Abstract
Molecular-scale computing has been explored since 1989 owing to the foreseeable limitation of Moore's law for silicon-based computation devices. With the potential of massive parallelism, low energy consumption and capability of working in vivo, molecular-scale computing promises a new computational paradigm. Inspired by the concepts from the electronic computer, DNA computing has realized basic Boolean functions and has progressed into multi-layered circuits. Recently, RNA nanotechnology has emerged as an alternative approach. Owing to the newly discovered thermodynamic stability of a special RNA motif (Shu et al. 2011 Nat. Nanotechnol. 6, 658–667 (doi:10.1038/nnano.2011.105)), RNA nanoparticles are emerging as another promising medium for nanodevice and nanomedicine as well as molecular-scale computing. Like DNA, RNA sequences can be designed to form desired secondary structures in a straightforward manner, but RNA is structurally more versatile and more thermodynamically stable owing to its non-canonical base-pairing, tertiary interactions and base-stacking property. A 90-nucleotide RNA can exhibit 490 nanostructures, and its loops and tertiary architecture can serve as a mounting dovetail that eliminates the need for external linking dowels. Its enzymatic and fluorogenic activity creates diversity in computational design. Varieties of small RNA can work cooperatively, synergistically or antagonistically to carry out computational logic circuits. The riboswitch and enzymatic ribozyme activities and its special in vivo attributes offer a great potential for in vivo computation. Unique features in transcription, termination, self-assembly, self-processing and acid resistance enable in vivo production of RNA nanoparticles that harbour various regulators for intracellular manipulation. With all these advantages, RNA computation is promising, but it is still in its infancy. Many challenges still exist. Collaborations between RNA nanotechnologists and computer scientists are necessary to advance this nascent technology.
Keywords: RNA computation, nanobiotechnology, computer, molecular-scale computing, RNA nanoparticles, RNA nanostructure
1. The end of Moore's law for the electronic computer
Silicon-based computer and embedded systems are becoming ubiquitous in our daily lives [1]. We need processors to implement computation, communication and control to meet the demands of new applications and paradigms. Computer chip manufacturers frantically compete to make future microprocessors that are increasingly difficult to continue scaling with Moore's law, the number of electronic devices on the microprocessor every 18 months, doubling the demand to break the quickest record. Silicon microprocessor speed and miniaturization will eventually reach their own limits. Sooner or later, this chip competition is bound to reach stalemate. Scientists and engineers are wondering whether Moore's law can be continued for the next 10 years, and whether the capacity of transistors in the unit area of a chip can be doubled every 2 years (or every 18 months). There are three reasons for this critical challenge: (i) the accuracy of computing will be affected if we make a transistor at the atomic level, because two wires in the circuit will be too close and they will affect each other; (ii) the heat generated in such a small area with too many concentrated transistors will greatly affect the functions of the transistors; (iii) the energy consumption to cool the circuit board would be too high a burden.
By 2011, US data centres were predicted to consume 100 billion kWh at a cost of $7.4 billion per year [2]. Unfortunately, much of this energy is wasted by systems that are idle when current servers still draw about 60% of peak power. In typical data centres, average utilization is only 20–30%. Chip temperature impacts circuit reliability, energy consumption and system cost. Research has shown that every 10–15°C increase in operating temperature reduces the lifetime of the chip by half. With increasing temperatures, the leakage current of a chip increases exponentially. In addition, the cooling cost increases significantly, which amounts to a considerable portion of the total cost of the whole computer system. The cross-sectional power density increases linearly with the number of stacked silicon layers, causing a serious thermal problem. Chip manufacturers need a new kind of material in order to produce faster computing microprocessors.
Living entities have the most complicated computational systems in the world. The calculation capacity of cells, living organisms and the human brain is incomparable with machines used in the outside world. The supercomputer of biological systems, including the human body, relies on the interaction of DNA, RNA and proteins. Currently, the computational mechanism of the human brain has not been completely elucidated, but it is believed that biomimetic approaches following the realism of RNA, DNA and protein would be a new horizon that would solve electronic computing problems.
2. Molecular-scale computing
Molecular computation uses bottom-up approaches to create biological and chemical computers at the nanoscale [3–7]. Comparatively, electronic-integrated circuit systems comprise logic gates that perform Boolean logic by receiving true (1, high voltage) and false (0, low voltage) values resulting in a Boolean output [8]. Although the computational DNA logic gate first appeared in 1989 [9,10], the actual beginning of molecular-scale computing was when Adleman's group, in 1994, used a DNA-based design to solve a seven-city Hamiltonian path problem [11]. This began the new era of DNA computing.
The basic mechanism of the DNA-based computer is a single-strand of DNA that undergoes a certain reaction upon the arrival of an input signal. This process is controllable, and has led to DNA strand displacement circuits [12–16]. The advantage of using DNA is that DNA is stable, and DNA hybridization reactions can be used to represent computational steps, with dynamic DNA nanostructures representing computational states. Figure 1 shows a detailed comparison between an electronic computer and a molecular-scale computer. The molecular-scale computer has many promising features that have the potential to overcome the restraints of Moore's law for electronic computers. Based on concepts borrowed from electronics, such as Boolean values, signal processing, signal amplification and feedback, multi-layered biocircuits have been created since the middle of the 1990s.
Figure 1.
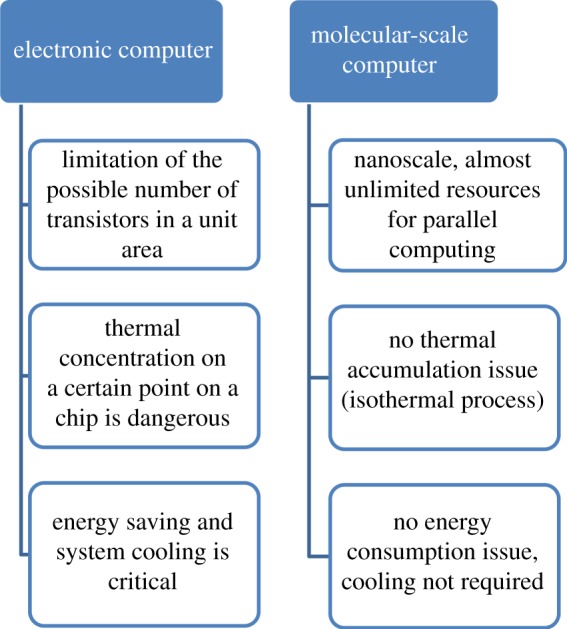
Comparison of the electronic computer and a molecular-scale computer.
Boolean frameworks have been used in the measurement of biological interactions, and the classification of genes has been represented under diseased conditions [17,18]. Currently, most Boolean logic gates operate by fluorescence detection, a method that is complex and physically inconvenient [14,19–23]. Alternatively, a homogeneous colorimetric detection method has been employed that uses high extinction coefficients and the distance-dependent optical properties of gold nanoparticles to reduce the complexity and cost of the procedure and increase the ease of operation [24,25]. As exemplified in figure 2, metal-ion-mediated DNA logic gates (using AND, NAND and NOR) have been fashioned based on electrochemical outputs that use the unique features of Ag+ ions that interact with the cytosine–cytosine mismatch, and of Hg2+ ions that interact with the thymine–thymine mismatch, in DNA duplexes [26]. The AND logic system was based on the proximity-dependent surface hybridization between thiolated T-/C-rich DNA on a gold electrode surface and T-/C-rich DNA labelled with ferrocenecarboxylic acid (Fc), in which the Hg2+ and Ag+ ions are used as inputs and the current of the Fc as output. Subsequently, an NAND logic gate was constructed based on the strand dissociation as well as the conformational switch of T-/C-rich DNA triggered by Ag+ and Hg2+ ions. In addition, it was found that the C−Ag+−C and T−Hg2+−T base pairs can trigger the structural conversion of multiple nucleic acid helices from triplexes to duplexes, which motivated the fabrication of another NOR logic gate.
Figure 2.

Logic-gate systems with Ag+ and Hg2+ ions as inputs and electrochemical signals as output detected by differential pulse voltammetry (DPV). (a) Schematic of the two-input logic gate and equivalent electronic circuit for the AND logic operations. (b) A schematic presentation of a ‘NAND’ gate. (c) Schematic of a ‘NOR’ gate. (Adapted from [26]. Copyright © 2013 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.)
DNA electrochemical logic gates that can be made with minimal reagents, fewer working steps and a simpler optical set-up are optimal. Compared with silicon-based elements used in electrical computation, nucleic acids are structurally simple, have straightforward sequence-specific hybridization between complementary strands, and can capture certain target molecules (e.g. metal ions, small molecules and proteins in a highly specific manner [27,28]; as such, in order to compute DNA, DNA logic gates must be created [29]). Zhang and co-workers [30] have designed a system of colorimetric logic gates (OR, AND and INHIBIT) using Pb2+ and Mg2+ ions as the DNAzyme cofactors to activate respective scission DNAzymes, and Zhang et al. have constructed a complete set of two-input logic gates by designing a series of circular substrates (OR, AND, INHIBIT, XOR, NOR, NAND and XNOR), where ion-dependent DNAzymes were the functional components and the respective cofactor ions were used as the inputs [31].
Another step forward was the introduction of toehold-mediated DNA strand displacement. A toehold is a single-stranded domain that leads to enzyme-free DNA machinery that is automated by hybridization [6,32]. The most recent results are from the Caltech laboratory [33]. Qian and Winfree [33] used a DNA hybridization reaction to represent the logic operations AND and OR. The digital values were controlled through threshold gates. The signal recognition is amplified through fluorophores and quenchers. The designed seesaw circuits with three layers of gates demonstrated the possible progression of DNA computation to more layers of gates.
