Summary
Very small embryonic-like stem cells (VSELs) isolated from bone marrow (BM) have been reported to be pluripotent. Given their nonembryonic source, they could replace blastocyst-derived embryonic stem cells in research and medicine. However, their multiple-germ-layer potential has been incompletely studied. Here, we show that we cannot find VSELs in mouse BM with any of the reported stem cell potentials, specifically for hematopoiesis. We found that: (1) most events within the “VSEL” flow-cytometry gate had little DNA and the cells corresponding to these events (2) could not form spheres, (3) did not express Oct4, and (4) could not differentiate into blood cells. These results provide a failure to confirm the existence of pluripotent VSELs.
Highlights
-
•
We rigorously evaluated the mouse VSEL hypothesis
-
•
Most CD45−/intLin−SCA-1+ events were neither “very small” nor diploid cells
-
•
No pluripotency was detected in CD45−/intLin−SCA-1+ cells, regardless of their size
-
•
Existence of adult mouse VSELs in the bone marrow remains dubious
Introduction
During normal development, pluripotent cells from the inner cell mass give rise to several types of tissue-committed stem cells (TSCs), restricted in their differentiation potential. TSCs can self-renew and produce downstream progenitors and mature cells throughout life. It remains controversial (Wagers et al., 2002; Wagers and Weissman, 2004) whether some pluripotent cells self-renew as pluripotent stem cells (PSCs) and persist after birth (Beltrami et al., 2007; Check, 2007; Jiang et al., 2002; Kögler et al., 2004; Krause et al., 2001; Serafini et al., 2007).
One group has recently identified bone marrow (BM)-residing very small embryonic-like stem cells (VSELs) in both human and mouse as a putative nonembryonic source for PSCs (Drukała et al., 2012; Kucia et al., 2006b; Wojakowski et al., 2009; Zuba-Surma et al., 2011). Mouse VSELs have been characterized to: (1) be very small (3–5 μm), (2) have a CD45−Lineage(Lin)−SCA-1+ phenotype, (3) express pluripotent marker genes (e.g., Oct4, Nanog), and (4) when cultured on the myoblast C2C12 cell line, form embryoid body-like spheres and then differentiate into multi-germ-layer cells. Moreover, these cells were reported to be directly obtainable from BM or mobilized peripheral blood without culture. Thus, their pluripotency should be reproducible in independent laboratories, a requirement for a scientific finding to be generally accepted (Jasny et al., 2011). However, recent studies have shown the lack of stem cell characteristics in VSELs isolated from mouse (Szade et al., 2013) or human cord blood (CB) (Danova-Alt et al., 2012), while another study confirmed the existence of mouse VSELs and their ability to give rise to lung cells (Kassmer et al., 2013).
We sought to recapitulate the previous findings on relating to mouse VSELs, focusing on the DNA content of the small-sized fraction and on its hematopoietic lineage potential. We discovered an anomaly in separating cells by forward scatter (FSC) using different types of cell sorters and, more significantly, could not find PSCs within the VSEL fraction of mouse BM.
Results
Identifying and Characterizing Candidate VSEL Cells
We sought to identify VSELs from mouse BM by using commonly reported VSEL characteristics (Kucia et al., 2006a; Ratajczak et al., 2011; Zuba-Surma et al., 2008). We fractionated BM samples using fluorescence-activated cell sorting (FACS). Dead cells and debris were excluded as Ratajczak and colleagues previously reported (Zuba-Surma et al., 2008). To minimize the risk of missing candidate cells, SCA-1+ cells (including SCA-1lo) beyond the threshold defined by the fluorescence minus one (FMO) method (Herzenberg et al., 2006) were included, and only the obvious Lin+ cells were excluded from the Lin− fraction (which included Linlo cells). Consequently, the Lin−SCA-1+ gate was more inclusive than that described elsewhere for VSELs and included all cells reported to be in the VSEL fraction (Kucia et al., 2006a; Ratajczak et al., 2008a, 2011). Lin−SCA-1+ events were subdivided into three fractions according to CD45 expression (Figure 1A; Table S1 available online).
Figure 1.
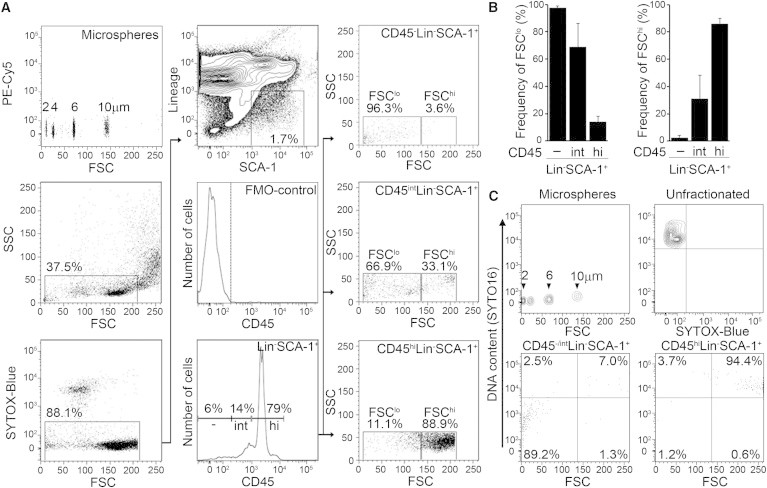
Adult Mouse Bone Marrow CD45−Lin−SCA-1+ Cells Enriched in the FSClo Region Contain Little DNA
(A) FACS plots of BM cells from wild-type mice. The initial gate (left middle) was based on defined-size microspheres (left upper) and expected to include both HSCs and VSELs. After excluding dead cells (left bottom), we focused on the Lin−SCA-1+ fraction (center upper), subdividing these into FMO-defined CD45− (dashed line in center middle), CD45int, and CD45hi fractions (center bottom). The frequency of FSClo (<10 μm microspheres) and FSChi (>10 μm microspheres) cells in each subfraction is indicated (right panels).
(B) Frequency of FSClo (left) or FSChi (right) cells among the CD45−, CD45int, and CD45hi subfractions of Lin−SCA-1+ events. The mean ± SD of data from 22 independent experiments are shown.
