface marker profile (A) and trilineage differentiation phenotype (B) for bone marrow (BM) multipotent MSCs and placental MSCs (P-MSCs). Adipo, adipogenic lineage (stained with oil red O to assess for oil droplet formation); chondro, chondrogenic lineage (stained with Alcian blue to assess for the presence of glucosaminoglycans); osteo, osteogenic lineage (stained with alizarin red to assess calcium deposition). Scale bar: 200 μm.
To test whether MSCs can expand MDSCs, we cocultured MSCs with human peripheral blood leukocytes (PBLs) and assayed for MDSCs, which in the human system are most commonly characterized as CD14−CD11b+CD33+ leukocytes with a strong suppressor function (Ostrand-Rosenberg and Sinha, 2009; Poschke and Kiessling, 2012). We found that both bone marrow and placental MSCs could increase the number of CD14−CD11b+CD33+ cells from PBLs (Figure 2A). Both cell percentage and numbers were increased significantly after PBL coculture with MSCs, with CD11b+ cells showing an increase from a baseline of 8.9% ± 0.6% (10,179 ± 926 cells; averages ± SEM) to 19.2% ± 1.2% (19,508 ± 1,258 cells) after coculture with MSCs, and CD14−CD11b+CD33+ cells increasing from 1.3% ± 0.1% (1,052 ± 90 cells) at baseline to 2.4% ± 0.2% (2,343 ± 147 cells) after coculture with MSCs (Figure 2B). To assess whether these MSC-expanded MDSCs were functional, we sorted MSC-induced CD14−CD11b+CD33+ cells and cocultured these cells with allogeneic proliferating lymphocytes to assess for suppressive capacity. We found that the MSC-expanded CD14−CD11b+CD33+ cells could suppress lymphocyte proliferation in a dose-dependent manner (Figure 2C). Human MDSCs are known to express ARG1 and iNOS (Ochoa et al., 2007; Rodríguez and Ochoa, 2008), and we found that MSC-induced MDSCs also expressed these enzymes and at significantly higher cell numbers compared with baseline (Figures 2D and 2E, respectively). MDSCs have also been shown to express IL-10 and TGFβ in some reports (Poschke and Kiessling, 2012), but we did not find any expression of these molecules at baseline or after MSC coculture (Figure S1A available online). CD14−CD11b+CD33+ MDSCs have been reported to induce Tregs (Dugast et al., 2008; Huang et al., 2006), and we also found that MSC-induced CD14−CD11b+CD33+ cells were able to induce high numbers of CD4+CD25highCD127low Tregs from stimulated PBL (Figures 2F and S1B). Thus, MSC-expanded CD14−CD11b+CD33+ cells have multiple immunomodulatory functions.
Figure 2.

Human MSCs Expand the Number of Functional CD14−CD11b+CD33+ MDSCs in Allogeneic PBLs
(A) Expansion of CD14−CD11b+CD33+ cells from PBLs by MSCs. Allogeneic PBLs (40 donors) were cocultured alone (top panel) or with MSCs (lower panel; three donors of BM-MSCs and four donors of P-MSCs; representative dot plots/ histogram shown). PBLs were gated on forward scatter (FSC)/side scatter (SSC) (R1, 100,000 events for all analyses), analyzed for CD14− and CD11b+ (R2), and further analyzed for CD33+ (R3).
(B) Quantification of percentage (left-side charts) and cell number (right-side charts) of PBL CD11b+ (upper graphs) or CD14−CD11b+CD33+ MDSCs (lower graphs) before and after coculture with MSCs. Averages ± SEM; 57 independent experiments; ∗∗∗p < 0.001 compared with PBL only.
(C) Suppressive function of MSC-induced MDSCs. Allogeneic PBLs (T, target cells; eight donors) were stained with CFSE for assessment of cell division and stimulated with anti-CD3/28 (α-CD3/28) without or with the addition of MSC-expanded MDSCs (from three donors) at various E:T ratios. Flow-cytometric analysis was performed to assess PBL cell proliferation/division as evidenced by decreasing CFSE staining. The chart on the right is a quantitative summary of experimental results; x axis: ratio of MSC-expanded MDSCs to activated PBL; y axis: percentage suppression of PBL proliferation by MSC-expanded MDSCs; eight independent experiments; ∗p < 0.05 for trend. E, effector cells.
(D and E) Expression of iNOS (D) and ARG1 (E) by MSC-expanded MDSCs (five donors of MSCs; representative histograms shown). Negatively selected CD14− cells from PBLs (eight donors) were cultured alone (top panels) or with MSCs (lower panels). CD14− cells were gated on FSC/SSC (R1), analyzed for CD11b+ and CD33+ (R2), and further analyzed for either (D) iNOS or (E) ARG1 (shaded gray areas in histogram; unfilled black line: isotype control). Average cell numbers ± SEM are indicated in the upper-right corner of the histogram; ∗p < 0.05 comparing CD14− + MSCs with CD14− only.