However, there are many limitations of DNA-based computation. The most obvious is that the execution time of a logic gate such as AND or OR is too long to tolerate. It usually takes 1–2 h to finish one AND or OR operation through biochemical reactions. Three-layer seesaw circuits require more than 6–10 h to reach a stable state and to achieve the final value represented. The second challenge of DNA-based computation is the limiting proteins required in the DNA reactions, such as DNA-binding and DNA-cutting proteins. Nevertheless, scientists and engineers have started to use molecular-scale computing devices to build new-style computers, such as quantum computing and neurocomputing. But, DNA-based computation is still a promising approach.
3. Historical evolution of RNA nanotechnology
Adleman [11] proposed and introduced the idea of using DNA to solve complex mathematical problems. DNA coding is very similar to how permanent personal genetic information is stored in an electronic computer's hard drive. DNA Logic is a team from Rochester, NY, whose DNA-based logic gate achieved the first step in the creation of computing systems that have the same structure as a conventional computer. They used an electronic signal instead of a DNA genetic code to carry out a calculation. They used the genetic materials as input and a combination of two fragments as an output. Two DNA inputs were linked and crossed by a chemical to form an end-to-end concatemer structure to serve as an ‘AND GATE’. They reported that these logic gates could be combined with DNA microchips to execute a new approach for DNA computation.
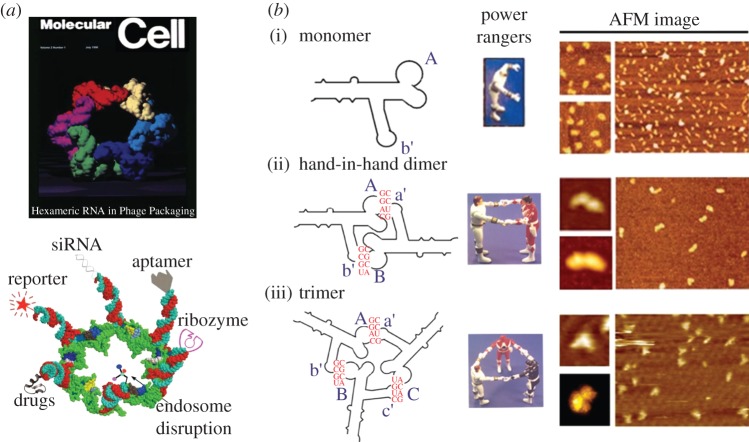
The main driving force of RNA-based computing lies in the concept of RNA nanotechnology. To uncover the role of RNA nanotechnology in network computing devices, it is important to briefly provide terminology and a history of the RNA nanotechnology concept. RNA nanotechnology is a relatively recent field of science that uses bottom-up or top-down approaches to build artificial RNA architectures that are nanometres in scale. It involves the characterization of the physical, chemical, biological and pharmaceutical properties of artificial RNA scaffolds or nanoparticles that can be used in nanobiotechnology, synthetic biology, nanomedicine and computing devices. RNA nanotechnology is a unique field that is distinct from the classical studies on RNA structure. The concept began in the late 1990s with the pioneering work led by Dr Peixuan Guo and his laboratory. In 1998, they demonstrated the construction of RNA nanoparticles using the re-engineered natural packaging RNA (pRNA) derived from a bacteriophage ϕ29 DNA packaging motor to self-assemble by hand-in-hand interaction into multimeric RNA nanoparticles (figure 3a). This finding was published in Zhang et al. [39], and was featured in Hendrix [40]. In 2004, the same group reported the systematic formation of pRNA nanoparticles using technologies of palindrome sequence-mediated self-annealing [37] (figure 3b). In the following years, they successively showed that pRNA molecules could be conjugated with various therapeutic functionalities, including aptamers, siRNA, ribozymes and microRNA, and serve as polyvalent vehicles to deliver these molecules [35,38,41–50] (figure 3c). These findings have paved the way for RNA nanotechnology to develop into a novel area of therapeutics for the treatment of various diseases such as cancer, viral infections and genetic diseases. This is pioneer work to prove the concept of RNA nanotechnology and intracellular computation and raises the possibility of intracellular computation.
Figure 3.
Historical outline of RNA nanotechnology. (a) Re-engineered pRNAs form hexamers, one of the first pieces of evidence for the application of the RNA nanotechnology concept [34,35]. (Upper panel, adapted with permission from F. Major and from © Elsevier 1998; lower panel, adapted from [35] with permission from © Mary Ann Liebert, Inc. 2005.) (b) Re-engineered pRNA (i) monomer can assemble to (ii) dimers and (iii) trimers [36–38]. AFM, atomic force microscopy. (Adapted from [38] with permission from © Elsevier 2010.)
The development of multi-valent pRNA nanoparticles in the Guo laboratory is just one facet of the rapidly emerging field of RNA nanotechnology and therapeutics. Elucidation of the structure and folding mechanism of RNA motifs and junctions has laid a foundation for the further development of RNA nanotechnology. In its early stage, significant contributions to the fundamental studies of RNA structural motifs were made by Eric Westhof, Neocles Leontis, Luc Jaeger, David Lilley and their laboratories [51–65]. Advances in RNA three-dimensional computation from traditional intra-molecular interaction to inter-molecular interaction were promoted by Bruce Sharpiro and co-workers (figure 3d) and have brought new features into the RNA nanotechnology field [59,66–69].
Currently, RNA nanotechnology is becoming a popular and rapidly developing branch of science, as evidenced by the burst of publications on RNA nanostructures in the past 5 years, indicating a strong interest in RNA nanotechnologies in diverse fields such as chemistry, biophysics, biochemistry, structural biology, microbiology, cancer biology, pharmacy, cell biology and nanomedicine. New perspectives on the application of the RNA nanotechnology concept are slowly developing into a more sophisticated era of RNA computing.
4. Significance and uniqueness of RNA nanotechnology
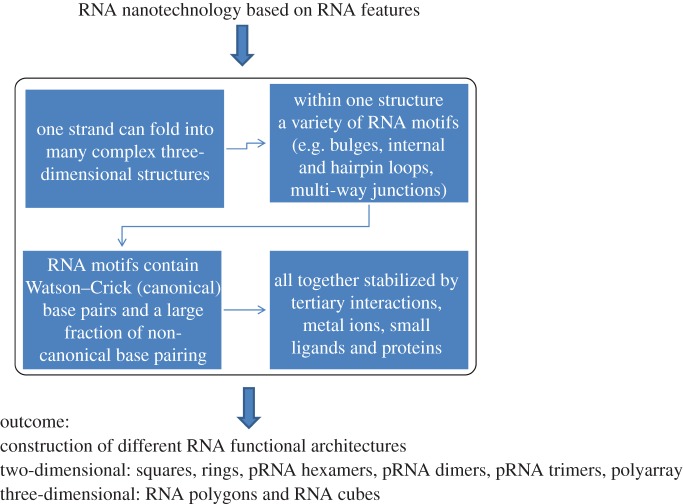
RNA biopolymers are very important to RNA nanotechnology, beginning at the atomic level of these intriguing molecules. Composed of four different nucleotides—A, C, G and U—RNA is capable of Watson–Crick base pairing (as DNA does) as well as a variety of non-canonical base pairing [55,58,70–72]. This property mainly promotes RNA folding into either rigid or flexible structures containing tertiary interactions that are distinct from those of double-stranded DNA (figure 4) [54,55,73–76]. RNA tertiary interactions mediate bulges, internal hairpin loops, multi-way junctions and inter- and intra-RNA–RNA components. A 90-nucleotide RNA can display up to 490 different sequences with a very large number alternative secondary and tertiary structures. Such a huge pool of rich structural conformations could ease the search for viable partners in particle assembly, substrate binding, architecture building and manufacture engineering.
Figure 4.
Significance and uniqueness of RNA nanotechnology.
In addition, the versatility and low-energy folding of RNA deliver a significant advantage over DNA, such as high thermodynamic stability [77–79] and various in vivo attributes [34,39,45,80–84]. At the same time, RNA can be designed and manipulated with a level of simplicity characteristic of DNA. But it displays a structural flexibility and functional diversity similar to that of proteins, including enzymatic activities. Although RNA nanotechnology can be regarded as a subdivision of nucleic acid nanotechnology, the uniqueness of RNA properties when compared with DNA will advance the emerging field of RNA nanotechnology. The discovery of diverse RNA functions in ribozyme, riboswitch, aptamer, siRNA and miRNA and the methods to produce fluorogenic RNA in the cell [85,86] suggest the immense potential of intracellular computation.
5. Fabrication of RNA nanoparticles with potential as computer parts
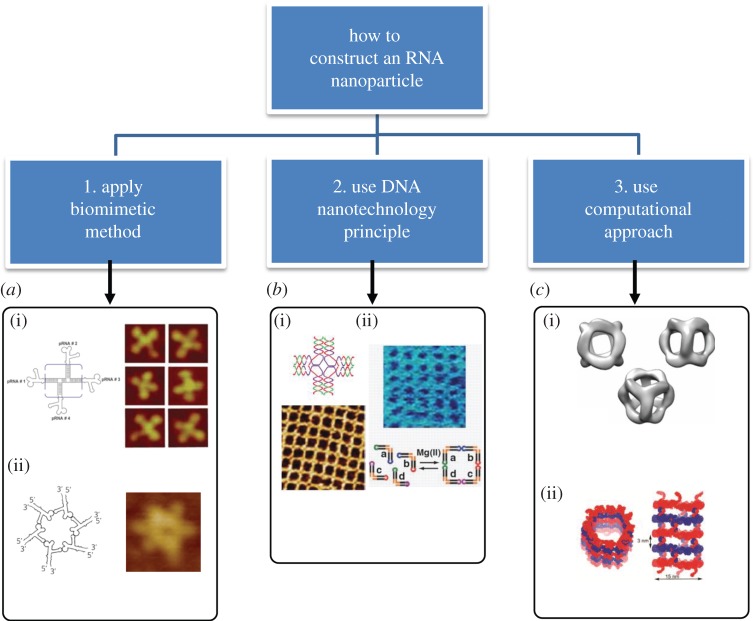
Artificial construction and assembly of RNA molecules into more complex and functional systems requires the use of programmable, addressable and predictable building blocks. The thermodynamic stability, specificity, affinity, flexibility and folding rules of RNA structural motifs need to be known, so that possible advantages can be found or difficulties overcome. RNA folding into various three-dimensional structures dictating its function is the result of complex hierarchical self-organization of modular elements. Self-assembly of RNA building blocks in a predefined manner to form larger two- and three-dimensional structures is a prominent bottom-up approach and represents an important means by which biological techniques and biomacromolecules can be successfully integrated into nanotechnology [37,35,43]. Figure 5 provides the main concepts of RNA nanoparticle fabrication based on the following methods [54,55,73–76,91].
