Abstract
Background
Cervical vagal nerve (CVN) stimulation may improve left ventricular ejection fraction in patients with heart failure.
Objectives
To test the hypothesis that sympathetic structures are present in the CVN and to describe the location and quantitate these sympathetic components of the CVN.
Methods
We performed immunohistochemical studies of the CVN from 11 normal dogs and simultaneously recorded stellate ganglion nerve activity, left thoracic vagal nerve activity, and subcutaneous electrocardiogram in 2 additional dogs.
Results
A total of 28 individual nerve bundles were present in the CVNs of the first 11 dogs, with an average of 1.87 ± 1.06 per dog. All CVNs contain tyrosine hydroxylase-positive (sympathetic) nerves, with a total cross-sectional area of 0.97 ± 0.38 mm2. The sympathetic nerves were nonmyelinated, typically located at the periphery of the nerve bundles and occupied 0.03%–2.80% of the CVN cross-sectional area. Cholineacetyltransferase-positive nerve fibers occupied 12.90%–42.86% of the CVN cross-sectional areas. Ten of 11 CVNs showed tyrosine hydroxylase and cholineacetyltransferase colocalization. In 2 dogs with nerve recordings, we documented heart rate acceleration during spontaneous vagal nerve activity in the absence of stellate ganglion nerve activity.
Conclusions
Sympathetic nerve fibers are invariably present in the CVNs of normal dogs and occupy in average up to 2.8% of the cross-sectional area. Because sympathetic nerve fibers are present in the periphery of the CVNs, they may be susceptible to activation by electrical stimulation. Spontaneous activation of the sympathetic component of the vagal nerve may accelerate the heart rate.
Keywords: Cervical vagus nerves, Sympathetic nerves, Ganglion cells, Heart failure, Vagal nerve stimulation
Introduction
Cervical vagal nerve (CVN) stimulation has been available for the treatment of resistant partial-onset seizures in Europe since 1994 and in the United States since 1997.1–4 Animal studies in heart failure models have shown improvement in left ventricular ejection fraction, ventricular arrhythmia suppression, and survival with CVN stimulation.5–8 The early results of a human heart failure clinical trial showed that CVN stimulation improves left ventricular ejection fraction and New York Heart Association functional class for up to 1-year follow-up.9 However, in the same study, CVN stimulation was associated with 26 serious adverse events that occurred in 13 of 32 (40.6%) patients, including deaths and cardiac arrhythmias. In addition to heart failure therapy, the clinicaltrials.gov Web site lists a number of active clinical trials that use CVN for other cardiac and noncardiac diseases. Two of these ongoing trials involve CVN stimulation in patients with heart failure. While there is much enthusiasm for CVN in treating human diseases, only limited information is available regarding the anatomy of the CVN. Foly and DuBois10 have shown that within felines the CVN is 65%–80% sensory and 20%–35% motor. On average, there are more nerve fibers on the left CVN than on the right CVN. Agostoni et al11 state that myelinated nerves compose about 16% of the CVN. While the CVN is largely considered to be a parasympathetic structure that sends fibers to numerous parts of the body (heart, esophagus, and stomach),12 previous research has noted a sympathetic component.13–18 Only one of these studies used specific immunohistochemical markers to identify the sympathetic nerves in the CVN.18 This single study reported the presence of tyrosine hydroxylase (TH)-positive structures in human vagus nerves, but the study did not provide quantitative information. Studies from our laboratory recently confirmed these findings by documenting the presence of both TH-positive nerve fibers and ganglion cells in the CVN of dogs that underwent rapid pacing to induce atrial fibrillation.19 However, because rapid atrial pacing is known to cause cardiac sympathetic nerve sprouting and sympathetic hyperinnervation,20,21 these observations may not be applicable to dogs without rapid atrial pacing. That study did not quantify the amount of TH-positive nerves in the canine CVN. The purpose of the present study was to perform immunohistochemical studies on canine CVN to test the hypothesis that sympathetic nerves are invariably present in all CVN and that the location of these nerves makes them susceptible to activation by CVN stimulation. A secondary aim is to provide quantitative information on the amount of sympathetic nerve fibers in the CVN. We also recorded stellate ganglion nerve activity (SGNA) and thoracic vagal nerve activity (VNA) in ambulatory dogs to document that VNA alone can be associated with heart rate acceleration.
