Abstract
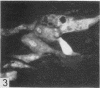
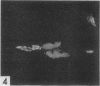
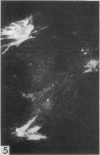
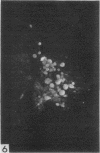
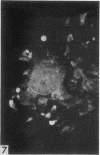
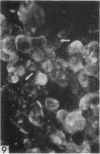
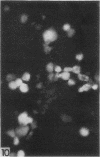
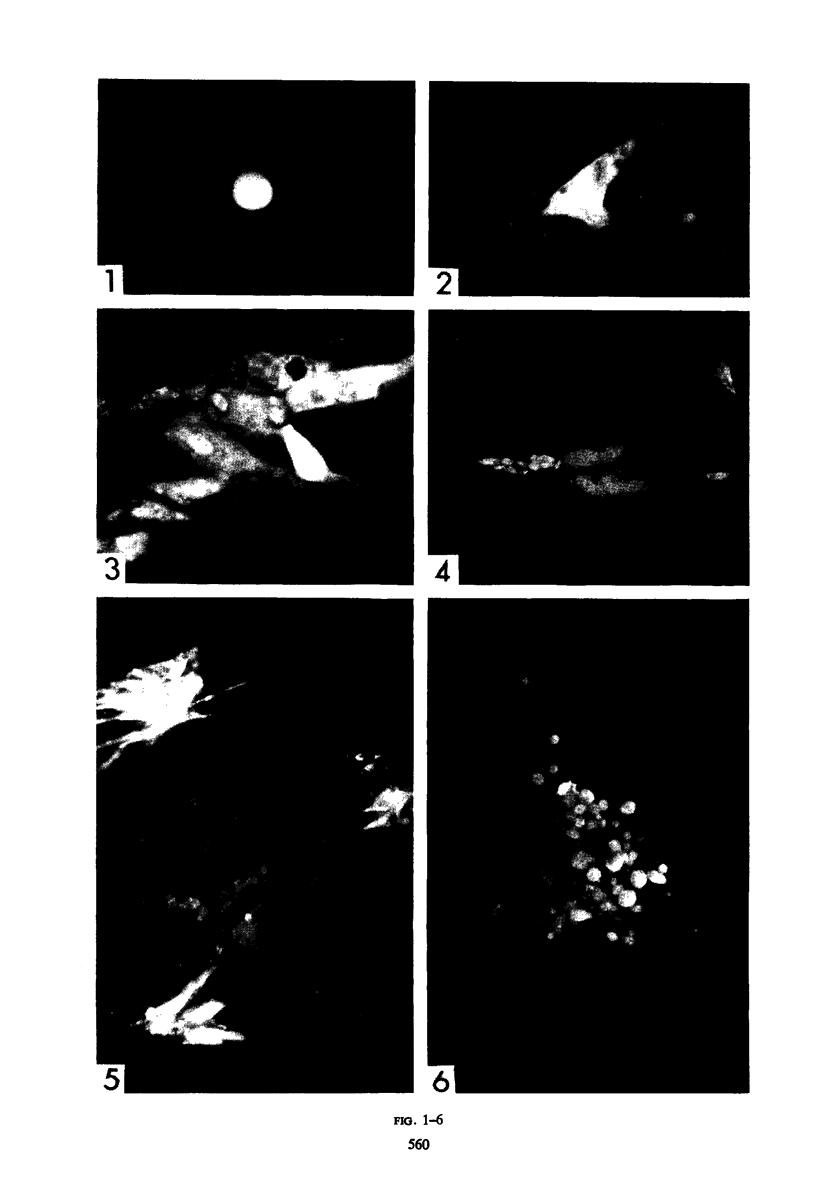
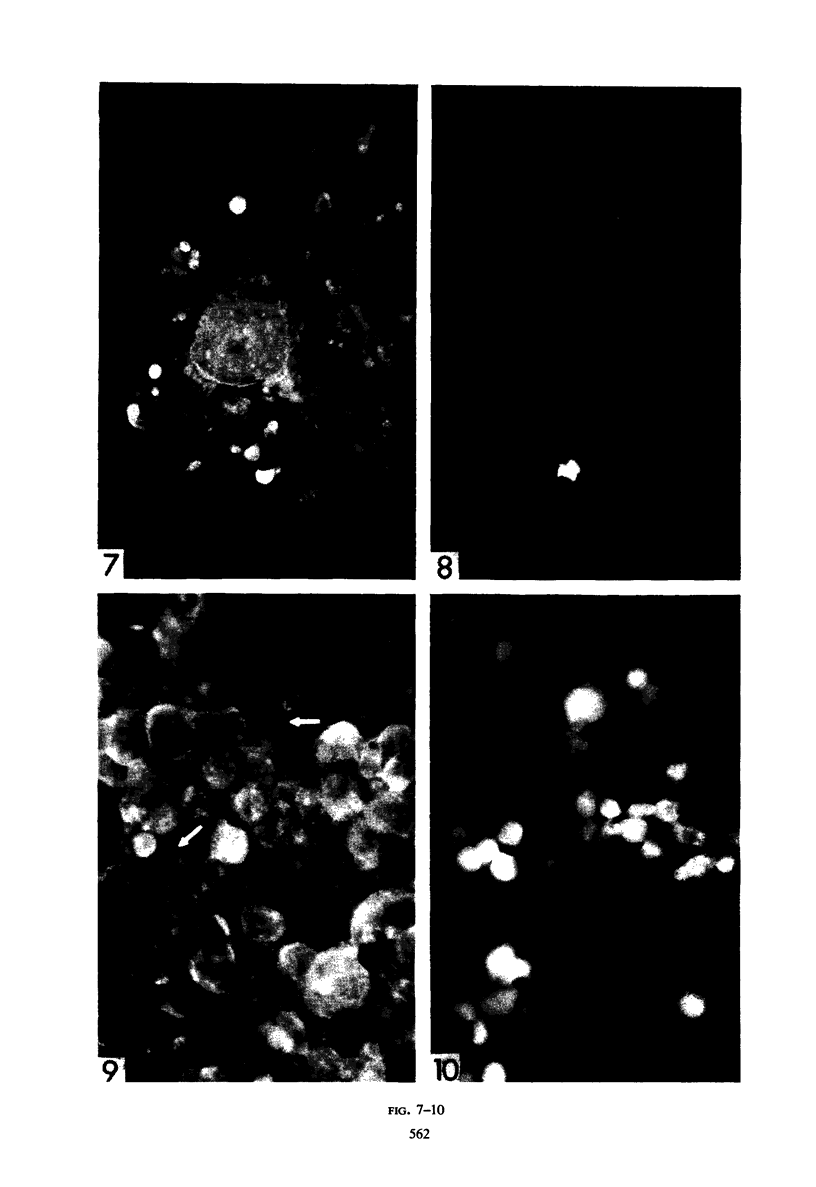
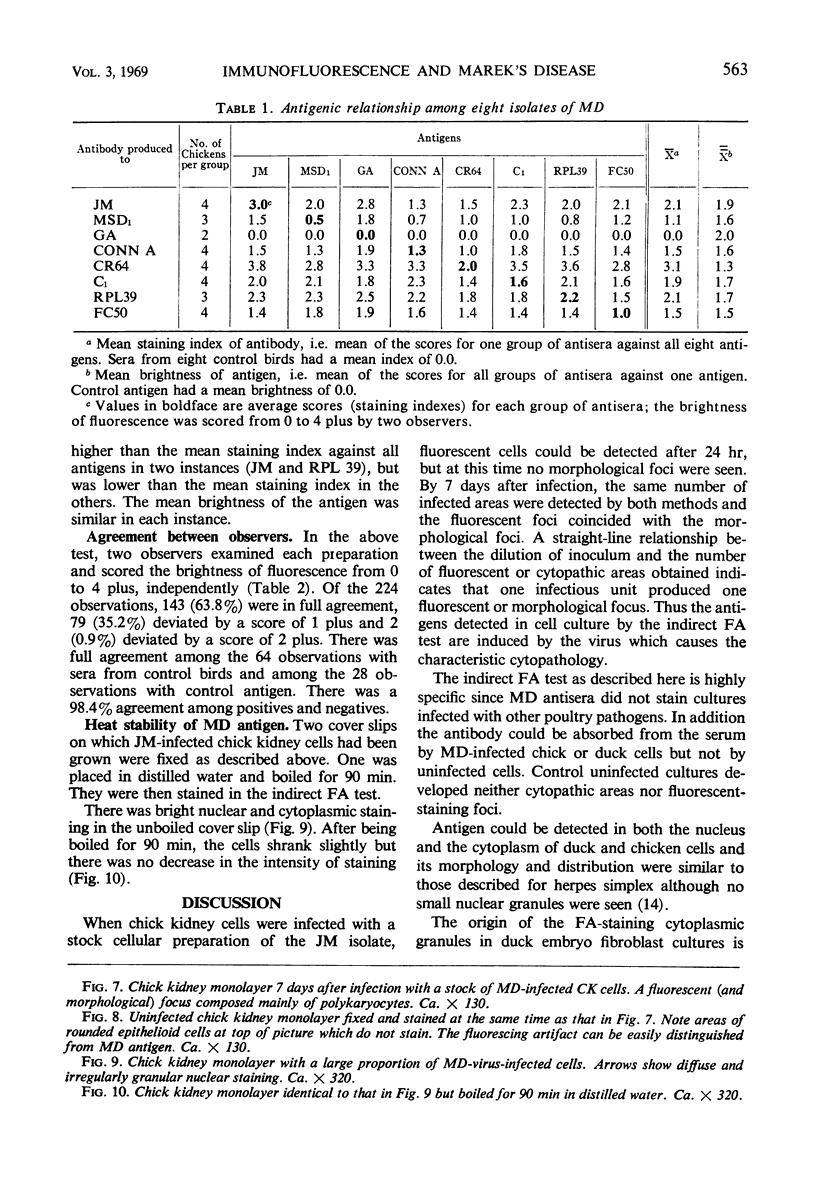
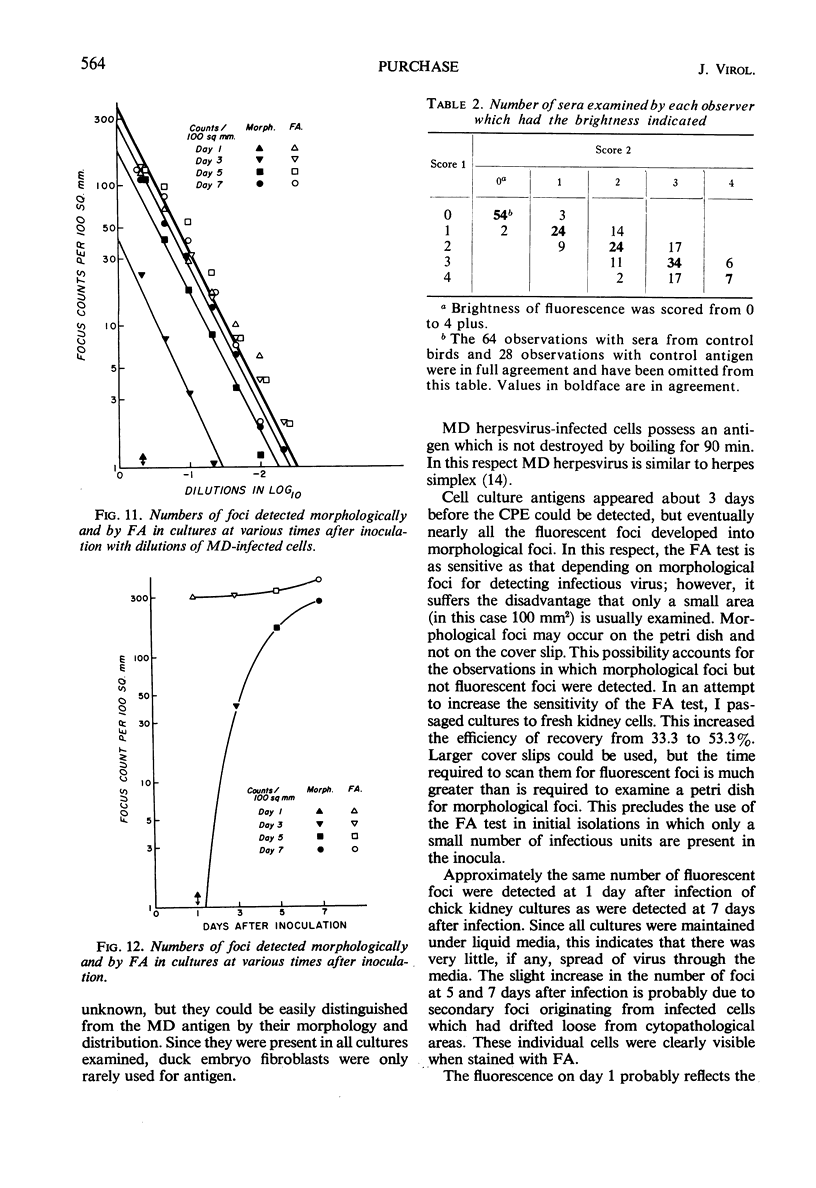
The indirect fluorescent-antibody (FA) test was applied to the detection of Marek's disease (MD) antigen in cell culture and antibody in the serum of birds. For the detection of antigen, sera were obtained from birds hyperimmunized with the JM strain of MD. MD antigen could be detected in the nucleus and in the cytoplasm of duck and chick embryo fibroblasts and in those of chick kidney cells infected with material known to contain the MD virus. Uninoculated cultures of chicken cells were always free of MD antigen. When chick kidney cells were infected with a stock cellular preparation of MD virus, infected cells could be detected after 24 hr with the FA test. At this time no cytopathological areas were seen by conventional light microscopy. By 7 days after infection, the same number of infected areas were detected by both methods, and the fluorescent areas