Figure 5.
Fabrication of RNA nanoparticles. (a) (i) pRNA four-way junction constructed by mimicking the observed natural pRNA 3WJ core motif [42]. (Adapted from [42], © 2012 with permission from Elsevier.) (ii) pRNA hexamer was built via loop–loop interaction by naturally occurring pRNA hexamers [34,87]. (Left panel, adapted from [34]. © 1998 with permission from Elsevier. Right panel, adapted from [88]. © 2013 with permission from Elsevier.) (b) Illustration of the similarity between DNA and RNA structural arrays: (i) AFM image of cross-shaped tiles possessing a four-arm formation through the sticky end [89,90]. (Adapted from [89]. © 2008 with permission from the American Association for the Advancement of Science.) (ii) An example of RNA square-based structural arrays obtained from six sticky nucleotides [62]. (Adapted from [62]. © 2008 with permission from the American Association for the Advancement of Science.) (c) Examples of RNA nanostructures designed with the help of computer programs. (i) Computer-aided design of an RNA nanocube [59]. (Adapted from [59]. © 2010 with permission from Nature Publishing Group.) (ii) An example of an RNA nanotube [66]. (Figure courtesy of Dr Bruce A. Shapiro.)
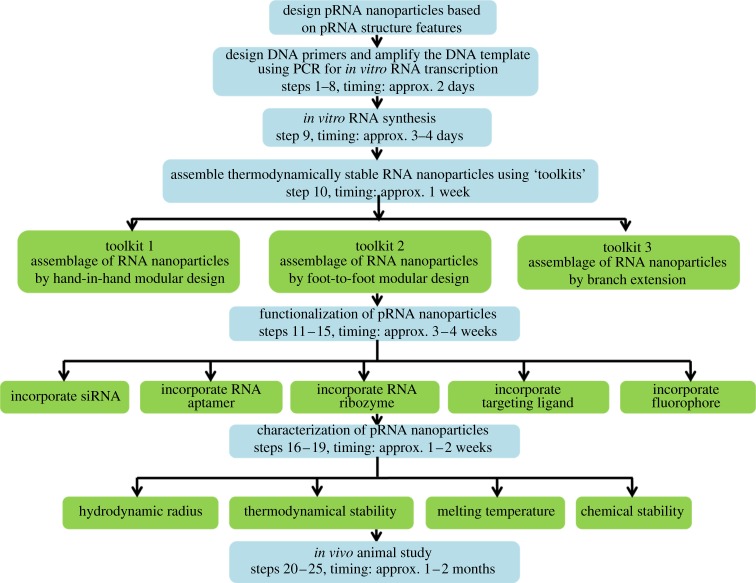
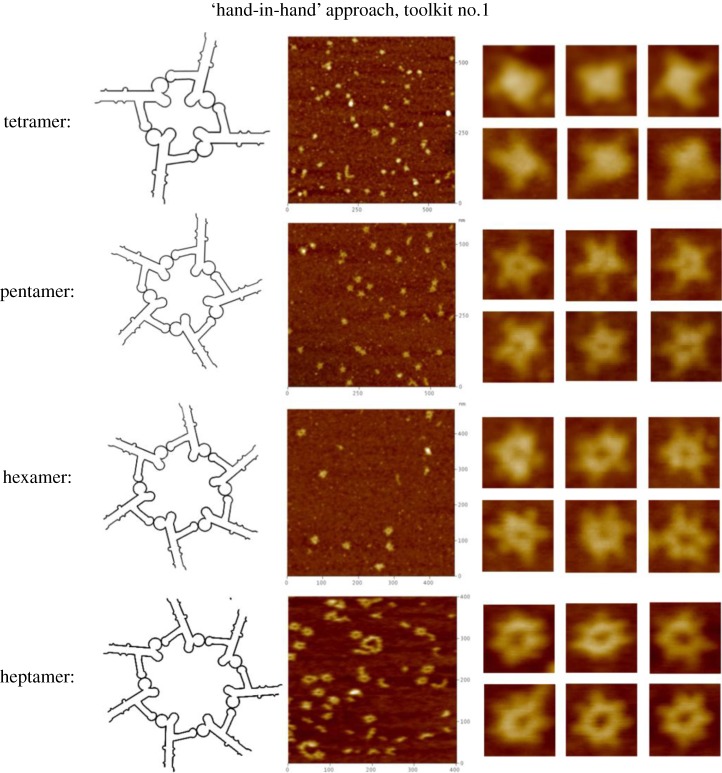
First is the biomimetic method, which mimics RNA constructs from naturally occurring atomic resolution X-ray or NMR structures. The structures of RNA motifs and the mechanisms of RNA folding and sequence interactions have been investigated for many years. A rich resource of well-developed databases can be used to extract known RNA structural units for construction of novel RNA nanoparticles with the desired properties [82,92,93]. One such example is based on the structural features of the pRNA of the bacteriophage ϕ29 DNA packaging motor, which uses a hexameric RNA ring to gear the machine [94–96]. The basic methodology to produce different RNA nanoparticles based on the pRNA sequence is illustrated in figure 6 (Shu Yi nature protocols). Thus, Guo's group has extensively re-engineered pRNA to form dimers, trimers, tetramers, hexamers (with proteins) and arrays via hand-in-hand or foot-to-foot interactions between two interlocking loops of pRNA, as summarized in figure 7 [36,37]. In addition, the dimer and trimer nanoparticles have been used successfully as polyvalent vehicles to deliver a variety of therapeutic molecules as well as for constructing RNA arrays [37].
Figure 6.
Workflow diagram of functional RNA construction based on the pRNA molecule. PCR, polymerase chain reaction.
Figure 7.
Illustration of toolkits to obtain RNA nanoparticles. (a) pRNA extended interlocking loop–loop interaction ‘hand-in-hand’, toolkit no. 1; (b) pRNA interaction via a palindrome sequence introduced at the 3′-end ‘foot-to-foot’, toolkit no. 2; and (c) 3WJ motif of pRNA 3WJ used to construct branched RNA nanoparticles, toolkit no. 3 [87]. (Adapted from [87]. © 2013 with permission from Cold Spring Harbor Laboratory Press.)
Another example that uses the structures of known RNA structural elements is called ‘RNA architectonics’ [62]. The strategy is based on the rational design of artificial three-dimensional RNA constructs guided by specific loop–loop interactions. This can be decomposed and reassembled to create new RNA nanoscopic architectures by inverse folding.
Application of a three-way junction (3WJ) [41] and a four-way junction (4WJ) [42] that are selected from known RNA structures is another exciting example of a biomimetic approach to create functional nanoparticles [60,67]. There are several examples in the literature: RNA structural motif (from rRNA) to guide the tetramer assembly of L-shaped tectoRNAs; 3WJ motif (from 23S rRNA) to construct a T-shaped arrangement of three helices; and tRNA motifs consisting of 4- and 5-WJ to fold L-shaped tertiary structures [60,82]. However, crystallography and NMR are not the only sources of RNA functional elements—many of them have been identified by in vitro selection, for instance aptamers [97,98].
The second method is to inherit the principles of DNA nanotechnology. Because DNA and RNA share some common structural and chemical features, DNA methods can provide viable models for RNA nanotechnology development. Direct DNA self-assembly is predictable, and complex nanostructures can be created with precise addressability. This is demonstrated by assembling DNA into a variety of elegant shapes with precise control over their geometries, periodicities and topologies (figure 5). Branched DNA tiles, tensegrity triangles (rigid structures in periodic array form) [99], algorithmic self-assembled Sierpinski triangles (aperiodic arrays of fractal patterns) [100], nanotubes, helix bundles [101], polycatenated DNA ladders [102] and three-dimensional cubes, polyhedrons, prisms and buckyballs are some of the examples.
Rothemund's [103] DNA origami is an exciting demonstration of the addressable and programmable property of DNA. Behind the principle of DNA origami lies a long single-stranded viral DNA which is used as a scaffold for binding shorter strands to generate well-defined two- and three-dimensional configurations. This strategy was applied to build three-dimensional boxes that can be locked and unlocked [104], nanoarrays for label-free detection of substrates [105], multi-layered three-dimensional DNA nanostructures and for structure elucidation of organized proteins [106]. Rationally designed supramolecular DNA assemblies can be conjugated with organic and inorganic molecules, such as conjugation of porphyrins on parallel DNA helix bundles [107], nanomagnets [108] and elegant nanomachines [109,110]. Recently, DNA fold-and-cut methodology was used to build a reconfigurable topological surface with only one side and only one boundary called a Möbius strip [111].
The above-mentioned DNA nanotechnology principles have been successfully applied to RNA nanotechnology (figure 5). The formation of jigsaw puzzles and bundles was demonstrated in both RNA and DNA [37,61,62,112–114]. To introduce branching into structures, multi-helix junctions are used as monomers in DNA constructs [115,116], enabling similar novel and diverse RNA-based architectures to be built [55,60].