(C) Analysis of DNA content by SYTO16 staining. The threshold of high SYTO16 positivity was determined to include 99% of unfractionated BM cells (upper right). The vertical line in the lower plots indicates the position of 10 μm microspheres.
We used the FSC intensity of 10 μm microspheres to define the cutoff point between FSClo and FSChi events. As previously reported (Zuba-Surma et al., 2008), the CD45− fraction contained many more FSClo cells than FSChi cells. Conversely, the CD45hi fraction contained many more FSChi cells than FSClo cells (Figure 1B; Tables S1 and S2).
FSClo events could include erythrocytes, vesicles, or cell fragments and/or debris. Since the original group has stated several times that VSELs are diploid (Kucia et al., 2008; Ratajczak et al., 2007, 2008b), we analyzed DNA content of FSClo events using SYTO16, a cell-membrane permeable DNA dye. We found that SYTO16hi events, representative of diploid cells, were present in about 98% of the CD45hiLin−SCA-1+ fraction but only ∼10% of the CD45−/intLin−SCA-1+ fraction (Figure 1C). The remaining events in the CD45−/intLin−SCA-1+ gate showed much lower intensity for SYTO16, indicating that these were likely to be cell fragments and not diploid cells. Furthermore, most of the CD45−/intLin−SCA-1+SYTO16hi cells were in the FSChi gate (Figure 1C). This indicated that our VSEL candidates were the relatively larger cells in the population analyzed, unlike the VSELs described in previous reports (Kucia et al., 2006a; Ratajczak et al., 2011; Wojakowski et al., 2009; Zuba-Surma et al., 2008, 2009).
Flow Cytometry in Assessing Size of Candidate VSELs
We hypothesized that this discrepancy was due to the type of flow cytometer used: Ratajczak et al. (2011) mainly used a MoFlo (Beckman Coulter) (Zuba-Surma et al., 2008, 2009), but we used a FACSAria (BD Biosciences). Thus, we analyzed the same BM sample on a FACSAria and MoFlo. Based on the FSC intensity of unfractionated BM, 10 μm microspheres were positioned to the left of lymphocytes on the FACSAria but in between lymphocytes and granulocytes on the MoFlo (Figure 2A). Using the FACSAria and the MoFlo, we sorted the BM sample into three subpopulations, based on the FSC intensity of defined-size microspheres. We then reanalyzed each of these subpopulations on both the FACSAria and MoFlo. Critically, a given population of cells seemed smaller (based on microsphere reference FSC intensities) on the MoFlo than on the FACSAria (Figures 2B and S1A). However, fluorescence intensity of the cell-to-cell comparison did not differ between the sorters (Figure S1B). Finally, we evaluated the FSC of candidate VSELs and hematopoietic stem/progenitor cells on FACSAria and MoFlo. Notably, more than 70% of candidate VSELs were detected in the FSChi gate on FACSAria, whereas >80% of them were in the FSClo gate on MoFlo (Figure 2C).
Figure 2.
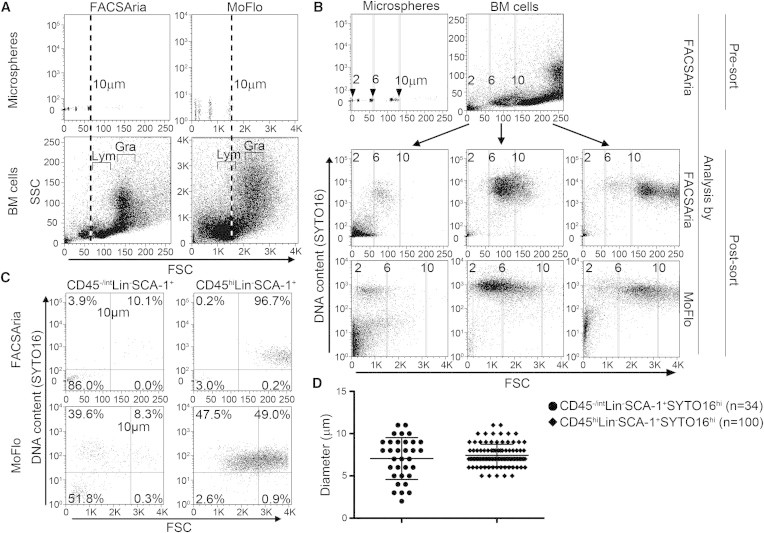
Evaluation of Cell Size by Different Cell Sorters
(A) Defined-size microspheres (upper) and whole BM cells (lower) were analyzed by FACSAria (left) and MoFlo (right). Dashed lines indicate the FSC of 10 μm microspheres. The positions of lymphocytes (Lym) and granulocytes (Gra) are indicated in the lower panels.
(B) On a FACSAria, BM cells were sorted by expected size (2–6, 6–10, and >10 μm) based on their FSC relative to microspheres (upper) and then reanalyzed on the same FACSAria (middle) and on a MoFlo (lower). Vertical lines indicate the positions of 2, 6, and 10 μm microspheres on each machine.
(C) FACS plots of diploid (SYTO16hi) CD45−/intLin−SCA-1+ cells (candidate VSELs; left) and CD45hiLin−SCA-1+ cells (HSCs; right) analyzed by FACSAria (upper) or MoFlo (lower). Vertical lines indicate the position of 10 μm microspheres in each sorter, and horizontal lines indicate the threshold of SYTO16 positivity.
(D) Comparison of the sizes of candidate VSELs (n = 34) and HSCs (n = 100) determined with an image flow cytometer. Each dot represents a cell, and each line with error bars represents the mean ± SD.
See also Figure S1.
To address the concern that flow cytometer FSC based on microspheres is not a reliable indicator of absolute size, we measured the size of cells with an image flow cytometer (FlowSight, Amnis). We captured 2 × 106 BM cells and identified 34 events having the immunophenotype corresponding to the candidate VSELs (Figure S1C). Their mean diameter was nonsignificantly smaller than that of a randomly selected population of 100 CD45hiLin−SCA-1+SYTO16hi events (7.06 versus 7.51 μm; p = 0.82; Figure 2D). However, only the candidate VSEL fraction contained few very small-sized (<5 μm) particles, as previously shown in fetal liver-resident VSELs (Zuba-Surma et al., 2009).