Methods
Histological studies of the CVN
We studied CVN from 11 normal healthy dogs weighing 25–30 kg. Both the right and left CVNs were available for analysis from 4 dogs, while from additional 7 dogs, 4 right and 3 left CVNs were available for study. A total of 8 right and 7 left nerves were analyzed. The nerves were fixed with 4% formalin for 45–60 minutes before transfer to 70% ethyl alcohol.22 CVN sections from formalin-fixed, paraffinembedded, 5-μm-thick cross sections were stained with TH for sympathetic (adrenergic) nerves23 and cholineacetyltransferase (ChAT) for acetylcholine-positive (cholinergic) nerves.24 Because acetylcholine is a neural transmitter for both motor and parasympathetic nerves, the ChAT stain is not specific to parasympathetic nerves. Luxol Fast Blue was used to identify myelinated nerves.25 The programs Adobe Photoshop CS5.1 and Magic Wand were used to localize the TH- and ChAT-positive portions and calculate their cross-sectional areas, respectively. These data were expressed as mean ± SD. A difference comparison was performed by using paired t tests. A P value of ≤.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS Statistics 19.
Ambulatory monitoring of vagal nerve activities
Two male mongrel dogs (weighing 29 and 26 kg, respectively) were used in this part of the study. First (sterile) surgeries were performed under isoflurane inhalation general anesthesia. Left thoracotomy was performed through the third intercostal space for the implantation of a radiotransmitter (D70-EEE, Data Sciences International, St Paul, MN) according to methods described elsewhere.26 The first pair of electrodes was inserted beneath the fascia of the left stellate ganglion. A second pair of bipolar leads was attached to the left thoracic vagal nerve at the level 2–4 cm above the aortic arch. A third pair of bipolar electrodes was used to record subcutaneous electrocardiogram, with 1 electrode each inserted under the subcutaneous space of left thorax and left lower abdomen. The transmitter and remaining wires were inserted into a subcutaneous pocket. The original study design was to use these dogs to develop a model of pacing-induced heart failure. Therefore, a pacing lead was fixed to the right ventricular free wall and connected to an Itrel neurostimulator (Medtronic, Minneapolis, MN) in a subcutaneous pocket. The chest wall was then closed. After 2 weeks of recovery, the radiotransmitter was turned on to record nerve signals and electrocardiograms at baseline. The dogs then underwent intermittent rapid ventricular pacing to induce heart failure. The tissues were then harvested for analyses. The nerve recordings obtained during baseline state (prior to rapid pacing) were analyzed and included in this report. The data from these 2 dogs were not included in any previous report from our laboratory.
Results
Histological studies of CVN
As shown in Figure 1A, each CVN may contain more than 1 nerve bundle. There are, on average, 1.87 ± 1.06 distinctly isolated nerve bundles per CVN (range 1–5 bundles). Among a total of 28 individual nerve bundles, 25 contained sympathetic components. Figures 1B and 1D show TH-positive nerves located in the upper edge of the small and large bundles, respectively. Figures 1C and 1E show ChAT stains. The ChAT-positive nerve structures were widely distributed in the vagus nerves. While the TH-positive nerve structures were typically present at the periphery of the nerve bundles, occasionally they were also seen in the center of the nerve bundles (Figure 1F, arrow).
Figure 1.