The final method is to use computational tools to design RNA nanoparticles. As mentioned above, RNA molecules display diverse structures mediated by both canonical and non-canonical base pairing. In contrast to traditional methods in which raw materials are harvested from three-dimensional databases rather than designed for a given application, the next generation of building blocks can be designed a priori for programmed assembly and synthesis. RNA secondary structures are stabilized largely by nearest-neighbour interactions that can be measured in model systems [77,78].
Application of this method, however, requires a large data bank of parameters for the nearest-neighbour interactions. Fortunately, advances in RNA synthesis make it possible to study molecules with many different structural motifs. The use of computational methodologies can significantly reduce the time and expense required to build RNA-based functional nanoparticles to address experimental needs. However, prediction of RNA structure or folding for particle assembly is a great challenge, owing to the unusual folding properties involving non-canonical interactions. Single-base modifications can result in folding alterations and loss of function. Currently, using Zuker's RNA secondary structure dynamic programming algorithm, typically only 70% of the two-dimensional folding prediction is accurate [117,118]. Obviously, predicting the RNA three-dimensional structures is even more difficult.
Although there are novel strategies that predict the self-assembly of nucleotide sequences into three-dimensional RNA nanoparticles, presently, new and efficient computational approaches are required. Generally, there are two steps in building RNA nanoparticles: (i) the computational approach (e.g. using Kinefold) [119], using the spontaneous self-folding property of RNA into defined structures via base–base interactions based on their characteristic ΔG, and (ii) spontaneous assembly of the resulting RNA building blocks into larger assemblies based on the predicted architecture. This creates an effective computational pipeline for generating molecular models of RNA nanostructures. The RNA junction database [82], NanoTiler [67], RNA2D3D algorithms [120], RNA dynamics [121,122] and FR3D [123] are used to build RNA nanoparticles that incorporate individual RNA motifs to defined user specifications [66] and have been shown to self-assemble in vitro (e.g. nanocubes; figure 4 and [59,68]).
6. Application of RNA nanotechnology for computer design
Thus far, we have shown the capabilities of RNA nanotechnology to provide one of the few ways to form designed, complex structures with precise control over nanoscale properties. The field is beginning to see application in the design of logic gates as building blocks for computer construction. Here, we briefly emphasize some important aspects of why it is critical to apply RNA nanotechnology into ‘RNA computers’.
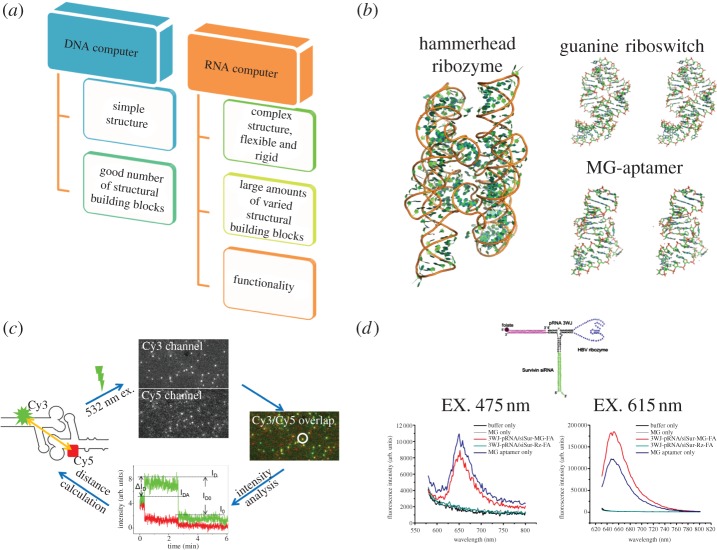
One of the main advantages of RNA is that it can carry catalytic (e.g. ribozymes [124]) and gene regulation functions (e.g. riboswitches [124]) within the cell as well as performing detection, signalling and sensing functions (e.g. aptamers). Thus, the RNA molecule has advantages over the DNA molecule not only in versatility of different structures but also in functionality (figure 8a–d). Unlike traditional electronic computers, which use electric current as inputs and outputs, these RNAs use the concentrations of specific chemical species as signals. These RNAs can replace DNA to implement logic functions and to build up the multiple layer Boolean networks with AND/OR/NOT logic gates [91,127,128].
Figure 8.
Application RNA nanotechnology for computer design. (a) Comparison between DNA and RNA computers. (b) Atomic resolution structures of some functional RNAs (shown are the hammerhead ribozyme PDB ID: 1GID, guanine riboswitch PDB ID: 3GER and malachite green (MG) aptamer PDB ID: 1F1 T). (c) Example of the application of RNA nanoparticles to calculate a distance [125]. (Adapted from [125]. © 2010 with permission from the American Chemical Society.) (d) Application of MG aptamer-functionalized 3WJ pRNA nanoparticles in vitro, showing the different emissions based on different excitation wavelengths [126]. (Adapted from [126]. © 2011 with permission from Nature Publishing Group.)
The design and synthesis of basic functional circuits are the fundamental tasks for developing a fully functional computing device. Before it is possible to engineer higher order genetic networks that can perform complex functions, basic functions should first be realized. RNA nanotechnology-based devices can perform cellular information-processing operations from standard components. These devices can exhibit logic operations (AND, NOR, NAND or OR gates) and signal filtering. RNA-based devices process and transmit molecular inputs to targeted protein outputs, linking computation to gene expression and thus the potential to control cellular function. Several information-processing systems have been developed based on RNA computing. For example, protein-based systems can perform logic operations to convert molecular inputs to regulated transcriptional events. In addition, a framework for the construction of single input–single output RNA devices based on the assembly of three functional components—a sensor component which is made of an RNA aptamer; an actuator component, made of a hammerhead ribozyme; and a transmitter component, made of a sequence that couples the sensor and actuator components [129]—was proposed.
RNA nanostructures will represent different inputs. The output, such as the activation of a pathway, is based on logic functions of input RNA concentrations. The beauty of the RNA approach is that there are many more variations in RNA than in DNA, as emphasized in figures 9– 11. As an example, using only one specific type of RNA structure as an input, we can construct a tremendous variety of different structures and functionalities through different methods, e.g. hand-in-hand (figure 9), foot-to-foot (figure 10) and junction core extension toolkits (figure 11). RNA logic gates can lead to a computer capacity that is comparable to the world's currently most powerful supercomputer. The future RNA computer will be compact; for example, a 1 cm3 space can hold 10 trillion RNA molecules. With this small amount of RNA, a computer would be able to hold 10 terabytes of data, and perform 10 trillion calculations at a time. By adding more RNA, more calculations could be performed. The RNA computer will be fast. In contrast to conventional computers, which operate linearly, RNA computers can perform parallel calculations. Such parallel computation allows RNA to solve in 1 h complex mathematical problems that would require hundreds of years for conventional computers to compete the task.
Figure 9.
AFM images of pRNA nanoparticles obtained by ‘hand-in-hand’ interaction following toolkit no. 1 [87]. (Adapted from [87]. © 2013 with permission from Cold Spring Harbor Laboratory Press.)
Figure 11.
AFM images of pRNA nanoparticles obtained by ‘foot-to-foot’ interaction following toolkit no. 3 [87]. (Adapted from [87]. © 2013 with permission from Cold Spring Harbor Laboratory Press.)
Figure 10.
AFM images of pRNA nanoparticles obtained by ‘foot-to-foot’ interaction following toolkit no. 2 [87]. (Adapted from [87]. © 2013 with permission from Cold Spring Harbor Laboratory Press.)
In addition, there are numerous small RNA regulators available [130,131]. By the trans- and cis-actions, we can use varieties of small RNA regulators to build in vivo products and functional pathways and control them with induction or repression. Three types of working methods are generally available: cooperative, synergistic and antagonistic, to produce computational logic circuits as conjunctive or disjunctive normal forms, or other kinds of logic operation [127,91,128].
7. RNA in vivo computation
Recent advances in RNA-based therapeutics have broadened the scope of therapeutic targets for a variety of human diseases ranging from genetic disorders to HIV infection and extending to various cancers. The concept of nanotechnology application to living systems has already been proven when molecular computers were found to be able to operate in, and directly communicate with, a biological environment [6,14].
In vitro demonstration of this computing device has been shown before [4] when DNA was the software encoded with input and output and DNA-manipulating enzymes were the hardware. Simple tasks could be performed; for example, determining whether a list of zeroes and ones had an even number of ones. Later, it was programmed to sense specific biomolecular concentrations (messenger RNA (mRNA)); perform a simple computation; and release a biomolecule (DNA) in response [19]. From this, abnormal concentrations of mRNA could be sensed, cancer diagnosed and an agent released to treat it in vitro. Seelig et al. [14] inserted Boolean logic gates over microRNA (miRNA) by DNA strand displacement and created multi-layered circuits based on electronic concepts such as signal restoration, amplification and feedback.
Rinaudo et al. [132] was able to program a biomolecular computing device to work inside a living cell. They inserted a plasmid with specifically encoded DNA and used an external program with the ability to control the computation inside the host cell. The mRNA was used to encode a fluorescent protein, and the target sequences for small interfering RNA (siRNA). They found that siRNA was able to control the level of fluorescence exhibited by the cell because of mRNA degradation that allowed siRNA the control to do so.
Win & Smolke [129] developed in vivo programming with computation independent of the cell machinery, yet that can respond to both endogenous and exogenous molecular signals. In their work, they used a combination of ribozymes and RNA aptamers [133,134]. The whole idea was simply to use the cleaving ability of ribozyme, which was regulated by binding aptamer RNA to a specific molecule, so that aptamer binding allowed cleavage or blocked cleavage by the ribozyme. They demonstrated Boolean logic operations using the concentrations of two proteins as input and the expression of green fluorescent protein (GFP) as output, implemented by the ribozyme–aptamer molecules using yeast. Their modular and easy to program system consisted of mRNA that encoded GFP with a modified control region (3′ untranslated region); one or more ribozyme–aptamer molecules were embedded in the region.