Assessment of VSEL Candidates for Indicators of Pluripotency
To determine whether the CD45−/intLin−SCA-1+ fraction contained any pluripotent cells, we compared Oct4 expression levels among freshly purified CD45−/intLin−SCA-1+ and CD45hiLin−SCA-1+ cells and a mouse embryonic stem cell (ESC) line using quantitative RT-PCR with four different primer pairs. We could not detect Oct4 expression in CD45−/intLin−SCA-1+ (both SYTO16hi and SYTO16lo) cells at all (Figure 3A). These results contrast with previous reports (Kassmer et al., 2013; Kucia et al., 2006a; Ratajczak et al., 2011; Shin et al., 2010).
Figure 3.
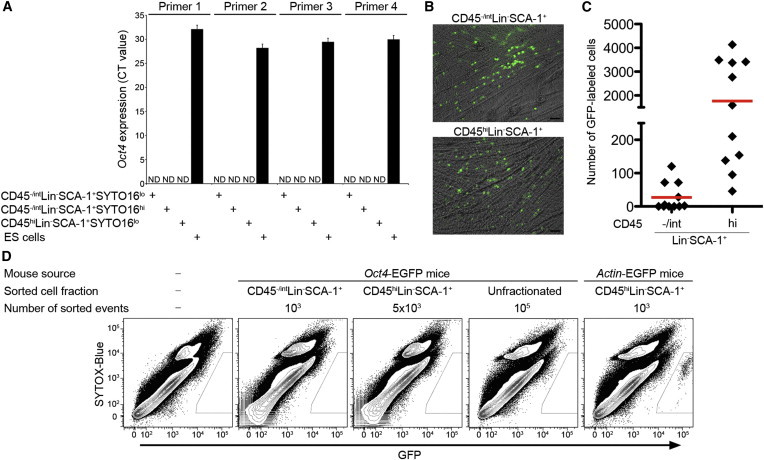
No Evidence for the Pluripotency of CD45−/intLin−SCA-1+ Cells
(A) Real-time RT-PCR analysis of Oct4 expression in subfractions of Lin−SCA-1+ cells and in ESCs (BM cells were prepared as a pool of four mice and then sorted in quadruplicate and subjected to RT-PCR; the data are shown as mean ± SD). ND, not detected.
(B) Representative images of 8 day progeny of FACS-purified Lin−SCA-1+ populations from Actin-EGFP mice. Cells were cocultured with C2C12 cells in DMEM supplemented with 2% FCS. Scale bar, 50 μm.
(C) The total number of GFP+ cells detected by FACS analysis at day 8 coculture of either 1,000 CD45−/intLin−SCA-1+ events or 1,000 CD45hiLin−SCA-1+ events (n = 11 for each from three independent experiments); the red line indicates the mean.
(D) FACS analysis of Oct4-derived EGFP expression on culture day 8. On day 0, the following were plated on C2C12 cells: no cells (far left column) or BM cells from Oct4-EGFP or Actin-EGFP mice, sorted by the indicated phenotypes. Data shown are representative of three independent experiments.
Next, we performed a sphere-forming assay using a mouse myoblastic C2C12 cell line (Kucia et al., 2008; Shin et al., 2010). The C2C12 cell line is known to initiate its own differentiation and form muscle tubules when cultured in low serum concentrations (Yaffe and Saxel, 1977). We observed that, when cultured alone, C2C12 cells aggregated spontaneously and sometimes formed sphere-like structures (Figure S2A). Therefore, to distinguish between spheres originating from candidate VSELs and spontaneous aggregation of C2C12 cells, we isolated candidate VSELs from Actin-EGFP transgenic mice and cocultured them with C2C12 cells. Some GFP+ cells proliferated and formed small clusters (Figure 3B) but never the spheres described in previous reports (Kucia et al., 2008; Shin et al., 2010). Eight-day progeny was harvested and analyzed for the absolute number of GFP+ cells by flow cytometry. The mean number of GFP+ cells initiated from the CD45−/intLin−SCA-1+ fraction was significantly lower than that from the CD45hiLin−SCA-1+ fraction: 27.2 versus 1764; p = 0.0002, respectively (Figure 3C). We repeated this assay with BM samples harvested from Oct4-EGFP transgenic mice. However, we could not detect any GFP+ cells in day 8 cultures of the following originally plated cells: 1,000 CD45−/intLin−SCA-1+, 5,000 CD45hiLin−SCA-1+, or 105 unfractionated BM cells (Figure 3D). The addition of leukemia inhibitory factor and/or usage of mouse embryonic fibroblasts instead of C2C12 cells did not affect the results (data not shown).
Grown GFP+ cells from both CD45−/intLin−SCA-1+ and CD45hiLin−SCA-1+ fractions had similar morphologies: an unlobulated nucleus and many vacuoles in voluminous cytoplasm, suggestive of macrophages (Figure S2B). Furthermore, flow cytometry analysis confirmed these GFP+ cells expressed high levels of CD45 (a hematopoietic lineage marker) and CD11b (a macrophage marker; Figure S2C). Since C2C12 cells are reported to secrete macrophage-colony stimulating factor (M-CSF) into the culture supernatant (Ghosh-Choudhury et al., 2006), it is possible that they may induce macrophage differentiation to either CD45−/intLin−SCA-1+ or CD45hiLin−SCA-1+ cells via M-CSF signaling.
To demonstrate the pluripotency of a particular cell, clonal expansion without differentiation from a single cell in vitro is an indispensable step. However, the C2C12 cells did not work to induce this expansion in candidate VSELs. Therefore, we sought another way to demonstrate that CD45−/intLin−SCA-1+ cells are pluripotent or at least could even generate any tissue-specific lineage. We focused on testing hematopoietic potential because (1) a previous report showed that transplantation of cultured VSELs could reconstitute the hematopoietic system (Ratajczak et al., 2011) and (2) methods of purification and functional analyses of hematopoietic stem cells (HSCs) have been established (Spangrude et al., 1988; Uchida and Weissman, 1992). If hematopoietic potential cannot be recapitulated, the cells in the VSEL fraction must not be pluripotent.