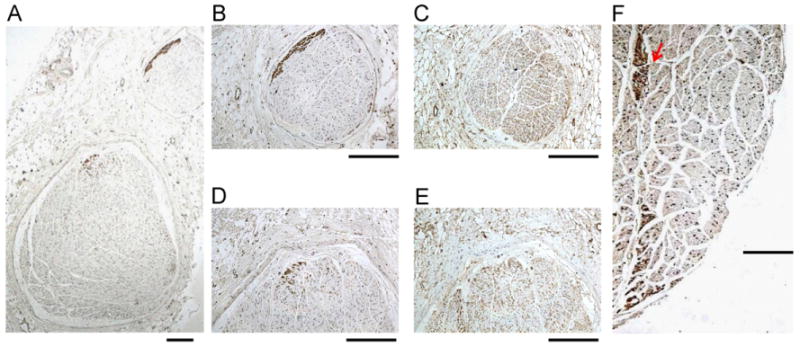
Tyrosine hydroxylase (TH) and cholineacetyltransferase (ChAT) staining of the cervical vagus nerves (CVNs). A: A low power view of the right CVN stained with TH. There are 2 distinct nerve bundles in this nerve. B, D: The TH stain of the smaller (B) and the larger (D) bundles. The brown color identifies the positively stained nerves. Note that TH-positive nerves are located in the periphery of the nerve bundle. C, E: ChAT staining of the same structures as in panels B and D, respectively. Note that ChAT-positive components are widely distributed in the CVN. F: The TH-positive nerve structure (red arrow) in the middle of the CVN. The objective lens used in panel A was 4×, with a calibration bar of 0.2 mm in length. The objective lens used in panels B–F was 20×, with a calibration bar of 0.2 mm in length.
Figure 2 illustrates the methods used for analyzing the CVN. Figure 2A shows the TH-positive nerve that was located in the periphery of both nerve bundles. The TH-positive nerve structures (Figure 2B) and the total cross-sectional area of the CVN (Figure 2C) were identified, and the red color was used to indicate the positive identification. The ratio between the red area in Figure 2B and the red area in Figure 2C was used to find the percentage of TH-positive nerve structures in the CVN. The same methods were used to identify the percentage of ChAT-positive nerves in the CVN.
Figure 2.
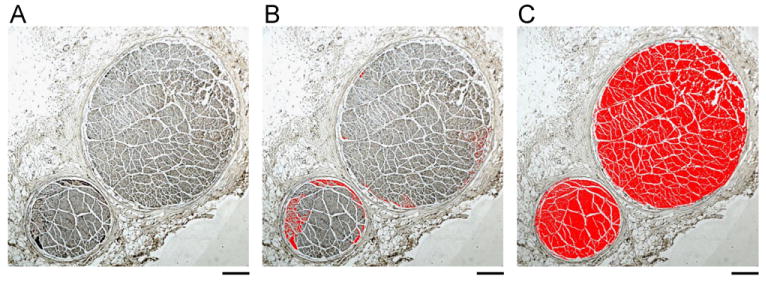
Quantification of the tyrosine hydroxylase (TH)-positive nerves. A: A TH-stained left cervical vagus nerve (CVN). B: The TH-positive nerves identified by the software (red). C: The total nerve cross-sectional area as identified by the software. Only the area colored red was included as the nerve bundles. The area of panel B divided by the area of panel C was used to find the percentage of sympathetic fibers in the CVN. The objective lens in panels A, B, and C was 4×, with a calibration bar of 0.2 mm in length.
Table 1 summarizes the results of all dogs. All CVN were found to have a sympathetic nerve component. The total surface area of the cross section measured 0.97 ± 0.38 mm2 (0.37–2.02 mm2). The sympathetic nerves (TH-positive nerve fibers) comprise 1.19% ± 0.87% (0.03%–2.80%) of its CVN cross-sectional area. The left CVN has an average cross-sectional surface area of 1.02 ± 0.50 mm2 (0.37–2.02 mm2) and a sympathetic component of 1.39% ± 1.09% (0.10%–2.80%). The rght CVN has an average cross-sectional surface area of 0.93 ± 0.27 mm2 (0.51–1.33 mm2) and a sympathetic component of 1.02% ± 0.65% (0.03%–2.00%). All TH-positive nerves were Luxol Fast Blue-negative, indicating that they are nonmyelinated sympathetic nerve fibers (Figure 3). The cholinergic nerves occupy 25.70% ± 10.00% (12.90%– 42.86%) of its CVN cross-sectional surface area. The left CVN has a cholinergic component of 19.20% ± 6.62% (12.90%– 31.29%), And the right CVN has a cholinergic component of 31.38% ± 9.09% (14.33%–42.86%).