Recently, Auslander et al. [135] used the advances in RNA synthetic biology and designed standardized control devices using trigger-controlled transcription factors and RNA-binding proteins. These combinatorial circuits can be integrated as a two-molecule input and can perform digital computations with NOT, AND, NAND and N-IMPLY expression logic in single mammalian cells. Importantly, they showed that individual mammalian cells capable of executing basic molecular arithmetic functions isolated or coordinated to metabolic activities in a predictable, precise and robust manner. As the outcome, this information may provide new treatment strategies and bioelectronic interfaces in future gene-based and cell-based therapies.
Lou et al. [136] developed a memory module which is a toggle switch with two mutually repressed repressors, CI and CI434 genes from the lambda and 434 phages, respectively. The memory module is expected to function as follows: when CI is present, it activates transcription and represses the transcription of CI434, thus establishing a stable high CI/low CI434 state, which is defined as the ‘ON’ state of the memory module. In this case, red fluorescent protein is expressed. Alternatively, when CI434 is present, it can repress the transcription of CI, thus maintaining the transcription of itself and establishing a stable low CI/high CI434 state, which is defined as the ‘OFF’ state. Another approach used a scalable transcriptional/post-transcriptional synthetic regulatory circuit composed of HeLa cancer cell ‘classifier’ that senses expression levels of a customizable set of endogenous microRNAs and triggers a cellular response only if the expression levels match a predetermined profile of interest. This cancer cell classifier selectively identifies HeLa cells and triggers apoptosis without affecting non-HeLa cell types [137].
Based on these developments, we believe that RNA computing will provide a way forward for future biocomputing. Artificial RNA computers operating inside living cells would enable us to control cellular physiology. Our knowledge of RNA-based cell regulations is the most promising tool to construct in vivo computers. Nature has already given us all the information required to translate these RNA functions into digital molecular networks that embody standardized forms of logic functions.
8. Challenges for future RNA-based computation
RNA-based computation has the advantage of more variations and flexibility than DNA-based computation. But, the biochemistry reaction time is still a critical challenge. Inspired by the results and progress in DNA-based computing, RNA-based computing is promising. Xie et al. [138] reported that the observed rate constants of the siRNA output production are one to two orders of magnitude lower than those measured with model DNA strand-exchange substrates. But they have not built a scalable RNA computer and determined whether the RNA computer is slower or faster than the DNA computer at system level.
The interdisciplinary approach combining computer engineering and biochemistry is critical to the success of RNA-based computation. There are two complementary motivations for constructing molecular computing [138]. One is to solve the famous ‘NP-hard’ computational problem [11,139,140], the other is to build autonomous molecular computers that could potentially operate in vivo [6]. Figure 12 shows the development path of molecular-scale computing. We can borrow many concepts from electronic computer science and engineering to learn how to build a complicated molecular-based computer. Although by designing the logic circuit of AND/OR/NOT gates in a cell an ‘RNA computer’ can theoretically be designed and implemented in bacterial, yeast and mammalian systems [127], the way to harness the computer in a cell to our benefit is still a very long way off. The biggest challenge is that protein engineering is still in its infancy [141] and we are still very far away from building a molecular-based computer comparable to current electronic computers.
Figure 12.
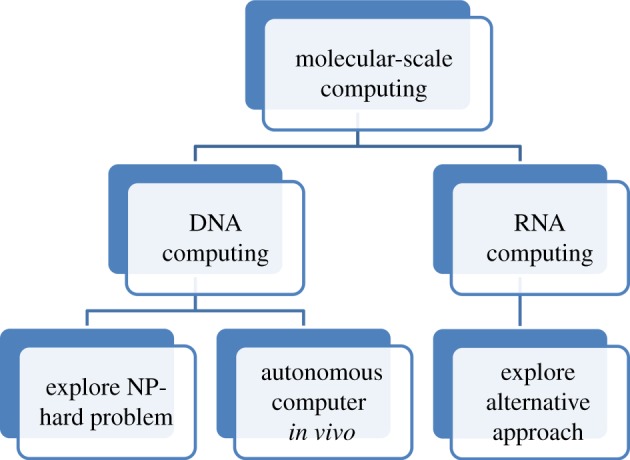
The development path of molecular-scale computing.
One of the biggest challenges associated with RNA-based intracellular computation is its instability. Despite the fact that RNA therapeutics such as ribozymes, aptamers and siRNAs have been shown to demonstrate exceptional versatility inside the body, delivery vehicles are still required for therapeutic moieties to efficiently transport them to the targeted cells. Because natural RNA is prone to RNase degradation this has hindered its application as a delivery vehicle. However, some progress has already been reported. This includes artificial modification of the RNA bases, phosphate backbones and the C-2′ functional group [68,142]. The introduction of peptide nucleic acids, locked nucleic acids (LNAs) and their respective derivatives polycarbamate nucleic acids [143] or LNA with a bridge at different positions (2′–4′, 1′–3′) also significantly improves the chemical and enzymatic stability of RNA structures [144]. However, it is believed that the most prominent is the replacement of the 2′-hydroxyl group with fluorine as this has a minimal detrimental effect on RNA folding and functions.
In addition to instability issues, the challenges of in vivo computation using RNA [127,145] include scaling the logic operations with a large number of inputs, extending input signal types and non-specific actions resulting in targeting unexpected or undesired pathways resulting in toxicity effects. The results of modifications related to RNA folding and in vivo toxicity of the nucleotide derivatives remain to be explored. Owing to the metabolism and biocompatibility issues, the most stable RNA might not necessarily be the most desirable; retention of particles within an appropriate time period is more attractive.
Another challenge associated with RNA ‘computers’ is the yield and cost of RNA production. As has been reported before [91], commercial RNA chemical synthesis can only offer 40 (conservative) to 80 (with low yield) nucleotides. Acetalester 2′-OH protecting groups, such as pivaloyloxymethyl, have been reported to enhance chemical synthesis of RNA. RNase ligase II has been shown to be a good alternative to the traditional T4 DNA ligase to generate longer RNA by ligation of two shorter synthetic RNA fragments [146]. Heterogeneity of the 3′-end of RNA products obtained during in vitro transcription is another issue [147]; this can be addressed by extending the transcribed sequence beyond the intended end and then cleaving the RNA at the desired site using ribozymes, DNAzymes or RNase H [146–148]. Large-scale RNA complexes produced in bacteria escorted by a tRNA vector have also been reported [149,150]. Based on the rapid reduction of cost over the history of DNA synthesis, it is expected that the cost of RNA synthesis will gradually decrease with the development of industrial-scale RNA production technologies.
In conclusion, natural or synthetic RNA molecules can fold into predefined structures that can spontaneously assemble into nanoparticles with multiple functionalities. The field of RNA nanotechnology is emerging but will play more and more important roles in medicine, biotechnology, synthetic biology and nanotechnology.
Funding statements
This paper is partially supported by NSF CNS-1249223 (M.Q.), NIH grants nos. R01 EB003730 (P.G.), R01 EB012135 (P.G.) and U01 CA151648 (P.G.). P.G. is a co-founder of Kylin Therapeutics, Inc., and Biomotor and Nucleic Acids Nanotech Development, Ltd.
Acknowledgements
We thank Thomas D. Schneider (National Institutes of Health) for his valuable comments, Jeannie Haak for editing this review, and Dr Franois Major for permission to use figure 3(a).