Freshly isolated CD45−Lin−SCA-1+ BM cells (including both FSClo and FSChi) did not proliferate under hematopoietic culture conditions (Figures S3A and S3B). A recent report indicated that 5-day coculture with the OP9 stromal cell line (i.e., OP9 priming) is critical for VSELs to be designated for a hematopoietic lineage (Ratajczak et al., 2011). In accordance with previous reports (Nakano et al., 1994; Seiler et al., 2011), we found that, when cocultured with OP9 cells, an ESC line was capable of hematopoietic differentiation (data not shown); this served as a positive control. However, CD45−Lin−SCA-1+ cells could not generate hematopoietic colonies in the methylcellulose assay, even after OP9 priming (Figures 4A and 4B). The addition of various hematopoietic cytokines during the OP9 priming did not affect the results (data not shown). Furthermore, CD45−Lin−SCA-1+ cells failed to produce any hematopoietic cells under the following additional conditions: (1) serial plating (Ratajczak et al., 2011); (2) including SYTOX-Blue positive fraction; (3) using only the SYTO16hi cells obtained from ten mice; (4) using cells from H2K-BCL-2 transgenic mice (Domen et al., 1998); or (5) using cells sorted on a MoFlo machine (data not shown). These observations indicate that the FMO-defined CD45−Lin−SCA-1+ fraction, at least in vitro, lacks hematopoietic potential, as recently described both for mouse VSELs (Szade et al., 2013) and their human counterparts (Danova-Alt et al., 2012).
Figure 4.
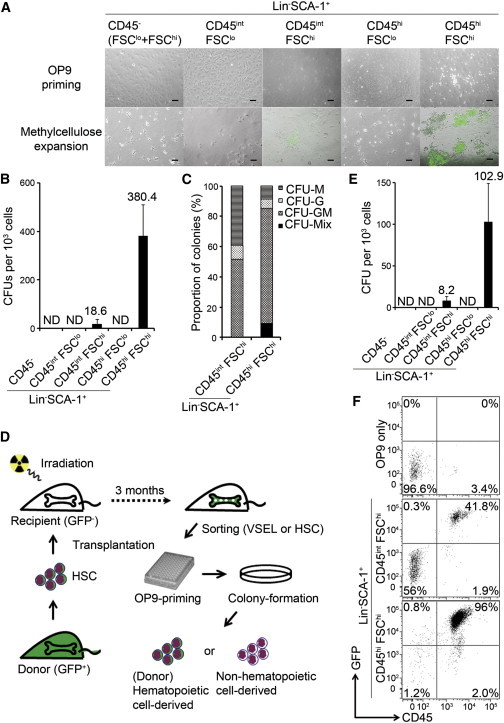
HSCs Are the Only Contributor to Postnatal Mouse Hematopoiesis
(A) Representative images on culture days 5 and 10. Each FACS-purified BM Lin−SCA-1+ fraction from Actin-EGFP mice was cocultured with OP9 cells for the first 5 days (i.e., OP9 priming) and transferred to methylcellulose for an additional 5 days (i.e., methylcellulose expansion). The threshold between FSClo and FSChi was defined by 10 μm microspheres. Scale bar, 100 μm.
(B) The number of colony-forming units (CFUs) from 103 cells of each Lin−SCA-1+ fraction. Data shown are mean ± SD of four independent experiments. ND, not detected.
(C) Proportion of colonies. From five independent colony assays, 199 colonies derived from CD45intLin−SCA-1+FSChi cells and 250 colonies derived from CD45hiLin−SCA-1+FSChi cells were picked up, cytospun, and stained with the May-Giemsa method to determine the cell types included. CFU-M, CFU-macrophage; CFU-G, CFU-granulocyte; CFU-GM, CFU-granulocyte/macrophage; CFU-Mix, CFU-erythroid and myeloid cells.
(D) Schematic of in vivo experiments.
(E) The number of CFUs from 103 cells of each Lin−SCA-1+ fraction. Data shown here are mean ± SD.
(F) Ten day progeny of CD45intLin−SCA-1+FSChi or CD45hiLin−SCA-1+FSChi cells were harvested with OP9 stromal cells and analyzed by FACS. Live cells were gated and tested for the expression of CD45 and GFP. A total of 5 × 104 events were recorded. Data were similar in three independent experiments.
See also Figures S3 and S4.
CD45intLin−SCA-1+FSChi cells with Limited Hematopoietic Potential Originated from HSCs
Within the remaining CD45hiLin−SCA-1+ fraction, 38.0% of CD45hiLin−SCA-1+FSChi cells but no CD45hiLin−SCA-1+FSClo cells formed hematopoietic colonies. Also, 1.86% of CD45intLin−SCA-1+FSChi cells formed hematopoietic colonies (Figure 4B). Many colonies from the CD45intLin−SCA-1+FSChi fraction contained fewer cells than those from the CD45hiLin−SCA-1+FSChi fraction, and their differentiation potential was restricted to nonerythroid cells and tended to skew to the monocyte/macrophage lineage (Figures 4C and S3C). In addition, when compared to CD45hiLin−SCA-1+FSChi cells, CD45intLin−SCA-1+FSChi cells showed a more indented nucleus, lower levels of SCA-1, and higher levels of Lin and side scatter (Figures S3D and S3E). These findings suggest that the CD45intLin−SCA-1+FSChi cells are at a lineage stage downstream of the CD45hiLin−SCA-1+FSChi cells. However, the exact lineage stage from which granulocyte-macrophage progenitors can develop may vary depending on conditions such as the type of cytokine cocktail (Rieger et al., 2009). Therefore, we cannot definitively determine the stage of the initially plated cells that gave rise mainly to macrophages in this assay.