Table 1. Cervical vagus nerves of normal canines.
| Dogs (N = 11) | Average nerve cross-sectional area* (mm2) | Bundles per Nerve (L, R) | Average TH% per nerve | Average ChAT% per nerve |
|---|---|---|---|---|
| 1 | 0.82 | (–, 3) R | 0.03 | 32.30 |
| 2 | 0.93 | (1, –) L | 0.36 | 14.80 |
| 3 | 0.88 | (–, 2) R | 0.37 | 27.77 |
| 4 | 1.05 | (1, –) L | 0.50 | 12.90 |
| 5 | 0.88 | (–, 2) R | 0.81 | 33.24 |
| 6 | 0.89 | (2, 1) Both | 0.91 | 24.98 |
| 7 | 0.87 | (–, 2) R | 1.16 | 42.06 |
| 8 | 0.78 | (5, 2) Both | 1.20 | 35.89 |
| 9 | 1.06 | (2, 1) Both | 1.71 | 13.77 |
| 10 | 1.67 | (1, 1) Both | 2.09 | 22.01 |
| 11 | 0.37 | (2, –) L | 2.80 | 23.86 |
ChAT = cholineacetyltransferase; L = left; R = right; TH = tyrosine hydroxylase.
Bundle cross-sectional areas were added together to give the total area of the nerve. The nerve size was then averaged between the right and the left nerve for each dog to find the average nerve size per dog. The same process was done for Average TH% and ChAT%.
Figure 3.
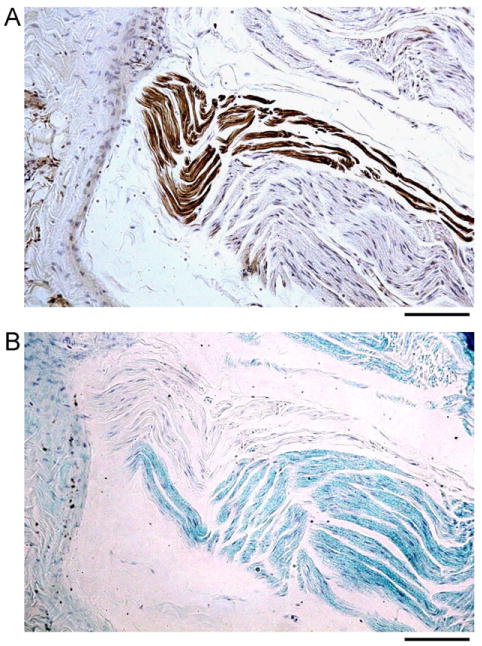
Tyrosine hydroxylase (TH) and Luxol Fast Blue (LFB) staining. A, B: A right cervical vagus nerve (CVN) cut longitudinally and stained with TH (brown) and LFB (blue), respectively. TH-positive portions of the nerve (brown color in panel A) were LFB negative in panel B, indicating that TH-positive nerves are nonmyelinated. The objective lens in panels A and B was 20×, with a calibration bar of 0.1 mm in length.
In the 4 dogs that had both left and right CVNs available for the analysis, the percentage of sympathetic nerves between the right and the left CVN was variable. The right CVN may have less, similar, or more TH-positive nerves than the left CVN (Table 2). There was a trend toward a higher percentage of ChAT-positive nerves in the right CVN than in the left CVN (Table 2), but the difference did not reach statistical significance (P = .056).