coincided with the cytopathological areas. This indicates that the fluorescent areas and the areas with cytopathology are caused by the same agent. A straight-line relationship between the dilution of inoculum and the number of fluorescent or morphological foci obtained indicates that one infectious unit produced one fluorescent or morphological focus. In addition, this time sequence study confirmed the cell association of the virus and demonstrated the cell-to-cell spread of infection. Cell cultures inoculated with eight different isolates of MD were tested in all combinations with sera prepared against the same isolates. The antigens were indistinguishable from one another, indicating that either the strains are antigenically identical or there is a common antigen or contaminant in all of them so that they stained equally well. The FA test can detect MD antigen before cytopathological areas develop in cell culture; however, the small size of the area usually examined precludes its use in initial isolations in which only a small number of infectious units are present in the inoculum. MD-infected cells contain a heat-stable antigen similar to that found in herpes simplex-infected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomiak T. W., Luginbuhl R. E., Helmboldt C. F., Kottaridis S. D. Marek's disease. I. Propagation of the Connecticut A (Conn-A) isolate in chicks. Avian Dis. 1967 Nov;11(4):646–653. [PubMed] [Google Scholar]
- Churchill A. E., Biggs P. M. Agent of Marek's disease in tissue culture. Nature. 1967 Jul 29;215(5100):528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B. Avian tumor viruses: prospects for control. Worlds Poult Sci J. 1968 Jan-Mar;24(1):18–36. doi: 10.1079/wps19680006. [DOI] [PubMed] [Google Scholar]
- Culling C. F. Permanent mounting method for fluorescent antibody preparations. Nature. 1967 Jun 10;214(5093):1140–1140. doi: 10.1038/2141140a0. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY R. M. Use of dimethyl sulphoxide for preservation of tissue culture cells by freezing. Nature. 1962 Feb 10;193:550–552. doi: 10.1038/193550a0. [DOI] [PubMed] [Google Scholar]
- Eidson C. S., Schmittle S. C. Studies on acute Marek's disease. I. Characteristics of isolate GA in chickens. Avian Dis. 1968 Aug;12(3):467–476. [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Kottaridis S. D., Luginbuhl R. E. Marek's disease. 3. Immunofluorescent studies. Avian Dis. 1968 Aug;12(3):383–393. [PubMed] [Google Scholar]