To directly evaluate whether the colony-forming cells in the CD45intLin−SCA-1+FSChi fraction were progeny of HSCs or independent of hematopoietic lineage cells, we engrafted EGFP-HSCs into uncolored mice. The experimental design, summarized in Figure 4D, was similar to that described in a previous report (Hall et al., 2007). Three months after the mice underwent transplantation of GFP-expressing HSCs, 98% of their peripheral blood cells (not shown) and 96.8% of their BM granulocytes were GFP+ (Figure S4A). The frequencies of CD45−, CD45int, and CD45hi fractions within Lin−SCA-1+ gate in the chimeric mice were comparable to those in age-adjusted nonirradiated syngeneic mice (Figure S4B). We evaluated the frequency of colony-forming cells in each fraction. As in the experiments with mice that did not receive transplants, a limited number of colony-forming cells were detectable in the fraction of CD45intLin−SCA-1+FSChi and CD45hiLin−SCA-1+FSChi cells (Figure 4E). Analysis by fluorescent microscopy and flow cytometry confirmed that all of the colonies derived from CD45intLin−SCA-1+FSChi cells (81/81) and CD45hiLin−SCA-1+FSChi cells (926/926) expressed GFP (Figure 4F). This indicates that the CD45intLin−SCA-1+FSChi cells that demonstrated hematopoietic potential originated from HSCs. Furthermore, recipient CD45−/int cells, including the previously reported highly radiation-resistant VSELs (Ratajczak et al., 2011), failed to respond to this critical myelosuppressive condition and generate HSCs and/or their downstream progenitors in vivo. In other words, HSCs, reported to have little developmental plasticity (Wagers et al., 2002), are the only contributors to postnatal mouse hematopoiesis.
Discussion
The existence of pluripotent cells after the preimplantation blastocyst stage has not been demonstrated, except in the case of germline stem cells. Therefore, VSELs and other PSCs in postnatal mice must be independently verified to change how we view events in embryogenesis and fetal development. PSCs in postnatal humans, if they exist, would have an extremely significant impact on society because of their potential applications in research and regenerative medicine. In fact, the discovery of VSELs has already led to a commercial venture, Neostem, to bring their potential to human therapies. One of its financial backers, the Vatican, has announced in two symposia at the Vatican that these cells represent an ethical alternative to ESCs derived from humans (International Vatican Conference, November 9–11, 2011 and April 11–13, 2013, http://www.stemforlife.org/vatican-initiative).
In contrast to these optimistic findings, some groups have recently reported on their failure to detect VSELs. To accept a discovery as a scientific fact in the stem cell field, we previously proposed that all of the following three criteria are critical (Weissman, 2007): (1) the initial discovery must be published in fully peer-reviewed journals; (2) the experiment as published must be repeatable in many independent laboratories; and (3) the phenomenon described should be so robust that other experimental methods must reveal it. And in the case of stem cells, the regeneration derived from their transplantation should be rapid, robust, and lifelong.
It is not infrequent that, although the initial findings are peer-reviewed, subsequent attempts to independently replicate the findings fail (Begley and Ellis, 2012). We therefore sought to employ a logical strategy to examine all the cells within the CD45−/intLin−SCA-1+ population in mouse BM, even going as far as to include dead cells and cell debris in the analysis. Besides surface markers, the main criteria for the VSELs as defined by the original group are the following two points: (1) very small in size (3–5 μm) and (2) pluripotency. First, we tried to clarify whether this fraction contains any viable cells of the reported small size. We found that about 90% of all the events in this gate are indeed relatively small and have much less DNA than that of diploid cells according to SYTO16 staining measured on FACSAria (Figure 1C). This suggests that these relatively very small events are simply cell debris or fragments; subcellular particles, such as exosomes and blebs from dying cells, could be included. As mentioned above, it is unreliable to determine absolute cell size by FSC intensity (Figure 2). Furthermore, any modifications such as fixation or cytospin have the potential to affect the cell size. Therefore, we used an image flow cytometer and directly measured the size of candidate events without any modification. This experiment revealed that most of the SYTO16lo events lack the round shape of cells and are jagged, suggestive of dead cells and/or cell debris. SYTO16hi cells, both from CD45−/intLin−SCA-1+ and CD45hiLin−SCA-1+ are not statistically different in size and the majority are not small (<5 μm). However, we were still able to detect an exceedingly rare population of CD45−/intLin−SCA-1+SYTO16hi events, which we sought to evaluate for pluripotency.
Since the original group reported that VSELs can survive and differentiate to the hematopoietic lineage on OP9 cells, we sought to evaluate these candidate VSELs by the same assay, namely blood colony formation. When we cultured all the SYTOX-Blue-positive cells in the CD45−/intLin−SCA-1+ fraction (without size discrimination), no colony formation was detected. This indicates logically that SYTOX-Blue staining method can separate dead cells functionally; there are no viable VSELs in the SYTOX-Blue-positive fraction. We were unable to detect any hematopoietic cells among the SYTOX-Blue-negative CD45−Lin−SCA-1+ cells—the ideal candidates for VSEL cells—under the following additional conditions: (1) serial plating (previous reports showed serial plating would recover cell growth; Ratajczak et al., 2011); (2) using only the SYTO16hi cells obtained from ten mice; (3) using cells from H2K-BCL-2 transgenic mice (to improve viability by inhibiting programmed cell death; Domen et al., 1998); or (4) using cells sorted on a MoFlo machine (data not shown).
Since we have shown that size may not be a reliable parameter in isolating VSEL, we broadened our search by not using the size criteria while maintaining the immunophenotype (CD45−/intLin−SCA-1+FSChi). Compared to CD45hiLin−SCA-1+FSChi cells, CD45intLin−SCA-1+FSChi cells showed much less hematopoietic differentiation potential, and it never matched CD45hiLin−SCA-1+FSChi cells, even after serial plating, in contrast to the original group’s report (Figure 4B; data not shown; Ratajczak et al., 2011). However, given our observation of some hematopoietic output from the CD45intLin−SCA-1+FSChi fraction, we sought to determine the developmental origin responsible: HSC or candidate VSEL. All of the hematopoietic differentiation potential in the CD45intLin−SCA-1+FSChi fraction were derived from prospectively isolated and transplanted HSCs (Figure 4F), suggesting that the only cells with hematopoietic differentiation potential in the CD45intLin−SCA-1+FSChi cells are hematopoietic stem/progenitor cells.