Table 2. Comparing left and right CVNs in 4 dogs.
| Dogs (N = 4) | Left TH% | Left ChAT% | Left nerve area (mm2) | Right TH% | Right ChAT% | Right nerve area (mm2) | Average TH% | Average ChAT% |
|---|---|---|---|---|---|---|---|---|
| 6 | 0.10 | 18.89 | 0.93 | 1.73 | 32.08 | 0.85 | 0.91 | 24.98 |
| 8 | 1.41 | 31.29 | 1.06 | 0.99 | 42.86 | 0.51 | 1.20 | 35.89 |
| 9 | 2.37 | 13.21 | 0.80 | 1.04 | 14.33 | 1.33 | 1.71 | 13.77 |
| 10 | 2.18 | 19.45 | 2.02 | 2.00 | 26.44 | 1.32 | 2.09 | 22.01 |
ChAT = cholineacetyltransferase; CVN = cervical vagus nerve; TH = tyrosine hydroxylase.
Ganglia (N = 17) were observed in 4 specimens. We attempted to match the cells in serial tissue sections, as shown in Figure 4. Among the 17 cells identified, 12 stained positive for both TH and ChAT while the other 5 were TH-negative. Among the TH-negative ganglion cells, 2 were ChAT-positive. The remaining 3 TH-negative cells were not identified on the ChAT-stained slides. Thus, it was unclear whether those TH-negative cells were ChAT-positive.
Figure 4.
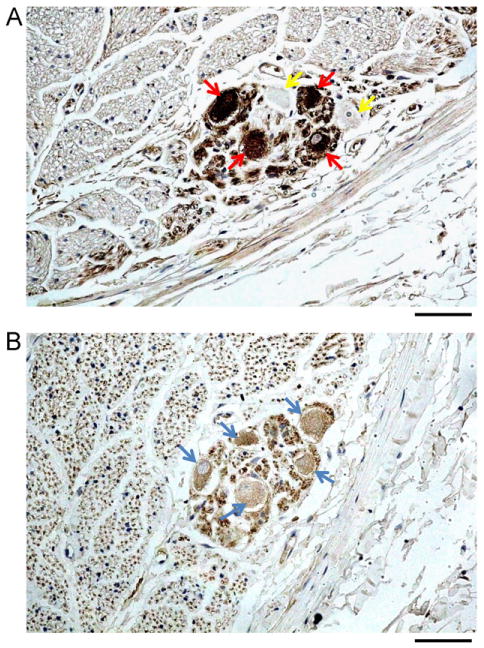
Tyrosine hydroxylase (TH) and cholineacetyltransferase (ChAT) staining of an autonomic ganglion in the left cervical vagus nerve (CVN). A: Four ganglion cells stained positive for TH (red arrows) and 2 stained negative (yellow arrows). B: The same nerve with 5 ganglia stained ChAT positive (blue arrows). The objective lens in panels A and B was 40×, with a calibration bar of 0.05 mm in length.
Ambulatory monitoring of VNAs
We found in both dogs episodes of VNA causing heart rate acceleration. Figure 5 shows an example in which thoracic VNA was active while the SGNA was quiescent. The VNA activity showed a burst-termination pattern typical for neural activity.27,28 There was immediate heart rate acceleration during the VNA bursts, suggesting that these nerve activities came from sympathetic component within the thoracic vagal nerve. We identified 62 such episodes in dog 1 and 33 episodes in dog 2. The heart rate was increased from 50 ± 24 beats/min immediately prior to the VNA bursts to 122 ± 19 beats/min (P < .001) during VNA bursts in dog 1 and from 66 ± 52 to 152 ± 30 beats/min (P < .001) in dog 2. Figure 6 shows the results of the TH staining of the left vagal nerve in the same dog. The CVN (A) contains fewer TH-positive nerve structures than does the thoracic vagal nerve (D). Figures 6B and 6C show that TH-positive ganglion cells are present in both the cervical and the thoracic vagal nerves, respectively.
Figure 5.
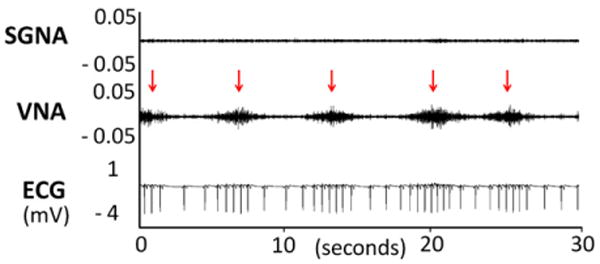
Vagal nerve activity (VNA) and heart rate acceleration. The nerve recordings show intermittent spontaneous VNAs (arrows) are associated with heart rate acceleration. Note the absence of stellate ganglion nerve activity (SGNA) in the same tracing. ECG = subcutaneous electrocardiogram.