- Nazerian K., Solomon J. J., Witter R. L., Burmester B. R. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proc Soc Exp Biol Med. 1968 Jan;127(1):177–182. doi: 10.3181/00379727-127-32650. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spring S. B., Roane P. R., Jr Cellular compartmentalization of herpesvirus antigens during viral replication. J Virol. 1967 Feb;1(1):181–192. doi: 10.1128/jvi.1.1.181-192.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J. J., Witter R. L., Nazerian K., Burmester B. R. Studies on the etiology of Marek's disease. I. Propagation of the agent in cell culture. Proc Soc Exp Biol Med. 1968 Jan;127(1):173–177. doi: 10.3181/00379727-127-32649. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Burgoyne G. H., Solomon J. J. Evidence for a herpesvirus as an etiologic agent of marek's disease. Avian Dis. 1969 Feb;13(1):171–184. [PubMed] [Google Scholar]
- Witter R. L., Burgoyne G. H., Solomon J. J. Preliminary studies on cell cultures infected with Marek's disease agent. Avian Dis. 1968 Feb;12(1):169–185. [PubMed] [Google Scholar]
- Witter R. L., Burmester B. R. Transmission of Marek's disease with oral washings and feces from infected chickens. Proc Soc Exp Biol Med. 1967 Jan;124(1):59–62. doi: 10.3181/00379727-124-31666. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Solomon J. J., Burgoyne G. H. Cell culture techniques for primary isolation of Marek's disease-associated herpesvirus. Avian Dis. 1969 Feb;13(1):101–118. [PubMed] [Google Scholar]