Failure to detect hematopoietic lineage potential seriously calls into question the existence of pluripotent stem cells in CD45−Lin−SCA-1+ cells. However, this finding does not deny the existence of other multi- or oligopotent stem cells with other lineage potentials. For instance, Morikawa et al. (2009) reported that CD45−Lin−SCA-1+platelet-derived growth factor receptor α+ cells in adult mouse BM can give rise to mesenchymal stromal cells both in vitro and in vivo.
At the time of our writing this report, a few independent groups have replicated select aspects of VSEL: Bhartiya et al., 2012 reported VSEL-derived TSCs existed in mammalian ovary or in human BM and CB (Parte et al., 2011) and Sovalat et al. (2011) claimed VSELs could be isolated from human BM or granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood. However, all of these other groups evaluated only the existence of VSEL by the analysis of the cell size and immunophenotype without evaluating the pluripotency criteria, as criticized by the others in the field (Ivanovic, 2012). In fact, other recent reports failed to detect the stem cell properties of mouse (Szade et al., 2013) or human VSELs (Danova-Alt et al., 2012). When only one or a few markers of cells rather than the function of cells themselves are assayed, artifactual or nonphysiological expression of single markers can lead to the interpretation that a cell type rather than a marker is being studied. The only assays acceptable for functionally identifying stem cells are those that assess (1) self-renewal; (2) differentiation to clonal progeny of the cell types inferred from the name of the cell; and (3), for adult tissue outcomes, robust and sustained regeneration. The transfer of donor markers to host cells could occur by cell fusion and, theoretically, by exosome transfer, to the extent that exosomes are shown to be physiological entities and not cell culture artifacts.
We conclude that most CD45−/intLin−SCA-1+ cells in mouse BM were not as small as previously reported. Moreover, even despite broadening our search to include larger cells as candidates, we could not find a single instance of CD45−/intLin−SCA-1+ cells that (1) expressed Oct4, (2) proliferated to form spheres in cultures, or (3) demonstrated the ability to generate cells of the hematopoietic lineage—three functional aspects of pluripotency that were previously reported. These results suggest the absence of embryonic-like pluripotent cells in postnatal mouse BM.
Additional rigorous data would be needed to demonstrate their existence both in humans and mouse before clinical application, including the derivation of the same repertoire of normal tissue cells currently demonstrated with ESC and/or induced pluripotent stem cells.
Experimental Procedures
Mice
C57Bl/Ka-Thy1.2 Ly5.1 (B/Ka), C57Bl/Ka-Thy1.1 Ly5.1 (BA), C57Bl/Ka-Thy1.1 Ly5.1-Tg (actin-EGFP), and C57Bl/Ka-Thy1.1 Ly5.1-Tg (pH2K-BCL-2) mouse strains were derived and maintained in our laboratory. The Oct4-EGFP transgenic strain (Tg[Pou5f1-EGFP]2Mnn/J) was purchased from the Jackson Laboratory. The C57Bl/6J-W41/W41 strain was kindly provided by Dr. Susan L. Hall, Loma Linda University. We used 4–12-week-old female and male mice. All animal procedures were performed in accordance with the International Animal Care and Use Committee and the Stanford University Administrative Panel on Laboratory Animal Care.
Cell Preparation and Staining
BM cells harvested from bilateral femurs and tibias with a flushing method were treated with BD Pharm Lyse Buffer (BD PharMingen). These cells were stained for 30 min with phycoerythrin (PE)-conjugated lineage antibodies (Lin), which consisted of anti-CD45R/B220 (clone; RA3-6B2, final concentration; 4 μg/ml), anti-T cell receptor (TCR) β (H57-597, 4 μg/ml), anti-TCR γδ (GL3, 4 μg/ml), anti-Ly-6G/C (RB6-8C5, 8 μg/ml), anti-CD11b (M1/70, 4 μg/ml), and anti-Ter119 (TER-119, 4 μg/ml); allophycocyanin-Cy7-conjugated anti-CD45 (30-F11, 8 μg/ml); and biotin-conjugated anti-Ly6A/E (SCA-1) (E13-161.7, 10 μg/ml) followed by streptavidin-PE-Cy5 (1 μg/ml). All the antibodies were purchased from BD. One micromolar SYTO16 (Life Technologies) was added to evaluate DNA quantity.
Flow Cytometry
Sorting and analyses were performed on multilaser-equipped FACSAria II and, where otherwise indicated, on a MoFlo cell sorter or FlowSight image flow cytometer. Dead cells were distinguished by adding 1 μM of SYTOX-Blue dead cell stain (Life Technologies). To minimize contamination, a second round of sorting was performed. The automatic cell deposition system was used for single-cell assays. Data were analyzed with FlowJo software (Tree Star) or IDEAS software (Amnis).
Isolation of HSCs and Candidate VSELs
By comparing the FSC of defined-size microspheres (Flow Cytometry Size Calibration Kit, Life Technologies) and that of BM cells, we evaluated relative cell size. The SCA-1 channel was separated between negative and positive by the FMO method. We further divided Lin−SCA-1+ cells into three subfractions according to the CD45 expression level: the upper limit of the CD45− subpopulation was defined by the FMO method; the CD45hi subpopulation by the obvious positive peak; and the CD45int area as that between CD45− and CD45hi. HSCs and candidate VSELs were expected to reside in the CD45hiLin−SCA-1+FSChi and CD45−Lin−SCA-1+ (and/or CD45intLin−SCA-1+) FSClo populations, respectively.
Sphere Formation Culture
A C2C12 mouse myoblast-derived cell line was maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% fetal calf serum (FCS). For the sphere formation assays, freshly isolated 1 × 103 BM Lin−SCA-1+ cells from Actin-EGFP mice were: (1) plated on the 1 × 105 C2C12 cells seeded the previous day on a 24 well plate and (2) cultured for 8 days in DMEM with 2% FCS. Half of the culture media was changed every day. The grown cells/clusters were trypsinized, harvested, passed through a 100 μm filter, and analyzed by FACSAria. The cells were also observed by fluorescence microscopy (DMI 6000B, Leica Microsystems).