Figure 6.
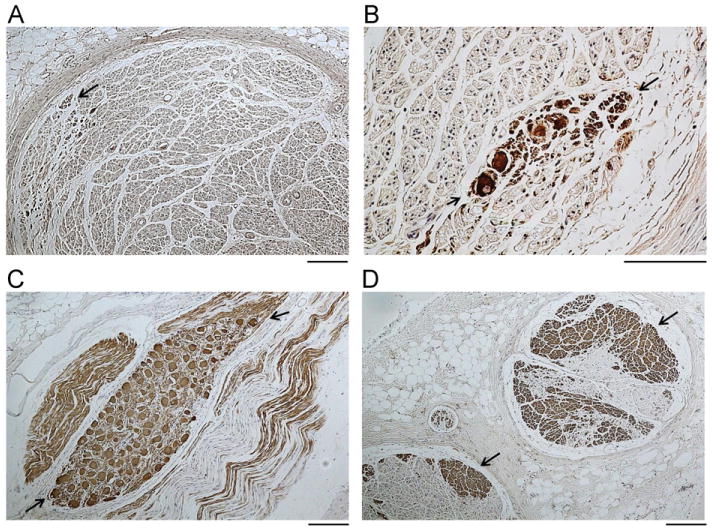
Tyrosine hydroxylase (TH) staining of the cervical (A and B) and thoracic (C and D) vagal nerves from the same dog as in Figure 5. Positive stains are brown (arrows). The TH-positive nerves in cervical vagal nerves are located in the periphery (A), while abundant TH-positive nerves are present in the thoracic vagal nerve both in the periphery and in the center (D). Note that TH-positive ganglion cells (between arrows) are present both in the cervical vagal nerve (B) and in the thoracic vagal nerve (C). The objective lens is 10× in panels A, C, and D and 40× in panel B. The calibration bars in panels A, C, and D are of 0.1 mm in length. The one in panel B is of 0.05 mm in length.
Discussion
Sympathetic component of the CVN
Previous studies have shown that there is a sympathetic component of the CVN. 13–17 Therefore, the CVN has also been referred to as the vagosympathetic trunk.29 Electrical stimulation of the vagosympathetic trunk heterogeneously shortens the atrial refractory periods, which was thought to underlie the mechanism of AF.30 More recent studies, however, show that sympathetic nerve activity is also important in the induction of AF and that simultaneous sympathovagal activation is particularly arrhythmogenic.26,31 It is reasonable to suggest that the presence of sympathetic nerve components in the CVN may have contributed to the induction of AF during vagal nerve stimulation. The sympathetic activation enhances Ca2+ entry, while the parasympathetic activation shortens the action potential duration. Simultaneous sympathovagal activation is arrhythmogenic26,28,31 by promoting Ca2+ transient triggered firing through the induction of late phase 3 early afterdepolarizations.32–34 While it is known that CVN contains sympathetic components, there are few data using immunohistochemical techniques to determine the amount of sympathetic nerves in the CVN. With the advent of clinical CVN stimulation as a treatment for epilepsy2–4,35 and the enthusiasm in applying this technology for the treatment of heart failure, a more thorough understanding of the structure of the CVN is warranted. In the present study, we found that most of the sympathetic nerve fibers are distributed in the periphery of the CVN. The location of the sympathetic nerves would appear to make them more susceptible to excitation from electrical stimulation and apt to influence its effects.