References
- 1.Qiu M, Sha EHM. 2009. Cost minimization while satisfying hard/soft timing constraints for heterogeneous embedded systems. ACM Trans. Des. Autom. Electron. Syst. 14, 25 ( 10.1145/1497561.1497568) [DOI] [Google Scholar]
- 2.US Environmental Protection Agency 2007. Report to Congress on server and data center energy efficiency. Public Law 109–431 Washington, DC: US Environmental Protection Agency. [Google Scholar]
- 3.Ogihara M, Ray A. 2000. Molecular computation: DNA computing on a chip. Nature 403, 143–144. ( 10.1038/35003071) [DOI] [PubMed] [Google Scholar]
- 4.Benenson Y, Paz-Elizur T, Adar R, Keinan E, Livneh Z, Shapiro E. 2001. Programmable and autonomous computing machine made of biomolecules. Nature 414, 430–434. ( 10.1038/35106533) [DOI] [PubMed] [Google Scholar]
- 5.Reif JH. 2002. Computing: successes and challenges. Science 296, 478–479. ( 10.1126/science.1070978) [DOI] [PubMed] [Google Scholar]
- 6.Benenson Y, Gil B, Ben-Dor U, Adar R, Shapiro E. 2004. An autonomous molecular computer for logical control of gene expression. Nature 429, 423–429. ( 10.1038/nature02551) [DOI] [PubMed] [Google Scholar]
- 7.De Silva AP. 2005. Molecular computation: molecular logic gets loaded. Nat. Mater. 4, 15–16. ( 10.1038/nmat1301) [DOI] [Google Scholar]
- 8.John PH. 1993. Introduction to digital logic design, Boston, MA: Addison-Wesley Longman. [Google Scholar]
- 9.Archer GP, Bone S, Pethig R. 1990. Dielectric studies of bistable ionic transitions in solid-state DNA: model of relevance to the development of molecular logic systems. J. Mol. Electron. 6, 199–207. [Google Scholar]
- 10.Overbeek R. 1989. Application of logic programming techniques to DNA-sequence gel-reading (logic programming, expert systems). Abstr. Pap. Am. Chem. Soc. 198, COM10. [Google Scholar]
- 11.Adleman LM. 1994. Molecular computation of solutions to combinatorial problems. Science 266, 1021–1024. ( 10.1126/science.7973651) [DOI] [PubMed] [Google Scholar]
- 12.Soloveichik D, Seelig G, Winfree E. 2010. DNA as a universal substrate for chemical kinetics. Proc. Natl Acad. Sci. USA 107, 5393–5398. ( 10.1073/pnas.0909380107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips A, Cardelli L. 2009. A programming language for composable DNA circuits. J. R. Soc. Interface 6, S419–S436. ( 10.1098/rsif.2009.0072.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seelig G, Soloveichik D, Zhang DY, Winfree E. 2006. Enzyme-free nucleic acid logic circuits. Science 314, 1585–1588. ( 10.1126/science.1132493) [DOI] [PubMed] [Google Scholar]
- 15.Zhang DY, Turberfield AJ, Yurke B, Winfree E. 2007. Engineering entropy-driven reactions and networks catalyzed by DNA. Science 318, 1121–1125. ( 10.1126/science.1148532) [DOI] [PubMed] [Google Scholar]
- 16.Yin P, Choi HMT, Calvert CR, Pierce NA. 2008. Programming biomolecular self-assembly pathways. Nature 451, 318–322. ( 10.1038/nature06451) [DOI] [PubMed] [Google Scholar]
- 17.Nagaraj SH, Reverter A. 2011. A Boolean-based systems biology approach to predict novel genes associated with cancer: application to colorectal cancer. BMC Syst. Biol. 5, 35 ( 10.1186/1752-0509-5-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Pelech S, Neve RM, Kuo WL, Ziyad S, Spellman PT, Gray JW, Speed TP. 2009. Sparse combinatorial inference with an application in cancer biology. Bioinformatics 25 ( 10.1093/bioinformatics/btn611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao C, LaBean TH, Relf JH, Seeman NC. 2000. Logical computation using algorithmic self-assembly of DNA triple-crossover molecules. Nature 407, 493–496. ( 10.1038/35035038) [DOI] [PubMed] [Google Scholar]
- 20.Elbaz J, Moshe M, Willner I. 2009. Coherent activation of DNA tweezers: a ‘SET-RESET’ logic system. Angew. Chem. Int. Ed. Engl. 48, 3834–3837. ( 10.1002/anie.200805819) [DOI] [PubMed] [Google Scholar]
- 21.Freeman R, Finder T, Willner I. 2009. Multiplexed analysis of Hg2+ and Ag+ ions by nucleic acid functionalized CdSe/ZnS quantum dots and their use for logic gate operations. Angew. Chem. Int. Ed. Engl. 48, 7818–7821. ( 10.1002/anie.200902395) [DOI] [PubMed] [Google Scholar]
- 22.Frezza BM, Cockroft SL, Ghadiri MR. 2007. Modular multi-level circuits from immobilized DNA-based logic gates. J. Am. Chem. Soc. 129, 14 ( 10.1021/ja0710149) [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi D, Inoue M, Sugimoto N. 2006. DNA logic gates based on structural polymorphism of telomere DNA molecules responding to chemical input signals. Angew. Chem. Int. Ed. Engl. 45, 7716–7719. ( 10.1002/anie.200602404) [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Xue X, Li T, Zeng H, Liu X. 2009. Ultrasensitive and selective colorimetric DNA detection by nicking endonuclease assisted nanoparticle amplification. Angew. Chem. Int. Ed. Engl. 48, 6849–6852. ( 10.1002/anie.200901772) [DOI] [PubMed] [Google Scholar]
- 25.Beissenhirtz MK, Elnathan R, Weizmann Y, Willner I. 2007. The aggregation of Au nanoparticles by an autonomous DNA machine detects viruses. Small 3, 375–379. ( 10.1002/smll.200600450) [DOI] [PubMed] [Google Scholar]
- 26.Zhang YM, Zhang L, Liang RP, Qiu JD. 2013. DNA electronic logic gates based on metal-ion-dependent induction of oligonucleotide structural motifs. Chemistry 19, 6961–6965. ( 10.1002/chem.201300625) [DOI] [PubMed] [Google Scholar]
- 27.Park KS, Seo MW, Jung C, Lee JY, Park HG. 2012. Simple and universal platform for logic gate operations based on molecular beacon probes. Small 8, 2129 ( 10.1002/smll.201290077) [DOI] [PubMed] [Google Scholar]
- 28.Mor-Piperberg G, Tel-Vered R, Elbaz J, Willner I. 2010. Nanoengineered electrically contacted enzymes on DNA scaffolds: functional assemblies for the selective analysis of Hg2+ ions. J. Am. Chem. Soc. 132, 6878–6879. ( 10.1021/ja1006355) [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wang L, Frutos AG, Condon AE, Corn RM, Smith LM. 2000. DNA computing on surfaces. Nature 403, 175–179. ( 10.1038/35003155) [DOI] [PubMed] [Google Scholar]
- 30.Bi S, Yan Y, Hao S, Zhang S. 2010. Colorimetric logic gates based on supramolecular DNAzyme structures. Angew. Chem. Int. Ed. Engl. 49, 4438–4442. ( 10.1002/anie.201000840) [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Zhang YM, Liang RP, Qiu JD. 2013. Colorimetric logic gates based on ion-dependent DNAzymes. J. Phys. Chem. C 117, 12 ( 10.1021/jp4027892) [DOI] [Google Scholar]
- 32.Elbaz J, Lioubashevski O, Wang FA, Remacle F, Levine RD, Willner I. 2010. DNA computing circuits using libraries of DNAzyme subunits. Nat. Nanotechnol. 5, 417–422. ( 10.1038/nnano.2010.88) [DOI] [PubMed] [Google Scholar]
- 33.Qian L, Winfree E. 2011. Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201. ( 10.1126/science.1200520) [DOI] [PubMed] [Google Scholar]
- 34.Guo P, Zhang C, Chen C, Trottier M, Garver K. 1998. Inter-RNA interaction of phage φ29 pRNA to form a hexameric complex for viral DNA transportation. Mol. Cell 2, 149–155. ( 10.1016/S1097-2765(00)80124-0) [DOI] [PubMed] [Google Scholar]
- 35.Guo S, Tschammer N, Mohammed S, Guo P. 2005. Specific delivery of therapeutic RNAs to cancer cells via the dimerization mechanism of ϕ29 motor pRNA. Hum. Gene Ther. 16, 1097–1109. ( 10.1089/hum.2005.16.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu D, Huang L, Hoeprich S, Guo P. 2003. Construction of ϕ29 DNA-packaging RNA (pRNA) monomers, dimers and trimers with variable sizes and shapes as potential parts for nano-devices. J. Nanosci. Nanotechnol. 3, 295–302. ( 10.1166/jnn.2003.160) [DOI] [PubMed] [Google Scholar]
- 37.Shu D, Moll WD, Deng Z, Mao C, Guo P. 2004. Bottom-up assembly of RNA arrays and superstructures as potential parts in nanotechnology. Nano Lett. 4, 1717–1723. ( 10.1021/nl0494497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu Y, Cinier M, Shu D, Guo P. 2011. Assembly of multifunctional ϕ29 pRNA nanoparticles for specific delivery of siRNA and other therapeutics to targeted cells. Methods 54, 204–214. ( 10.1016/j.ymeth.2011.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Lemieux S, Wu X, St -Arnaud S, McMurray CT, Major F, Anderson D. 1998. Function of hexameric RNA in packaging of bacteriophage ϕ29 DNA in vitro. Mol. Cell 2, 141–147. ( 10.1016/S1097-2765(00)80123-9) [DOI] [PubMed] [Google Scholar]
- 40.Hendrix RW. 1998. Bacteriophage DNA packaging: RNA gears in a DNA transport machine (minireview). Cell 94, 147–150. ( 10.1016/S0092-8674(00)81413-0) [DOI] [PubMed] [Google Scholar]
- 41.Shu D, Shu Y, Haque F, Abdelmawla S, Guo P. 2011. Thermodynamically stable RNA three-way junctions for constructing multifuntional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 6, 658–667. ( 10.1038/nnano.2011.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haque F, Shu D, Shu Y, Shlyakhtenko L, Rychahou P, Evers M, Guo P. 2012. Ultrastable synergistic tetravalent RNA nanoparticles for targeting to cancers. Nano Today 7, 245–257. ( 10.1016/j.nantod.2012.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khaled A, Guo S, Li F, Guo P. 2005. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 5, 1797–1808. ( 10.1021/nl051264s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo S, Huang F, Guo P. 2006. Construction of folate-conjugated pRNA of bacteriophage ϕ29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells. Gene Ther. 13, 814–820. ( 10.1038/sj.gt.3302856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoeprich S, Zhou Q, Guo S, Qi G, Wang Y, Guo P. 2003. Bacterial virus ϕ29 pRNA as a hammerhead ribozyme escort to destroy hepatitis B virus. Gene Ther. 10, 1258–1267. ( 10.1038/sj.gt.3302002) [DOI] [PubMed] [Google Scholar]