Nonfeeder Culture for Hematopoietic Differentiation
For liquid cultures, purified populations were suspended in 12 well plates with the following medium: Iscove’s modified Dulbecco’s medium (IMDM; Life Technologies) supplemented with 20% FCS, antibiotics, 10 ng/ml of mouse (m) recombinant interleukin 3 (IL-3), 10 ng/ml mouse stem cell factor (SCF), and 10 ng/ml mouse Flt3 ligand (R&D Systems). For clonogenic analyses of CD45hiLin−SCA-1+ (including HSCs) and CD45−/intLin−SCA-1+ (including VSELs) faction, cells were cultured 10 days in IMDM-based methylcellulose medium (Methocult GF M3434; StemCell Technologies), which contained FCS, bovine serum albumin, 2-mercaptoethanol, recombinant human (h) insulin and transferrin, as well as recombinant mSCF, mIL-3, hIL-6, and human erythropoietin. All cultures were incubated in a humidified chamber in 5% CO2. Colonies were scored and picked up for making cytospin preparations to define cell components.
OP9 Coculture Assay
OP9 coculture was performed as previously reported (Ratajczak et al., 2011). OP9 cells were maintained in α-MEM (MEMα Nucleosides Powder, Life Technologies) with 10% FCS. Freshly isolated Lin−SCA-1+ cells (CD45−, CD45int, or CD45hi) were plated on OP9 cells in 12 well plates with α-MEM plus 20% FCS and cultured for 5 days. Cultured cells were then trypsinized, centrifuged, and replated in methylcellulose medium with OP9 cells. For serial passage, methylcellulose cultures were trypsinized, centrifuged, and replated in new methylcellulose medium. Colonies were scored and picked up to make cytospin preparations and stained by the May-Giemsa method.
Purification of Long-Term HSCs and Transplantation
BM mononuclear cells from actin-EGFP mice were enriched for c-kit+ cells by using anti-c-kit microbeads (Miltenyi Biotec) and sorted for propidium iodide−Lin(CD3/CD4/CD8/Mac-1/Gr-1/B220/CD19/Ter119)−CD34−CD150+Flk2−c-kit+SCA-1+ long-term HSCs. Eight-week-old W41/W41-recipient mice (Reith et al., 1990), hematopoietic-deficient due to mutations in the W locus encoding the c-kit gene, received 400 cGy total body irradiation followed by intravenous injection of 100 sorted long-term HSCs via the retro-orbital vein. Peripheral blood and BM cells were analyzed by flow cytometry after transplantation to evaluate chimerism.
RNA Extraction and Real-Time RT-PCR
Prior to quantitative RT-PCR, 1 × 103 of each of the following cell types were sorted by flow cytometry: CD45−/intLin−SCA-1+SYTO16lo, CD45−/intLin−SCA-1+SYTO16hi, CD45hiLin−SCA-1+SYTO16hi, and SSEA-1hi ESCs. RNA extraction, reverse transcription, and PCR amplification were performed by using CellsDirect One-Step qRT-PCR Kit (Life Technologies) according to the manufacturer’s instructions. Gene-specific products were monitored by the 7900HT Fast Real-Time PCR System (Life Technologies). Four different design of primer pairs for Oct4 messenger RNA were used: (1) designed by Niwa group (Toyooka et al., 2008), (2 and 3) designed by Ratajczak group (Kucia et al., 2006a; Liu et al., 2009), and (4) TaqMan probe set Mm.PT.51.7439100.g (Integrated DNA Technologies). Primer sequences and amplicon sizes are listed in Table S2.
Statistical Analysis
The nonparametric Mann-Whitney test was applied to all comparisons of mean values after F-test evaluation of variances. All statistical analyses were performed on Prism 5 software (GraphPad).
Acknowledgments
We thank L. Jerabek and T. Storm for laboratory management; T.J. Naik for technical assistance; A. Mosley, C. Wang, and A. McCarty for animal care; Dr. S.L. Hall, Loma Linda University, for providing W41/W41 mice; and J. Ooehara, Y. Yamazaki, and M. Otsu for supporting experiments of side-by-side comparison between MoFlo and FACSAria.
This study was supported by fellowships from the Toyobo Biotechnology Foundation, the Uehara Memorial Foundation, Human Frontier Science Program (to M.M.), and the Japan Society for the Promotion of Science (to Y.M.) and by grants from the US National Institutes of Health (R01 CA086065 and U01 HL099999 to I.L.W.), the California Institute for Regenerative Medicine (RT2-02060 to I.L.W.), and the Leukemia & Lymphoma Society (700709 to I.L.W.). H.N. is a shareholder and a member of the scientific advisory board of ReproCELL and Megakaryon and a member of the scientific advisory board of Shionogi. I.L.W. is a member of the scientific advisory board of StemCells.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Begley C.G., Ellis L.M. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- Beltrami A.P., Cesselli D., Bergamin N., Marcon P., Rigo S., Puppato E., D’Aurizio F., Verardo R., Piazza S., Pignatelli A. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bhartiya D., Shaikh A., Nagvenkar P., Kasiviswanathan S., Pethe P., Pawani H., Mohanty S., Rao S.G., Zaveri K., Hinduja I. Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev. 2012;21:1–6. doi: 10.1089/scd.2011.0311. [DOI] [PubMed] [Google Scholar]
- Check E. Stem cells: the hard copy. Nature. 2007;446:485–486. doi: 10.1038/446485a. [DOI] [PubMed] [Google Scholar]
- Danova-Alt R., Heider A., Egger D., Cross M., Alt R. Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS ONE. 2012;7:e34899. doi: 10.1371/journal.pone.0034899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domen J., Gandy K.L., Weissman I.L. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- Drukała J., Paczkowska E., Kucia M., Młyńska E., Krajewski A., Machaliński B., Madeja Z., Ratajczak M.Z. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2012;8:184–194. doi: 10.1007/s12015-011-9272-4. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N., Singha P.K., Woodruff K., St Clair P., Bsoul S., Werner S.L., Choudhury G.G. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J. Biol. Chem. 2006;281:20160–20170. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- Hall S.L., Lau K.H., Chen S.T., Felt J.C., Gridley D.S., Yee J.K., Baylink D.J. An improved mouse Sca-1+ cell-based bone marrow transplantation model for use in gene- and cell-based therapeutic studies. Acta Haematol. 2007;117:24–33. doi: 10.1159/000096785. [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A., Tung J., Moore W.A., Herzenberg L.A., Parks D.R. Interpreting flow cytometry data: a guide for the perplexed. Nat. Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- Ivanovic Z. Human umbilical cord blood-derived very-small-embryonic-like stem cells with maximum regenerative potential? Stem Cells Dev. 2012;21:2561–2562. doi: 10.1089/scd.2012.0058. author reply 2563–2564. [DOI] [PubMed] [Google Scholar]