Large variations of the autonomic nerve distribution in the CVN
In a previous study, Hoffman and Kuntz17 documented 200–300 ganglion cells in the canine vagus nerve and its branches and 1700 in the human specimen. By using immunohistochemical techniques, Park et al36 confirmed the presence of adrenergic ganglion cells within the CVN. We found in our study that these ganglion cells could express both TH and ChAT, suggesting that the CVN can be a source of both sympathetic and parasympathetic tone. In addition to the variations of types of ganglion cells, a large variation in the sympathetic portion of the CVN was observed between dogs and in the same dog between the right and the left CVN. A vast majority of CVN stimulators for seizure treatment are placed on the left chest that stimulate the left CVN.2–4 However, in the recent human clinical trial of CVN stimulation in patients with heart failure, the CVN stimulators were implanted on the right chest that stimulated the right CVN.9 This is also the case in the 2 ongoing clinical studies of patients with heart failure (INOVATE-HF [phase 3 study] and NECTAR-HF [phase 2 study]). If our results are applicable to human patients, it might be difficult at the present time to deduce preoperatively which side will produce the least or the most sympathetic or parasympathetic effects. The CVN stimulation trial for patients with heart failure showed that CVN stimulation improves in New York Heart Association functional class and left ventricular ejection fraction with up to 1-year follow-up.9 However, 3 of 32 patients died and 13 of 32 patients suffered from serious adverse events, including worsening of heart failure leading to death, ventricular tachycardia, acute myocardial infarction, and atrial fibrillation. It is possible that increased sympathetic tone during CVN stimulation may contribute to both the beneficial and adverse effects observed in that clinical trial. On the other hand, it is also possible that these deaths and complications would have occurred even without CVN. Whether or not these complications are caused by CVN cannot be determined by the results of this study.
While it is possible that CVN may preferentially activate the sympathetic nerve owing to its location, we found that these sympathetic nerves are unmyelinated and hence might have a higher threshold of activation. In contrast, the myelinated fibers might have lower activation threshold and is preferentially activated by low level VNS to result in therapeutic effects. A suprathreshold VNS may also be beneficial by its anti-inflammatory effects.8
Vagal tone
The term “vagal tone” is commonly used to describe parasympathetic activation that results in bradycardia. The physiological data provided in this study show that vagal activation alone, without concomitant SGNA, can be associated with the heart rate acceleration. These findings suggest that the sympathetic component within the vagal nerve is physiologically active and can contribute to both sympathetic and parasympathetic tone of the heart. The term “vagal tone” should not be used as a surrogate of parasympathetic activation.
Clinical implications
The presence of sympathetic nerve fibers in the vagal nerve has important clinical implications. One of them is that VNS may activate sympathetic nerve fibers within the CVN. Sympathetic activation may play a role in both therapeutic and side effects of VNS. A second clinical implication is that VNS may capture both sympathetic and parasympathetic components of the CVN. Therefore, the effects of VNS should not be measured only by the heart rate changes.
Limitations
Because acetylcholine is also a neurotransmitter used by motor nerves, the ChAT-positive nerve fibers are not all parasympathetic nerve fibers. It has been shown that motor nerves are present in the CVN.10,37 The exact amount of parasympathetic nerve fibers in the CVN remains unclear. The number of dogs studied is small. Whether or not these findings are applicable to all dogs or humans remain unclear.
Conclusions
Sympathetic nerve fibers are invariably present in the CVN of normal dogs and occupy in average up to 2.8% of the cross-sectional area. The sympathetic nerve fibers within the vagal nerve may activate alone and accelerate heart rate. Because sympathetic nerve fibers are present in the periphery of the CVN, these sympathetic nerve fibers may be susceptible to activation by electrical stimulation.
Acknowledgments
We thank Lei Lin, Nicole Courtney, Jessica Warfel, and Janet Hutcheson for their assistance.
This article was processed by a guest editor. This study was supported in part by National Institutes of Health grants P01HL78931 and R01HL71140, a Piansky Endowment (to Dr Fishbein), a Medtronic-Zipes Endowment (to Dr Chen), and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Abbreviations
- ChAT
cholineacetyltransferase
- CVN
cervical vagus nerve
- SGNA
stellate ganglion nerve activity
- TH
tyrosine hydroxylase
- VNA
vagal nerve activity
Footnotes
Dr Chen is a consultant to Cyberonics. Our laboratory received equipment donations from Cyberonics, Medtronic, St Jude Medical, and Cryocath.