(F) Expansion of CD4+CD25high Tregs by MSC-expanded MDSCs. CD14−CD11b+CD33+ MSC-expanded MDSCs (three donors) were FACS sorted and cocultured with anti-CD3/28-stimulated allogeneic PBLs (three donors) at various ratios and assessed for induction of CD4+CD25high T cells. The chart on the right is a quantitative summary of the experimental results of three independent experiments. ∗p < 0.05 compared with anti-CD3/28-activated PBL (leftmost bar).
See also Figure S1.
Expansion of MDSCs by MSCs Is Mediated by Secreted HGF
We next investigated the mechanisms involved in MSC expansion of MDSCs. We found that the expansion of MDSCs by MSCs was not affected by transwell separation of cells, indicatig that cell-cell contact was not needed and implicating secreted factors in this process (Figure 3A). We therefore analyzed the conditioned medium of MSCs to assess for relevant secreted molecules. MSCs secrete a number of stromal-related factors, such as RANTES/CCL5, HGF, and IL-6, the latter of which has been implicated in the expansion of MDSCs (Bunt et al., 2007; Figure 3B). However, using both blocking antibodies and small interfering RNA (siRNA) knockdown studies, we did not find that IL-6 contributed to the expansion of MDSCs by MSCs (Figure S2).
Figure 3.
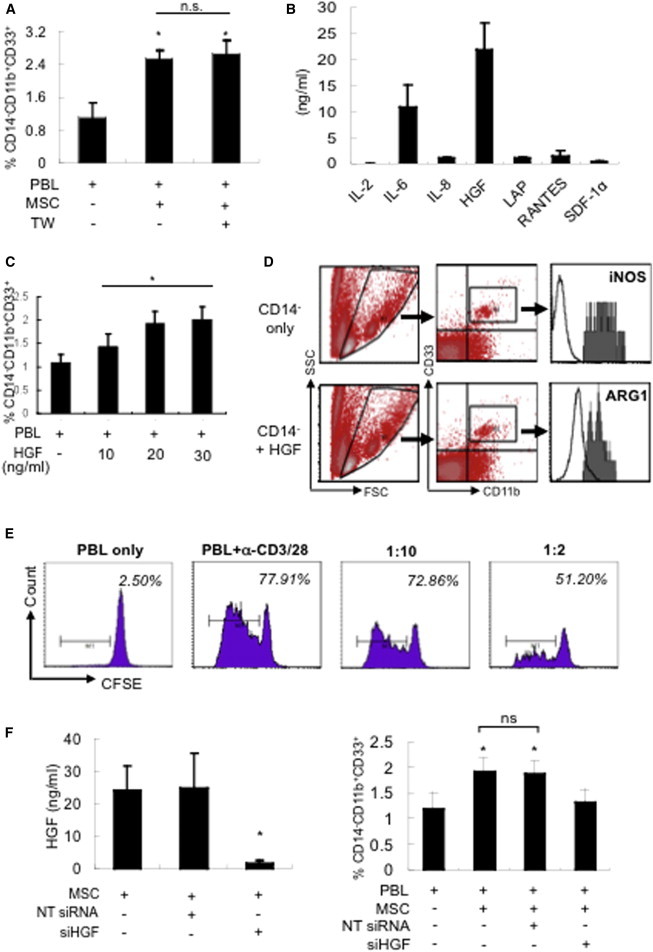
MSC Expansion of MDSCs Is Mediated by Secreted HGF
(A) MSC expansion of MDSCs is mediated by secreted factors. Allogeneic PBLs (five donors) were cocultured with MSCs either in direct contact (MSC; three donors) or separated by transwell (TW) and assessed for expansion of CD14−CD11b+CD33+ MDSCs; five independent experiments; ∗p < 0.05 compared with PBL only; n.s., not significant.
(B) Highly secreted factors of MSCs (four donors) as assessed by quantitative cytokine array. LAP, latency-associated peptide; SDF, stromal-derived factor.
(C and D) Exogenous addition of recombinant HGF to PBLs (five donors) increases the (C) percentage of MDSCs (∗p < 0.05 for trend) and (D) expression of iNOS (top panel) and ARG1 (lower panel) in HGF-expanded MDSCs (five donors; representative charts shown) as assessed by using negatively selected CD14− cells that were first gated on FSC/SSC (R1), analyzed for CD11b+ and CD33+ (R2), and then further analyzed for either iNOS (top histogram, shaded gray area) or ARG1 expression (bottom histogram, shaded gray area). Unfilled black line: isotype control; p = 0.051 for comparison of average number of CD14−CD11b+CD33+ cells (±SEM) expressing iNOS (367 ± 39 without HGF treatment compared with 409 ± 46 with HGF treatment); p < 0.05 for ARG1 expression (312 ± 53 without HGF treatment compared with 396 ± 56 with HGF treatment).
