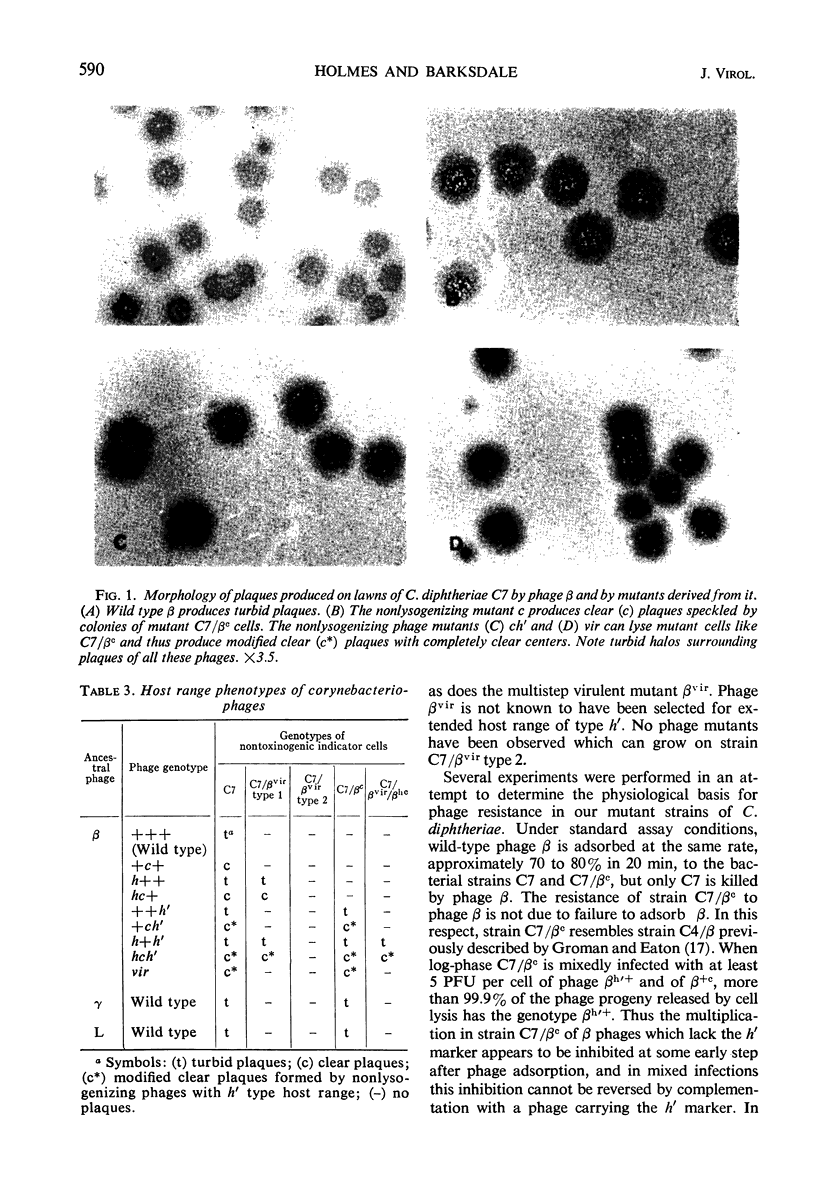
Abstract
A series of mutants derived from the temperate corynebacteriophages βtox+, γtox−, and Ltox+ was isolated and characterized. In three-factor crosses between mutant β phages the relative map order of the genetic markers determining extended host ranges (h and h′) and loss of ability to lysogenize (c) was found to be h--c--h′. Recombination between markers was observed in matings between phage β and the heteroimmune corynebacteriophages γ and L. In such matings between heteroimmune phages the c markers of phages β and γ failed to segregate from the imm markers which determine the specificity of lysogenic immunity in these phages. The factor which directs the synthesis of diphtherial toxin during infection of appropriate corynebacterial hosts by toxinogenic corynebacteriophages is designated tox+. It was possible to show that the tox+ determinant of phage β behaves as a single genetic element which occupies a position between the loci h and imm on the genetic map of this phage. Genetic recombination between mutants of phage β occurred at very low frequencies in biparental matings performed by mixed infection of Corynebacterium diphtheriae C7s(−)tox−. Considerably higher recombination frequencies were observed when lysogenic bacterial strains carrying one parental phage as prophage were induced by ultraviolet irradiation and then superinfected by the second parental phage. Maximal stimulation of genetic recombination between mutant β phages was detected when superinfection followed ultraviolet irradiation of the lysogenic cells within a limited period of time. In matings between phages with incomplete genetic homology, the stimulation of recombination by ultraviolet radiation was much less effective.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD R. K., MCGREGOR J. F., BAIRD K. M. Mutation to extended host range and the occurrence of phenotypic mixing in the temperate coliphage lambda. Virology. 1956 Aug;2(4):565–574. doi: 10.1016/0042-6822(56)90012-5. [DOI] [PubMed] [Google Scholar]
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BARDSDALE W. L., PAPPENHEIMER A. M., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. doi: 10.1128/jb.67.2.220-232.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKSDALE L., GARMISE L., HORIBATA K. Virulence, toxinogeny, and lysogeny in Corynebacterium diphtheriae. Ann N Y Acad Sci. 1960 Nov 21;88:1093–1108. doi: 10.1111/j.1749-6632.1960.tb20099.x. [DOI] [PubMed] [Google Scholar]
- BARKSDALE L., GARMISE L., RIVERA R. Toxinogeny in Corynebacterium diphtheriae. J Bacteriol. 1961 Apr;81:527–540. doi: 10.1128/jb.81.4.527-540.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKSDALE L. Sur quelques bactériophages de Corynebacterium diphtheriae et leurs hôtes. C R Hebd Seances Acad Sci. 1955 May 2;240(18):1831–1833. [PubMed] [Google Scholar]
- Baldwin R. L., Barrand P., Fritsch A., Goldthwait D. A., Jacob F. Cohesive sites on the deoxyribonucleic acids from several temperate coliphages. J Mol Biol. 1966 Jun;17(2):343–357. doi: 10.1016/s0022-2836(66)80146-8. [DOI] [PubMed] [Google Scholar]
- Barksdale L. I. : Lysogenic Conversions in Bacteria. Bacteriol Rev. 1959 Dec;23(4):202–212. doi: 10.1128/br.23.4.202-212.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H. A., Fuerst C. R., Siminovitch L., Thomas R., Lambert L., Pereira da Silva L., Jacob F. Genetics and physiology of defective lysogeny in K12 (lambda): studies of early mutants. Virology. 1966 Oct;30(2):224–241. doi: 10.1016/0042-6822(66)90098-5. [DOI] [PubMed] [Google Scholar]