- 46.Shu Y, Cinier M, Fox SR, Ben-Johnathan N, Guo P. 2011. Assembly of therapeutic pRNA–siRNA nanoparticles using bipartite approach. Mol. Ther. 19, 1304–1311. ( 10.1038/mt.2011.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shu Y, Shu D, Diao Z, Shen G, Guo P. 2009. Fabrication of polyvalent therapeutic RNA nanoparticles for specific delivery of siRNA, ribozyme and drugs to targeted cells for cancer therapy. In Proc. Life Science Systems and Applications Workshop (LiSSA 2009), Bethesda, MD, 910 April 2009 9–12Piscataway, NJ: IEEE; ( 10.1109/LISSA.2009.4906696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelmawla S, Guo S, Zhang L, Pulukuri S, Patankar P, Conley P, Trebley J, Guo P, Li QX. 2011. Pharmacological characterization of chemically synthesized monomeric pRNA nanoparticles for systemic delivery. Mol. Ther. 19, 1312–1322. ( 10.1038/mt.2011.35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye X, Hemida M, Zhang HM, Hanson P, Ye Q, Yang D. 2012. Current advances in ϕ29 pRNA biology and its application in drug delivery. Wiley Interdiscip. Rev. RNA 3, 469–481. ( 10.1002/wrna.1111) [DOI] [PubMed] [Google Scholar]
- 50.Ye X, Liu Z, Hemida MG, Yang D. 2011. Targeted delivery of mutant tolerant anti-coxsackievirus artificial microRNAs using folate conjugated bacteriophage ϕ29 pRNA. PLoS ONE 6, 21215 ( 10.1371/journal.pone.0021215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leontis NB, Westhof E. 2003. Analysis of RNA motifs. Curr. Opin. Struct. Biol. 13, 300–308. ( 10.1016/S0959-440X(03)00076-9) [DOI] [PubMed] [Google Scholar]
- 52.Lescoute A, Westhof E. 2006. Topology of three-way junctions in folded RNAs. RNA 12, 83–93. ( 10.1261/rna.2208106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jossinet F, Ludwig TE, Westhof E. 2007. RNA structure: bioinformatic analysis. Curr. Opin. Microbiol. 10, 279–285. ( 10.1016/j.mib.2007.05.010) [DOI] [PubMed] [Google Scholar]
- 54.Jaeger L, Westhof E, Leontis NB. 2001. TectoRNA: modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 29, 455–463. ( 10.1093/nar/29.2.455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leontis NB, Lescoute A, Westhof E. 2006. The building blocks and motifs of RNA architecture. Curr. Opin. Struct. Biol. 16, 279–287. ( 10.1016/j.sbi.2006.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lilley DM. 1999. Structure, folding and catalysis of the small nucleolytic ribozymes. Curr. Opin. Struct. Biol. 9, 330–338. ( 10.1016/S0959-440X(99)80044-X) [DOI] [PubMed] [Google Scholar]
- 57.McKinney SA, Declais AC, Lilley DMJ, Ha T. 2003. Structural dynamics of individual Holliday junctions. Nat. Struct. Biol. 10, 93–97. ( 10.1038/nsb883) [DOI] [PubMed] [Google Scholar]
- 58.Schroeder KT, McPhee SA, Ouellet J, Lilley DM. 2010. A structural database for k-turn motifs in RNA. RNA 16, 1463–1468. ( 10.1261/rna.2207910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afonin KA, Bindewald E, Yaghoubian AJ, Voss N, Jacovetty E, Shapiro BA, Jaeger L. 2010. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat. Nanotechnol. 5 ( 10.1038/nnano.2010.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Severcan I, Geary C, Verzemnieks E, Chworos A, Jaeger L. 2009. Square-shaped RNA particles from different RNA folds. Nano Lett. 9, 1270–1277. ( 10.1021/nl900261h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Severcan I, Geary C, Chworos A, Voss N, Jacovetty E, Jaeger L. 2010. A polyhedron made of tRNAs. Nat. Chem. 2, 772–779. ( 10.1038/nchem.733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chworos A, Severcan I, Koyfman AY, Weinkam P, Oroudjev E, Hansma HG, Jaeger L. 2004. Building programmable jigsaw puzzles with RNA. Science 306, 2068–2072. ( 10.1126/science.1104686) [DOI] [PubMed] [Google Scholar]
- 63.Jaeger L, Leontis NB. 2000. Tecto-RNA: one dimensional self-assembly through tertiary interactions. Angew. Chem. Int. Ed. Engl. 39, 2521–2524. () [DOI] [PubMed] [Google Scholar]
- 64.Liu B, Baudrey S, Jaeger L, Bazan GC. 2004. Characterization of tectoRNA assembly with cationic conjugated polymers. J. Am. Chem. Soc. 126, 4076–4077. ( 10.1021/ja031552v) [DOI] [PubMed] [Google Scholar]
- 65.Geary C, Chworos A, Jaeger L. 2010. Promoting RNA helical stacking via A-minor junctions. Nucleic Acids Res. 39, 1066–1080. ( 10.1093/nar/gkq748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yingling YG, Shapiro BA. 2007. Computational design of an RNA hexagonal nanoring and an RNA nanotube. Nano Lett. 7, 2328–2334. ( 10.1021/nl070984r) [DOI] [PubMed] [Google Scholar]
- 67.Bindewald E, Grunewald C, Boyle B, O'Connor M, Shapiro BA. 2008. Computational strategies for the automated design of RNA nanoscale structures from building blocks using NanoTiler. J. Mol. Graph. Model. 27, 299–308. ( 10.1016/j.jmgm.2008.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grabow WW, Zakrevsky P, Afonin KA, Chworos A, Shapiro BA, Jaeger L. 2011. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett. 11, 878–887. ( 10.1021/nl104271s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapiro BA, Bindewald E, Kasprzak W, Yingling Y. 2008. Protocols for the in silico design of RNA nanostructures. Methods Mol. Biol. 474, 93–115. ( 10.1007/978-1-59745-480-3_7) [DOI] [PubMed] [Google Scholar]
- 70.Ikawa Y, Tsuda K, Matsumura S, Inoue T. 2004. De novo synthesis and development of an RNA enzyme. Proc. Natl Acad. Sci. USA 101, 13 ( 10.1073/pnas.0405886101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumura S, Ohmori R, Saito H, Ikawa Y, Inoue T. 2009. Coordinated control of a designed trans-acting ligase ribozyme by a loop-receptor interaction. FEBS Lett. 583, 2819–2826. ( 10.1016/j.febslet.2009.07.036) [DOI] [PubMed] [Google Scholar]
- 72.Li X, Horiya S, Harada K. 2006. An efficient thermally induced RNA conformational switch as a framework for the functionalization of RNA nanostructures. J. Am. Chem. Soc. 128 ( 10.1021/ja0572093) [DOI] [PubMed] [Google Scholar]
- 73.Leontis NB, Westhof E. 2001. Geometric nomenclature and classification of RNA base pairs. RNA 7, 499–512. ( 10.1017/S1355838201002515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leontis NB, Westhof E. 1998. Conserved geometrical base-pairing patterns in RNA. Q. Rev. Biophys. 31, 399–455. ( 10.1017/S0033583599003479) [DOI] [PubMed] [Google Scholar]
- 75.Brion P, Westhof E. 1997. Hierarchy and dynamics of RNA folding. Annu. Rev. Biophys. Biomol. Struct. 26, 113–137. ( 10.1146/annurev.biophys.26.1.113) [DOI] [PubMed] [Google Scholar]
- 76.Westhof E, Masquida B, Jaeger L. 1996. RNA tectonics: towards RNA design. Fold. Des. 1, 78 ( 10.1016/S1359-0278(96)00037-5) [DOI] [PubMed] [Google Scholar]
- 77.Searle MS, Williams DH. 1993. On the stability of nucleic acid structures in solution: enthalpy–entropy compensations, internal rotations and reversibility. Nucleic Acids Res. 21, 2051–2056. ( 10.1093/nar/21.9.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. 1995. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 34, 11 ( 10.1021/bi00035a029) [DOI] [PubMed] [Google Scholar]
- 79.Freier SM, Kierzek R, Jaeger JA, Sugimoto N, Caruthers MH, Neilson T, Turner DH. 1986. Improved free-energy parameters for predictions of RNA duplex stability. Proc. Natl Acad. Sci. USA 83, 9373–9377. ( 10.1073/pnas.83.24.9373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laurenti E, et al. 2010. Inducible gene and shRNA expression in resident hematopoietic stem cells in vivo. Stem Cells 28, 1390–1398. ( 10.1002/stem.460) [DOI] [PubMed] [Google Scholar]
- 81.Chang KY, Tinoco I., Jr 1994. Characterization of a ‘kissing’ hairpin complex derived from the human immunodeficiency virus genome. Proc. Natl Acad. Sci. USA 91, 8705–8709. ( 10.1073/pnas.91.18.8705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bindewald E, Hayes R, Yingling YG, Kasprzak W, Shapiro BA. 2008. RNAJunction: a database of RNA junctions and kissing loops for three-dimensional structural analysis and nanodesign. Nucleic Acids Res. 36, 392 ( 10.1093/nar/gkm842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner C, Ehresmann C, Ehresmann B, Brunel C. 2004. Mechanism of dimerization of bicoid mRNA: initiation and stabilization. J. Biol. Chem. 279, 4560–4569. ( 10.1074/jbc.M306511200) [DOI] [PubMed] [Google Scholar]
- 84.Chen C, Sheng S, Shao Z, Guo P. 2000. A dimer as a building block in assembling RNA: a hexamer that gears bacterial virus ϕ29 DNA-translocating machinery. J. Biol. Chem. 275, 17 ( 10.1074/jbc.M909662199) [DOI] [PubMed] [Google Scholar]
- 85.Guo P, Erickson S, Anderson D. 1987. A small viral RNA is required for in vitro packaging of bacteriophage ϕ29 DNA. Science 236, 690–694. ( 10.1126/science.3107124) [DOI] [PubMed] [Google Scholar]
- 86.Reif R, Haque F, Guo P. 2013. Fluorogenic RNA nanoparticles for monitoring RNA folding and degradation in real time in living cells. Nucleic Acid Ther. 22, 428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shu Y, Haque F, Shu D, Li W, Zhu Z, Kotb M, Lyubchenko Y, Guo P. 2012. Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. RNA 19, 767–777. ( 10.1261/rna.037002.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartz C, De Donatis GM, Fang H, Guo P. 2013. The ATPase of the ϕ29 DNA packaging motor is a member of the hexameric AAA+ superfamily. Virology 443, 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aldaye FA, Palmer AL, Sleiman HF. 2008. Assembling materials with DNA as the guide. Science 321, 1795–1799. ( 10.1126/science.1154533) [DOI] [PubMed] [Google Scholar]