(E) Suppression of anti-CD3/28-stimulated PBL proliferation by HGF-expanded MDSCs as assessed by flow-cytometric analysis of CFSE staining for cell division (n = 3; a representative chart is shown).
(F) Knockdown of HGF secretion in MSCs with HGF-specific siRNA abrogates the expansion of MDSCs. Allogeneic PBLs (seven donors) were cocultured with MSCs (three donors) transfected with either NT siRNA or siHGF and assessed for CD14−CD11b+CD33+ cells. ∗p < 0.05 compared with MSCs only (leftmost bar) or PBLs only (rightmost bar); n.s., not significant (NT siRNA compared with siHGF).
See also Figure S2.
It was previously reported that STAT3 is critical for the expansion of MDSCs (Gabrilovich and Nagaraj, 2009). STAT3 is also an important molecule in the signal transduction pathway of HGF (Trusolino et al., 2010), a molecule that is known to be highly secreted by many cell types, including MSCs (Di Nicola et al., 2002; Takai et al., 1997; Trusolino et al., 2010). Since we also found that HGF was highly secreted by MSCs (Figure 3B), we assessed whether this molecule is involved in MSC expansion of MDSCs. We found that the addition of recombinant HGF alone could result in the expansion of MDSCs, and this effect was dose dependent up to a concentration of 30 ng/ml (Figure 3C), which is approximately the upper limit found in MSC-conditioned medium (Figure 3B). HGF-expanded MDSCs also expressed iNOS and ARG1, and at higher cell numbers compared with baseline (Figure 3D). Moreover, HGF-expanded MDSCs can suppress allogeneic lymphocytes proliferation as well (Figure 3E). To further ascertain the involvement of HGF in MSC expansion of MDSCs, we suppressed the secretion of HGF by MSCs with siRNA. Using siRNA specific for HGF, we were able to effectively suppress the secretion of the molecule by MSCs, and this abrogated the expansion of MDSCs by MSCs (Figure 3F). Thus, our data support that HGF secreted by MSCs is involved in the expansion of MDSCs.
HGF is a well-known and important mitogen for cancer cell growth (Mueller and Fusenig, 2004). Because MDSCs play a critical role in creating the immunoprivileged niche of tumors, we assessed whether cancer cell lines that secrete high levels of HGF could expand higher numbers of MDSCs. We found that, indeed, the level of HGF secreted by several cell lines correlated with the number of MDSCs expanded (Figures 4A and 4B). We then assessed the role of MSC-secreted HGF in the in vivo setting of the tumor microenvironment. When mice were inoculated with tumor cells admixed with MSCs silenced for HGF expression, there was a significant reduction in tumor-associated Gr-1+CD11b+ MDSCs (Figure 4C). Moreover, in both wild-type C57BL/6 and BALB/c mice, we found that the liver, an organ that is known to secrete high levels of HGF, contained a significantly higher proportion of Gr-1+CD11b+ MDSCs than the spleen (Figure 4D). Furthermore, when we inhibited the HGF/c-Met pathway in wild-type mice by injecting PHA-665752 (a second-generation c-Met inhibitor; Christensen et al., 2003), we observed a significant decrease in hepatic, but not splenic, MDSCs, even after we excluded other myeloid cells, such as macrophages and Kupffer cells (resident hepatic macrophages), using the surface marker F4/80 (Kinoshita et al., 2010; Lee et al., 1986; Figures 4E and S3). Critically, the HGF/c-Met pathway appears to be responsible for the high numbers and vast majority of hepatic Gr-1+CD11b+F4/80− MDSCs, since c-Met inhibition resulted in a significant loss of ∼70% of this population (Figure 4E), which was not observed for splenic MDSCs. These results are highly suggestive of a critical role for HGF in maintaining the high numbers of MDSCs in the liver. To further validate the in vivo relevance of our findings, we injected recombinant HGF intravenously into wild-type C57BL/6 mice. After 3 days, we found a significant increase of Gr-1+CD11b+ MDSCs in the bone marrow (where myeloid cells are most abundant; Gabrilovich et al., 2012) of these mice (Figure 4F), indicating that HGF can expand MDSCs in an in vivo setting. Collectively, these results indicate the relevance of HGF in tumor-associated MDSCs and in vivo settings.