References
- 1.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Heck CN, Levy ML, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–1276. doi: 10.1097/00006123-199812000-00001. discussion 1276–1280. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, et al. First International Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for treatment of partial seizures, 1: A controlled study of effect on seizures. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 4.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 6.Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7072–7075. doi: 10.1109/IEMBS.2005.1616135. [DOI] [PubMed] [Google Scholar]
- 7.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–178. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 9.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 10.Foly JO, DuBois FS. Quantitative studies of the vagus nerve in the cat, I: The ratio of sensory and motor fibers. J Comp Neurol. 1937;67:49–67. [Google Scholar]
- 11.Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59:S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 13.De Burgh Daly I, Hebb C. Pulmonary vasomotor fibres in the cervical vagosympathetic nerve of the dog. Q J Exp Physiol Cogn Med Sci. 1952;37:19–43. doi: 10.1113/expphysiol.1952.sp000978. [DOI] [PubMed] [Google Scholar]
- 14.Armour JA, Randall WC. Functional anatomy of canine cardiac nerves. Acta Anat. 1975;91:510–528. doi: 10.1159/000144411. [DOI] [PubMed] [Google Scholar]
- 15.Armour JA, Hopkins DA. Anatomy of the extrinsic efferent autonomic nerves and ganglia innervating the mammalian heart. In: Randall WC, editor. Nervous control of cardiovascular function. New York: Oxford University Press; 1984. pp. 21–45. [Google Scholar]
- 16.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. Am J Cardiol. 1986;57:299–309. doi: 10.1016/0002-9149(86)90908-2. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman HH, Kuntz A. Vagus nerve components. Anat Rec. 1957;127:551–567. doi: 10.1002/ar.1091270306. [DOI] [PubMed] [Google Scholar]
- 18.Kawagishi K, Fukushima N, Yokouchi K, Sumitomo N, Kakegawa A, Moriizumi T. Tyrosine hydroxylase-immunoreactive fibers in the human vagus nerve. J Clin Neurosci. 2008;15:1023–1026. doi: 10.1016/j.jocn.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Park HW, Shen MJ, Han S, et al. Neural control of ventricular rate in ambulatory dogs with pacing induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–580. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 21.Chang CM, Wu TJ, Zhou SM, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 22.Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 23.Uno T, Hisa Y, Murakami Y, Okamura H, Ibata Y. Distribution of tyrosine hydroxylase immunoreactive nerve fibers in the canine larynx. Eur Arch Otorhinolaryngol. 1992;249:40–43. doi: 10.1007/BF00175669. [DOI] [PubMed] [Google Scholar]
- 24.Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol. 1987;261:15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- 25.Bodhireddy SR, Lyman WD, Rashbaum WK, Weidenheim KM. Immunohistochemical detection of myelin basic protein is a sensitive marker of myelination in second trimester human fetal spinal cord. J Neuropathol Exp Neurol. 1994;53:144–149. doi: 10.1097/00005072-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.del Negro CA, Hsiao CF, Chandler SH. Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol. 1999;81:1478–1485. doi: 10.1152/jn.1999.81.4.1478. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res. 1974;8:647–655. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]
- 30.Alessi R, Nusynowitz M, Abildskov JA, Moe GK. Nonuniform distribution of vagal effects on the atrial refractory period. Am J Physiol. 1958;194:406–410. doi: 10.1152/ajplegacy.1958.194.2.406. [DOI] [PubMed] [Google Scholar]
- 31.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 33.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Connor DE, Jr, Nixon M, Nanda A, Guthikonda B. Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus. 2012;32:E12. doi: 10.3171/2011.12.FOCUS11328. [DOI] [PubMed] [Google Scholar]
- 36.Park HW, Shen MJ, Han S, et al. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–580. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat, I: The cervical vagus and nodose ganglion. J Comp Neurol. 1980;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]