- FREEMAN V. J., MORSE I. U. Further observations on the change to virulence of bacteriophage-infected a virulent strains of Corynebacterium diphtheria. J Bacteriol. 1952 Mar;63(3):407–414. doi: 10.1128/jb.63.3.407-414.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN V. J. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J Bacteriol. 1951 Jun;61(6):675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Conversion in Corynebacterium diphtheriae with phages originating from nontoxigenic strains. Virology. 1956 Dec;2(6):843–844. doi: 10.1016/0042-6822(56)90066-6. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M., BOOHER Z. K. Studies of mono- and polylysogenic Corynebacterium diphtheriae. J Bacteriol. 1958 Mar;75(3):320–325. doi: 10.1128/jb.75.3.320-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., EATON M. Genetic factors in Corynebacterium diphtheriae conversion. J Bacteriol. 1955 Dec;70(6):637–640. doi: 10.1128/jb.70.6.637-640.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Evidence for the active role of bacteriophage in the conversion of nontoxigenic Corynebacterium diphtheriae to toxin production. J Bacteriol. 1955 Jan;69(1):9–15. doi: 10.1128/jb.69.1.9-15.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. Evidence for the induced nature of the change from nontoxigenicity to toxigenicity in Corynebacterium diphtheriae as a result of exposure to specific bacteriophage. J Bacteriol. 1953 Aug;66(2):184–191. doi: 10.1128/jb.66.2.184-191.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B. The relation of bacteriophage to the change of Corynebacterium diphtheriae from avirulence to virulence. Science. 1953 Mar 20;117(3038):297–299. doi: 10.1126/science.117.3038.297. [DOI] [PubMed] [Google Scholar]
- HEWITT L. F. Mechanism of virulence transfer by bacterial viruses. J Gen Microbiol. 1954 Oct;11(2):272–287. doi: 10.1099/00221287-11-2-272. [DOI] [PubMed] [Google Scholar]
- HOWARD D. H., JANN G. J. The isolation and characterization of bacteriophage active against the diphtheria-like corynebacteria. J Bacteriol. 1954 Sep;68(3):316–319. doi: 10.1128/jb.68.3.316-319.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. III. Effet du rayonnement ultraviolet sur la recombinaison génétique. Ann Inst Pasteur (Paris) 1955 Jun;88(6):724–749. [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Liedke-Kulke M., Kaiser A. D. Genetic control of prophage insertion specificity in bacteriophages lambda and 21. Virology. 1967 Jul;32(3):465–474. doi: 10.1016/0042-6822(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Barksdale L. Phage-directed synthesis of diphtherial toxin in non-toxinogenic Corynebacterium diphtheriae. Nature. 1966 May 28;210(5039):911–913. doi: 10.1038/210911a0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Barksdale L. System for the investigation of the bacteriophage-directed synthesis of diphtherial toxin. J Bacteriol. 1967 Feb;93(2):722–730. doi: 10.1128/jb.93.2.722-730.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. A., Pappenheimer A. M., Jr, Doolittle W. F. Phage-host relationships in certain strains of Corynebacterium diphtheriae. Virology. 1966 Jul;29(3):410–425. doi: 10.1016/0042-6822(66)90217-0. [DOI] [PubMed] [Google Scholar]
- Miller P. A., Pappenheimer A. M., Jr Observations on the Weissensee G strain of Corynebacterium diphtheriae. J Gen Microbiol. 1967 Jan;46(1):43–45. doi: 10.1099/00221287-46-1-43. [DOI] [PubMed] [Google Scholar]
- PARSONS E. I. Induction of toxigenicity in non-toxigenic strains of C. diphtheriae with bacteriophages derived from non-toxigenic strains. Proc Soc Exp Biol Med. 1955 Oct;90(1):91–93. doi: 10.3181/00379727-90-21948. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A. B., Rao S. S. The role of phage in the transduction of the toxinogenic factor in Corynebacterium diphtheriae. J Gen Microbiol. 1965 Sep;40(3):421–429. doi: 10.1099/00221287-40-3-421. [DOI] [PubMed] [Google Scholar]
- VANHEYNINGEN W. E., ARSECULERATNE S. N. EXOTOXINS. Annu Rev Microbiol. 1964;18:195–216. doi: 10.1146/annurev.mi.18.100164.001211. [DOI] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]