Figure 4.

Cancer Cell-Secreted HGF and In Vivo Manipulation of HGF Expression in Mice Significantly Alter MDSC Numbers
(A and B) Level of HGF secretion by MSCs, MG63 (osteosarcoma cell line), human embryonic stem cell-derived mesenchymal progenitors (EMPs), and JEG-3 (choriocarcinoma cell line) (A), and fold expansion of MDSCs (B) after coculture of the four cell types with PBLs (three donors; three independent experiments; ∗p < 0.05 for trend).
(C) Knockdown of HGF expression in MSCs significantly decreases tumor-associated Gr-1+CD11b+ cells. Tumor growth was induced in nude mice by inoculating human colon cancer cells admixed with MSCs transfected with either control NT siRNA (n = 5 mice) or siHGF (n = 5 mice). Tumor-associated leukocytes were assessed for Gr-1+CD11b+ cells by flow-cytometric analysis (see Experimental Proceduresfor detailed description); ∗p < 0.05, NT siRNA compared with siHGF.
(D) Gr-1+CD11b+ cells (%) in the spleen and liver of C57BL/6 (B6) and BALB/c mice (n = 3 mice for each group); ∗p < 0.05, % of cells in liver compared with spleen.
(E) Treatment of C57BL/6 mice with the c-Met inhibitor PHA-665752 (c-Met inh) significantly decreases hepatic but not splenic Gr-1+CD11b+F4/80− cells (shown as a percentage of Gr-1+CD11b+ cells; average ± SEM; n = 3 mice for each group). ∗p < 0.05 for c-Met inhibitor treatment versus no treatment (see Experimental Procedures for a detailed description).
(F) Tail-vein injection of recombinant HGF (100 ng) into C57BL/6 mice and assessment of Gr-1+CD11b+ cells (fold change) in the peripheral blood, spleen, and bone marrow 1 day and 3 days after injection (n = 6 mice for each group); ∗p < 0.05 compared with day 0.
See also Figure S3.
Expansion of MDSCs by HGF Is Mediated by the HGF Receptor c-Met and Increased Phosphorylation of STAT3
To investigate the mechanism behind HGF-mediated expansion of MDSCs, we checked for expression of c-Met, the cognate receptor for HGF (Cecchi et al., 2010; Trusolino et al., 2010), on MDSCs. We found that CD14− leukocytes and MDSCs constitutively expressed low levels of c-Met (Figures 5A and S4), and when c-Met on PBLs was blocked with neutralizing antibodies, the expansion of MDSCs by HGF was abrogated (Figure 5B). To investigate whether the effects of the HGF/c-Met axis were mediated through STAT3, we checked for HGF-induced STAT3 phosphorylation, which indicates activation of the pathway, in MDSCs. We found that exogenous addition of HGF induced an increase over baseline levels of phosphorylated STAT3 (pSTAT3), most consistently and significantly at 1 hr after treatment (Figures 5C and S5). Moreover, when the STAT3 inhibitor cpd188 was added in the presence of exogenous HGF, the expansion of MDSCs was abrogated in a dose-dependent fashion (Figure 5D). Thus, HGF mediates the expansion of MDSCs by binding to its receptor, c-Met, which leads to increased phosphorylation of STAT3 (Fig. 6).
Figure 5.
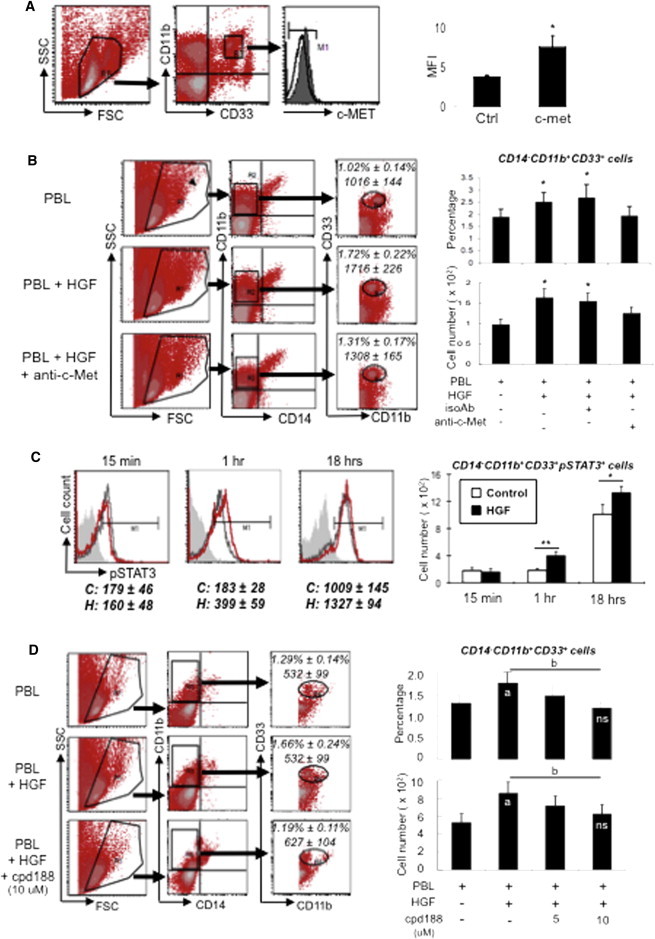
Expansion of MDSCs by HGF Is Mediated via c-Met, Its Receptor, and Increased Phosphorylation of STAT3
(A) Expression of c-Met on MDSCs as assessed by flow-cytometric analysis. PBLs (six donors) were first gated on FSC/SSC (R1), analyzed for CD11b+ and CD33+ (R2), and then further analyzed for c-Met expression (shaded gray area in histogram; unfilled black line: isotype control; the flow-cytometric analysis diagrams shown are representative). Right chart: quantification of mean fluorescent intensity (MFI); ∗p < 0.05 compared with isotype control (Ctrl).