- Jasny B.R., Chin G., Chong L., Vignieri S. Data replication & reproducibility. Again, and again, and again.... Introduction. Science. 2011;334:1225. doi: 10.1126/science.334.6060.1225. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kassmer S.H., Jin H., Zhang P.X., Bruscia E.M., Heydari K., Lee J.H., Kim C.F., Krause D.S. Very Small Embryonic-Like Stem Cells from the Murine Bone Marrow Differentiate into Epithelial Cells of the Lung. Stem Cells. 2013 doi: 10.1002/stem.1413. Published online May 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögler G., Sensken S., Airey J.A., Trapp T., Müschen M., Feldhahn N., Liedtke S., Sorg R.V., Fischer J., Rosenbaum C. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D.S., Theise N.D., Collector M.I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S.J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kucia M., Reca R., Campbell F.R., Zuba-Surma E., Majka M., Ratajczak J., Ratajczak M.Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M., Zhang Y.P., Reca R., Wysoczynski M., Machalinski B., Majka M., Ildstad S.T., Ratajczak J., Shields C.B., Ratajczak M.Z. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- Kucia M., Wysoczynski M., Ratajczak J., Ratajczak M.Z. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- Liu Y., Gao L., Zuba-Surma E.K., Peng X., Kucia M., Ratajczak M.Z., Wang W., Enzmann V., Kaplan H.J., Dean D.C. Identification of small SCA-1(+), Lin(-), CD45(-) multipotential cells in the neonatal murine retina. Exp. Hematol. 2009;37:1096–1107. doi: 10.1016/j.exphem.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Mabuchi Y., Kubota Y., Nagai Y., Niibe K., Hiratsu E., Suzuki S., Miyauchi-Hara C., Nagoshi N., Sunabori T. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Kodama H., Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Parte S., Bhartiya D., Telang J., Daithankar V., Salvi V., Zaveri K., Hinduja I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011;20:1451–1464. doi: 10.1089/scd.2010.0461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ratajczak M.Z., Machalinski B., Wojakowski W., Ratajczak J., Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Ratajczak M.Z., Zuba-Surma E.K., Machalinski B., Ratajczak J., Kucia M. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev. 2008;4:89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- Ratajczak M.Z., Zuba-Surma E.K., Shin D.M., Ratajczak J., Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp. Gerontol. 2008;43:1009–1017. doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J., Wysoczynski M., Zuba-Surma E., Wan W., Kucia M., Yoder M.C., Ratajczak M.Z. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp. Hematol. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A.D., Rottapel R., Giddens E., Brady C., Forrester L., Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Rieger M.A., Hoppe P.S., Smejkal B.M., Eitelhuber A.C., Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Seiler K., Soroush Noghabi M., Karjalainen K., Hummel M., Melchers F., Tsuneto M. Induced pluripotent stem cells expressing elevated levels of sox-2, oct-4, and klf-4 are severely reduced in their differentiation from mesodermal to hematopoietic progenitor cells. Stem Cells Dev. 2011;20:1131–1142. doi: 10.1089/scd.2010.0391. [DOI] [PubMed] [Google Scholar]
- Serafini M., Dylla S.J., Oki M., Heremans Y., Tolar J., Jiang Y., Buckley S.M., Pelacho B., Burns T.C., Frommer S. Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J. Exp. Med. 2007;204:129–139. doi: 10.1084/jem.20061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.M., Liu R., Klich I., Wu W., Ratajczak J., Kucia M., Ratajczak M.Z. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- Sovalat H., Scrofani M., Eidenschenk A., Pasquet S., Rimelen V., Hénon P. Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/ Lin(-)CD45(-) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp. Hematol. 2011;39:495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Spangrude G.J., Heimfeld S., Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Szade K., Bukowska-Strakova K., Nowak W.N., Szade A., Kachamakova-Trojanowska N., Zukowska M., Jozkowicz A., Dulak J. Murine Bone Marrow Lin(-)Sca-1(+)CD45(-) Very Small Embryonic-Like (VSEL) Cells Are Heterogeneous Population Lacking Oct-4A Expression. PLoS ONE. 2013;8:e63329. doi: 10.1371/journal.pone.0063329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- Uchida N., Weissman I.L. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J. Exp. Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers A.J., Weissman I.L. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wagers A.J., Sherwood R.I., Christensen J.L., Weissman I.L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Weissman I.L. In: Stem Cell Technology and Other Innovative Therapies. Sorondo M.S., Le Douarin N.M., editors. Pontifical Academia Scientiarum; Vatican City: 2007. pp. 49–88. [Google Scholar]
- Wojakowski W., Tendera M., Kucia M., Zuba-Surma E., Paczkowska E., Ciosek J., Hałasa M., Król M., Kazmierski M., Buszman P. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma E.K., Kucia M., Abdel-Latif A., Dawn B., Hall B., Singh R., Lillard J.W., Jr., Ratajczak M.Z. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J. Cell. Mol. Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma E.K., Kucia M., Rui L., Shin D.M., Wojakowski W., Ratajczak J., Ratajczak M.Z. Fetal liver very small embryonic/epiblast like stem cells follow developmental migratory pathway of hematopoietic stem cells. Ann. N Y Acad. Sci. 2009;1176:205–218. doi: 10.1111/j.1749-6632.2009.04562.x. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma E.K., Guo Y., Taher H., Sanganalmath S.K., Hunt G., Vincent R.J., Kucia M., Abdel-Latif A., Tang X.L., Ratajczak M.Z. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J. Cell. Mol. Med. 2011;15:1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