(B) Involvement of c-Met in HGF-mediated expansion of MDSCs. PBLs (six donors) were blocked with isotype control antibodies (IsoAb) or c-Met blocking antibodies (anti-c-Met), treated with recombinant HGF (20 ng/ml), and assessed for expansion of CD14−CD11b+CD33+ cells. PBLs were first gated on FSC/SSC (R1), analyzed for CD14− and CD11b+ (R2), and then further analyzed for CD33+ (R3). Average percentages and cell numbers ± SEM are indicated in dot plot diagrams (1.54% ± 0.20% and 1,544 ± 201 cells for isotype control). Charts on the right are quantitative summaries of the percentages (top right) and cell numbers (bottom right) of six independent experiments; ∗p < 0.05 compared with PBL only or anti-c-Met.
(C) Involvement of STAT3 in HGF-mediated expansion of MDSCs. Recombinant HGF (20 ng/ml) was added to negatively selected CD14− leukocytes (eight donors) that were stained for CD11b, CD33, and pSTAT3. Control cells (no HGF treatment; black unfilled line in histogram) and HGF-treated cells (red unfilled line) were collected for assessment by flow cytometry at the indicated time points (shaded gray area: isotype control); average cell numbers ± SEM for control (C) and HGF treatment (H) are indicated below the histograms. The chart on the right is a quantitative summary of eight independent experiments; ∗p < 0.05 and ∗∗p < 0.005.
(D) STAT3 inhibition abrogates HGF-mediated expansion of MDSCs. Recombinant HGF (20 ng/ml) was added to PBLs (six donors) with and without addition of cpd188 (STAT3 inhibitor) at the indicated doses and assessed by flow cytometry for MDSCs, with PBLs first gated on FSC/SSC (R1), analyzed for CD14− and CD11b+ (R2), and then further analyzed for CD33+ (R3). Average percentages and cell numbers ± SEM are indicated in dot plot diagrams (1.37% ± 0.22% and 717 ± 108 cells for 5 μM cpd188). Charts on the right are quantitative summaries of the percentages (top right) and cell numbers (bottom right) of CD14−CD11b+CD33+ cells for six independent experiments. a, p < 0.05 compared with PBL only; b, p < 0.05 for trend; n.s., not significant for 10 μM cpd188 compared with PBL only.
See also Figures S4 and S5.
Figure 6.

Summary of MSC-Mediated Expansion of Functional MDSCs
HGF secreted by MSCs binds to its cognate receptor, c-Met, expressed on CD14− PBLs. This leads to increased pSTAT3, a critical transcription factor in MDSC expansion. Both MSC- and HGF-expanded MDSCs are functional, expressing ARG1 and iNOS, and inhibit allogeneic lymphocyte proliferation while increasing the number of immunomodulatory Tregs.
Discussion
Recent reports have highlighted MDSCs as a prominent leukocyte subpopulation involved not only in tumor-associated immune suppression but also in regulation of the immune system at large (Almand et al., 2001; Gabrilovich and Nagaraj, 2009; Rodríguez and Ochoa, 2008). Previous data have shown that a number of proinflammatory mediators are important inducers of these cells, but there has been no report regarding the involvement of tumor-associated mitogenic growth factors in the process. Our data link HGF secreted by MSCs to the expansion of MDSCs. Our findings can help to explain the strong association of MDSCs with tumors, since it is well established that in the tumor microenvironment, HGF is highly secreted by both the cancer cells themselves and the supporting stromal cells, promoting cell survival and tumor growth (Cecchi et al., 2010; Mueller and Fusenig, 2004). While HGF has been implicated in immunoregulatory responses (Benkhoucha et al., 2010; Di Nicola et al., 2002), and the liver, which naturally secretes high levels of HGF, was reported to have some immunological functions (Sheth and Bankey, 2001), the specific molecular mechanisms underlying these observations have been largely unexplored and the reported effects have not been consistently replicated (Le Blanc et al., 2003). Our finding that high numbers of hepatic MDSCs in mice can be significantly altered by disruption of the HGF/c-Met pathway sheds some mechanistic light on this issue, and, overall, our data demonstrate that HGF mediates the expansion of functional MDSCs by engaging c-Met, its receptor, and increasing the phosphorylation of STAT3, one of its downstream molecules.
The immunomodulatory properties of MSCs have been highlighted as being therapeutic for autoimmune diseases and other immune-related diseases such as graft-versus-host disease (Abdi et al., 2008; Djouad et al., 2009; Keating, 2012; Le Blanc et al., 2008). As exciting as these findings are, some researchers have noted that the same immunomodulatory effects of MSCs can allow for the growth of tumors (Djouad et al., 2003). However, whether MSCs definitively enhance or inhibit tumor growth and cancer progression is still unresolved, since differences in the experimental design, cancer histologic cell type, and MSC isolation technique used can all affect the experimental outcome (Klopp et al., 2011; Yen and Yen, 2008). Moreover, to date, studies on MSC and cancer interactions have largely focused on the homing of MSCs to tumors, rather than the immunological aspects of MSCs (Elzaouk et al., 2006; Studeny et al., 2002; Xin et al., 2007). Although a considerable amount of data support MSC expansion of Tregs (Uccelli et al., 2008), and these immunosuppressive T lymphocytes are also found in tumors, MDSCs appear to play a more crucial role in maintaining the profound immune suppression of the tumor niche (Almand et al., 2001; Ostrand-Rosenberg and Sinha, 2009; Sinha et al., 2005; Young and Wright, 1992). Our data suggest that through HGF and the consequent expansion of MDSCs, MSCs may play a role in maintaining tumor growth.
While the association of MDSCs in a wide range of cancers has been known for some time, the mechanisms involved in expansion of these immunomodulatory cells are just beginning to be unraveled. IL-6 has been reported to mediate the expansion of MDSCs, and this cytokine is known to be secreted by both MSCs and stromal cells (Hung et al., 2007; Nemunaitis et al., 1989). However, in this work, we did not find IL-6 to be involved. This may be due to the lower levels of this cytokine and the masking of its effects by the much higher levels of HGF in our system (see Figure 3B). Moreover, the strong association of MDSCs with cancer suggests that multiple and redundant pathways are likely involved (Ostrand-Rosenberg and Sinha, 2009; Gabrilovich and Nagaraj, 2009). There is consensus, however, that STAT3 may be the final transcription factor involved in the expansion of MDSCs. Our finding that STAT3 activation is involved in HGF-mediated MDSC expansion further supports the importance of this molecule in inflammation and cancer (Yu et al., 2009).
In summary, we found that HGF secreted by MSCs can lead to the expansion of MDSCs. MDSCs expanded by coculture with MSCs or addition of HGF are functional, expressing iNOS and ARG1, as well as harboring suppressive function and inducing Tregs. Mechanistically, the MSC-mediated expansion of MDSCs occurs via the HGF/c-Met axis, with the downstream involvement of STAT3. Our findings not only further demonstrate the strong immunoregulatory nature of MSCs, but also show the involvement of a mitogenic, noninflammatory molecule in the expansion of MDSCs.
Experimental Procedures
Cell Culture
Human MSCs from bone marrow and placenta were cultured and expanded as we previously described (Chang et al., 2006; Yen et al., 2005). Human bone marrow MSCs were purchased from Cambrex and cultured according to manufacturer’s instructions. For human placental MSCs, term placentas (38–40 weeks of gestation) from healthy donor mothers were obtained with informed consent according to the procedures of the institutional review board. The cells were isolated as previously described (Yen et al., 2005). Differentiation studies were carried out as previously described (Chang et al., 2006; Liu et al., 2011; Yen et al., 2005). Human PBLs were isolated from the buffy coat of healthy donor blood samples (Taiwan Blood Services Foundation, Taipei Blood Center, Taipei, Taiwan) obtained with informed consent according to the procedures of the institutional review board, and cultured as previously described (Chang et al., 2006; Liu et al., 2011).
Immunophenotyping
Flow-cytometric analyses of cell surface markers were performed as previously described (Chang et al., 2006; Liu et al., 2011; Yen et al., 2005). All antibodies were purchased from BD Biosciences, except for CD33 and CD11b (BioLegend), ARG1 (R&D Systems), iNOS (Abcam), and c-Met (eBiosciences). All analyses were done on a BD FACSCalibur flow-cytometric system (BD Biosciences).
Expansion of MDSCs
PBLs were cocultured with MSCs or cancer cell lines (10:1 ratio) for 3 days and then assessed for CD14−CD11b+CD33+ cells, which also served as the cell surface markers for fluorescence-activated cell sorting (FACS) of MDSCs with the BD Aria Cell Sorter (BD Biosciences). Instead of PBLs, some experiments were performed with CD14− cells negatively selected with the use of magnetic beads (Miltenyi Biotec) according to the manufacturer’s instructions. Transwell studies were performed as previously described using 24-well transwell inserts (0.4 μm pores; BD Falcon) with MSCs cultured on the culture plates below and PBLs cultured in the inserts (Liu et al., 2011). Recombinant human HGF (R&D Systems) was added to PBLs at the indicated doses.
MDSC Suppression Analysis
Assessment of PBL cell division was performed as previously described (Chang et al., 2006). Briefly, allogeneic PBLs were labeled with 2.5 μmol/l of the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes/GIBCO-Invitrogen) for 10 min and then stimulated with anti-CD3/CD28 beads (GIBCO-Invitrogen). MSC-expanded MDSCs were FACS sorted for homogeneity and then cocultured with stimulated allogeneic PBL for 3 days at various effector-to-target (E:T) ratios. Flow-cytometric analysis was performed to assess for PBL cell division in terms of CFSE dye intensity.
Quantification of HGF Secretion
Supernatants were collected from cell cultures for detection of HGF with the use of a commercially available ELISA kit (R&D Systems) according to the manufacturer’s instructions.
RNAi Experiments
siRNAs specific for IL-6 and HGF were purchased from GIBCO-Invitrogen, and knockdown experiments were conducted according to the manufacturer’s instructions. The efficiency of siRNA knockdown of MSC-secreted factors was verified by ELISA.
In Vivo Experiments
All animal work was performed in accordance with protocols approved by the Animal Care and Use Committee of the National Health Research Institutes. Mice (4–8 weeks old) were obtained from the National Laboratory Animal Center of Taiwan (Taipei, Taiwan). Recombinant mouse HGF (100 ng; R&D Systems) was injected via the tail vein, and C57BL/6 mice were sacrificed 3 days after injection. Peripheral blood, bone marrow, and spleen were collected for flow-cytometric analysis of Gr-1+CD11b+ cells. For in vivo tumor studies, nude mice (BALB/c nu/nu) were inoculated subcutaneously with tumor cells (human colon cancer cell line HCT-116; 1 × 106 cells) admixed with human MSCs (5 × 105 cells at a 1:2 ratio to cancer cells) transfected with either control siRNA (NT siRNA) or HGF-specific siRNA (siHGF). The mice were sacrificed 3 weeks after inoculation, and tumors were excised and minced with scissors to obtain leukocytes, which were assessed for Gr-1+CD11b+ cells by flow-cytometric analysis. For c-Met inhibition studies, wild-type C57BL/6 mice were intraperitoneally injected with vehicle (DMSO, n = 3) or PHA-665752 (Tocris Bioscience), a c-Met inhibitor (0.15 mg, n = 3) as previously described (Gordin et al., 2010). The mice were sacrificed 5 days later and their livers and spleens were collected for flow-cytometric assessment of Gr-1+CD11b+ and Gr-1+CD11b+F4/80− cells.
Statistical Analysis
Statistical analysis was performed with SPSS 18.0 software (SPSS), with statistical significance defined as p < 0.05. Values were expressed as the mean ± SEM. Student’s t test was used for comparisons between two groups, and ANOVA was used for comparisons of multiple groups.
For further details regarding the materials and methods used in this work, see Supplemental Experimental Procedures.
Acknowledgments
This work was supported in part by funding from the National Health Research Institutes (102A1-CSPP06-014 to B.L.Y.) and the National Science Council of Taiwan (NSC101-2321-B-400-009 to B.L.Y.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Abdi R., Fiorina P., Adra C.N., Atkinson M., Sayegh M.H. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almand B., Clark J.I., Nikitina E., van Beynen J., English N.R., Knight S.C., Carbone D.P., Gabrilovich D.I. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Benkhoucha M., Santiago-Raber M.L., Schneiter G., Chofflon M., Funakoshi H., Nakamura T., Lalive P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S.K., Yang L., Sinha P., Clements V.K., Leips J., Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi F., Rabe D.C., Bottaro D.P. Targeting the HGF/Met signalling pathway in cancer. Eur. J. Cancer. 2010;46:1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.J., Yen M.L., Chen Y.C., Chien C.C., Huang H.I., Bai C.H., Yen B.L. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- Christensen J.G., Schreck R., Burrows J., Kuruganti P., Chan E., Le P., Chen J., Wang X., Ruslim L., Blake R. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P., Grisanti S., Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Djouad F., Plence P., Bony C., Tropel P., Apparailly F., Sany J., Noël D., Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Djouad F., Bouffi C., Ghannam S., Noël D., Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dugast A.S., Haudebourg T., Coulon F., Heslan M., Haspot F., Poirier N., Vuillefroy de Silly R., Usal C., Smit H., Martinet B. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 2008;180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- Elzaouk L., Moelling K., Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp. Dermatol. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Fricke I., Mirza N., Dupont J., Lockhart C., Jackson A., Lee J.H., Sosman J.A., Gabrilovich D.I. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- Friedenstein A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin M., Tesio M., Cohen S., Gore Y., Lantner F., Leng L., Bucala R., Shachar I. c-Met and its ligand hepatocyte growth factor/scatter factor regulate mature B cell survival in a pathway induced by CD74. J. Immunol. 2010;185:2020–2031. doi: 10.4049/jimmunol.0902566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I., Sayegh M.H. Maternal acceptance of the fetus: true human tolerance. J. Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- Huang B., Pan P.Y., Li Q., Sato A.I., Levy D.E., Bromberg J., Divino C.M., Chen S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Hung S.C., Pochampally R.R., Chen S.C., Hsu S.C., Prockop D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Klopp A.H., Gupta A., Spaeth E., Andreeff M., Marini F., 3rd Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Uchida T., Sato A., Nakashima M., Nakashima H., Shono S., Habu Y., Miyazaki H., Hiroi S., Seki S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Crocker P., Gordon S. Macrophage plasma membrane and secretory properties in murine malaria. Effects of Plasmodium yoelii blood-stage infection on macrophages in liver, spleen, and blood. J. Exp. Med. 1986;163:54–74. doi: 10.1084/jem.163.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Tammik L., Sundberg B., Haynesworth S.E., Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Liu K.J., Wang C.J., Chang C.J., Hu H.I., Hsu P.J., Wu Y.C., Bai C.H., Sytwu H.K., Yen B.L. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transplant. 2011;20:1721–1730. doi: 10.3727/096368911X580590. [DOI] [PubMed] [Google Scholar]
- Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Mueller M.M., Fusenig N.E. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J., Andrews D.F., Mochizuki D.Y., Lilly M.B., Singer J.W. Human marrow stromal cells: response to interleukin-6 (IL-6) and control of IL-6 expression. Blood. 1989;74:1929–1935. [PubMed] [Google Scholar]
- Ochoa A.C., Zea A.H., Hernandez C., Rodriguez P.C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S., Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poschke I., Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin. Immunol. 2012;144:250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rabinovich G.A., Gabrilovich D., Sotomayor E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez P.C., Ochoa A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Serafini P., Carbley R., Noonan K.A., Tan G., Bronte V., Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Sheth K., Bankey P. The liver as an immune organ. Curr. Opin. Crit. Care. 2001;7:99–104. doi: 10.1097/00075198-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Shojaei F., Wu X., Malik A.K., Zhong C., Baldwin M.E., Schanz S., Fuh G., Gerber H.P., Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Sinha P., Clements V.K., Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- Sinha P., Clements V.K., Fulton A.M., Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Swiecki M., Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K., Hara J., Matsumoto K., Hosoi G., Osugi Y., Tawa A., Okada S., Nakamura T. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood. 1997;89:1560–1565. [PubMed] [Google Scholar]
- Trusolino L., Bertotti A., Comoglio P.M. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Wing K., Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Xin H., Kanehira M., Mizuguchi H., Hayakawa T., Kikuchi T., Nukiwa T., Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- Yen B.L., Yen M.L. Mesenchymal stem cells and cancer: for better or for worse? J. Cancer Mol. 2008;4:5–9. [Google Scholar]
- Yen B.L., Huang H.I., Chien C.C., Jui H.Y., Ko B.S., Yao M., Shun C.T., Yen M.L., Lee M.C., Chen Y.C. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- Young M.R., Wright M.A. Myelopoiesis-associated immune suppressor cells in mice bearing metastatic Lewis lung carcinoma tumors: gamma interferon plus tumor necrosis factor alpha synergistically reduces immune suppressor and tumor growth-promoting activities of bone marrow cells and diminishes tumor recurrence and metastasis. Cancer Res. 1992;52:6335–6340. [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