- 90.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. 2003. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 301, 1882–1884 [DOI] [PubMed] [Google Scholar]
- 91.Guo P. 2010. The emerging field of RNA nanotechnology. Nat. Nanotechnol. 5, 833–842. ( 10.1038/nnano.2010.231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. 2005. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res 33, 121 ( 10.1093/nar/gki081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abraham M, Dror O, Nussinov R, Wolfson HJ. 2008. Analysis and classification of RNA tertiary structures. RNA 14, 2274–2289. ( 10.1261/rna.853208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen C, Zhang C, Guo P. 1999. Sequence requirement for hand-in-hand interaction in formation of pRNA dimers and hexamers to gear ϕ29 DNA translocation motor. RNA 5, 805–818. ( 10.1017/S1355838299990350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao F, Demeler B, Guo P. 2010. Assembly mechanism of the sixty-subunit nanoparticles via interaction of RNA with the reengineered protein connector of ϕ29 DNA-packaging motor. ACS Nano 4, 3293–3301. ( 10.1021/nn100158k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu D, Zhang H, Jin J, Guo P. 2007. Counting of six pRNAs of ϕ29 DNA-packaging motor with customized single molecule dual-view system. EMBO J. 26, 527–537. ( 10.1038/sj.emboj.7601506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellington AD, Szostak JW. 1990. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822. ( 10.1038/346818a0) [DOI] [PubMed] [Google Scholar]
- 98.Tuerk C, Gold L. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA ploymerase. Science 249, 505–510. ( 10.1126/science.2200121) [DOI] [PubMed] [Google Scholar]
- 99.Liu D, Wang M, Deng Z, Walulu R, Mao C. 2004. Tensegrity: construction of rigid DNA triangles with flexible four-arm DNA junctions. J. Am. Chem. Soc. 126, 2324–2325. ( 10.1021/ja031754r) [DOI] [PubMed] [Google Scholar]
- 100.Rothemund PW, Papadakis N, Winfree E. 2004. Algorithmic self-assembly of DNA Sierpinski triangles. PLoS Biol. 2, 424 ( 10.1371/journal.pbio.0020424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park SH, Barish R, Li H, Reif JH, Finkelstein G, Yan H, LaBean TH. 2005. Three-helix bundle DNA tiles self-assemble into 2D lattice or 1D templates for silver nanowires. Nano Lett. 5, 693–696. ( 10.1021/nl050108i) [DOI] [PubMed] [Google Scholar]
- 102.Weizmann Y, Braunschweig AB, Wilner OI, Cheglakov Z, Willner I. 2008. A polycatenated DNA scaffold for the one-step assembly of hierarchical nanostructures. Proc. Natl Acad. Sci. USA 105, 5289–5294. ( 10.1073/pnas.0800723105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rothemund PWK. 2006. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302. ( 10.1038/nature04586) [DOI] [PubMed] [Google Scholar]
- 104.Andersen ES, et al. 2009. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 459, 73–76. ( 10.1038/nature07971) [DOI] [PubMed] [Google Scholar]
- 105.Ke YG, Lindsay S, Chang Y, Liu Y, Yan H. 2008. Self-assembled water-soluble nucleic acid probe tiles for label-free RNA hybridization assays. Science 319, 180–183. ( 10.1126/science.1150082) [DOI] [PubMed] [Google Scholar]
- 106.Douglas SM, Chou JJ, Shih WM. 2007. DNA-nanotube-induced alignment of membrane proteins for NMR structure determination. Proc. Natl Acad. Sci. USA 104, 6644–6648. ( 10.1073/pnas.0700930104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Endo M, Seeman NC, Majima T. 2005. DNA tube structures controlled by a four-way-branched DNA connector. Angew. Chem. Int. Ed. Engl. 44, 6074–6077. ( 10.1002/anie.200501034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanaka K, Tengeiji A, Kato T, Toyama N, Shionoya M. 2003. A discrete self-assembled metal array in artificial DNA. Science 299, 1212–1213. ( 10.1126/science.1080587) [DOI] [PubMed] [Google Scholar]
- 109.Aldaye FA, Sleiman HF. 2007. Modular access to structurally switchable 3D discrete DNA assemblies. J. Am. Chem. Soc. 129, 13 ( 10.1021/ja075966q) [DOI] [PubMed] [Google Scholar]
- 110.Yurke B, Turberfield AJ, Mills AP, Jr, Simmel FC, Neumann JL. 2000. A DNA-fuelled molecular machine made of DNA. Nature 406, 605–608. ( 10.1038/35020524) [DOI] [PubMed] [Google Scholar]
- 111.Han D, Pal S, Liu Y, Yan H. 2010. Folding and cutting DNA into reconfigurable topological nanostructures. Nat. Nanotechnol. 5, 712–717. ( 10.1038/nnano.2010.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Endo M, Sugita T, Katsuda Y, Hidaka K, Sugiyama H. 2010. Programmed-assembly system using DNA jigsaw pieces. Chemistry 16, 5362–5368 [DOI] [PubMed] [Google Scholar]
- 113.Dietz H, Douglas SM, Shih WM. 2009. Folding DNA into twisted and curved nanoscale shapes. Science 325, 725–730. ( 10.1126/science.1174251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cayrol B, Nogues C, Dawid A, Sagi I, Silberzan P, Isambert H. 2009. A nanostructure made of a bacterial noncoding RNA. J. Am. Chem. Soc. 131, 17 ( 10.1021/ja906076e) [DOI] [PubMed] [Google Scholar]
- 115.Lin C, Liu Y, Yan H. 2009. Designer DNA nanoarchitectures. Biochemistry 48, 1663–1674. ( 10.1021/bi802324w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seeman NC. 2010. Nanomaterials based on DNA. Annu. Rev. Biochem. 79, 65–87. ( 10.1146/annurev-biochem-060308-102244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415. ( 10.1093/nar/gkg595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Markham NR, Zuker M. 2008. UNAFold: software for nucleic acid folding and hybridization. Methods Mol. Biol. 453, 3–31. ( 10.1007/978-1-60327-429-6_1) [DOI] [PubMed] [Google Scholar]
- 119.Shapiro BA. 2009. Computational design strategies for RNA nanostructures. J. Biomol. Struct. Dyn. 26, 820 [Google Scholar]
- 120.Martinez HM, Maizel JV, Shapiro BA. 2008. RNA2D3D: a program for generating, viewing, and comparing 3-dimensional models of RNA. J. Biomol. Struct. Dyn. 25, 669–683. ( 10.1080/07391102.2008.10531240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kasprzak W, Bindewald E, Kim TJ, Jaeger L, Shapiro BA. 2010. Use of RNA structure flexibility data in nanostructure modeling. Methods 54, 239–250. ( 10.1016/j.ymeth.2010.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paliy M, Melnik R, Shapiro BA. 2009. Molecular dynamics study of the RNA ring nanostructure: a phenomenon of self-stabilization. Phys. Biol. 6, 046003 ( 10.1088/1478-3975/6/4/046003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarver M, Zirbel CL, Stombaugh J, Mokdad A, Leontis NB. 2008. FR3D: finding local and composite recurrent structural motifs in RNA 3D structures. J. Math. Biol. 56, 215–252. ( 10.1007/s00285-007-0110-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westhof E. 2012. Ribozymes, catalytically active RNA molecules: introduction. Methods Mol. Biol. 848, 1–4. ( 10.1007/978-1-61779-545-9_1) [DOI] [PubMed] [Google Scholar]
- 125.Shu D, Zhang H, Petrenko R, Meller J, Guo P. 2010. Dual-channel single-molecule fluorescence resonance energy transfer to establish distance parameters for RNA nanoparticles. ACS Nano 4, 6843–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shu D, Shu Y, Haque F, Abdelmawla S, Guo P. 2011. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 6, 658–667. ( 10.1038/nnano.2011.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benenson Y. 2009. RNA-based computation in live cells. Curr. Opin. Biotechnol. 20, 471–478. ( 10.1016/j.copbio.2009.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Breaker RR. 2008. Complex riboswitches. Science 319, 1795–1797. ( 10.1126/science.1152621) [DOI] [PubMed] [Google Scholar]
- 129.Win MN, Smolke CD. 2008. Higher-order cellular information processing with synthetic RNA devices. Science 322, 456–460. ( 10.1126/science.1160311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mattick JS, Makunin IV. 2005. Small regulatory RNAs in mammals. Hum. Mol. Genet. 14, 121 ( 10.1093/hmg/ddi101) [DOI] [PubMed] [Google Scholar]
- 131.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43, 880–891. ( 10.1016/j.molcel.2011.08.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rinaudo K, Bleris L, Maddamsetti R, Subramanian S, Weiss R, Benenson Y. 2007. A universal RNAi-based logic evaluator that operates in mammalian cells. Nat. Biotechnol. 25, 795–801. ( 10.1038/nbt1307) [DOI] [PubMed] [Google Scholar]
- 133.Breaker RR. 2002. Engineered allosteric ribozymes as biosensor components. Curr. Opin. Biotechnol. 13, 31–39. ( 10.1016/S0958-1669(02)00281-1) [DOI] [PubMed] [Google Scholar]
- 134.Suess B, Weigand JE. 2008. Engineered riboswitches: overview, problems and trends. RNA Biol. 5, 24–29. ( 10.4161/rna.5.1.5955) [DOI] [PubMed] [Google Scholar]
- 135.Auslander S, Auslander D, Muller M, Wieland M, Fussenegger M. 2012. Programmable single-cell mammalian biocomputers. Nature 487, 123–127. ( 10.1038/nature11149) [DOI] [PubMed] [Google Scholar]
- 136.Lou C, et al. 2010. Synthesizing a novel genetic sequential logic circuit: a push-on push-off switch. Mol. Syst. Biol. 6, 350 ( 10.1038/msb.2010.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie Z, Wroblewska L, Prochazka L, Weiss R, Benenson Y. 2011. Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science 333, 1307–1311. ( 10.1126/science.1205527) [DOI] [PubMed] [Google Scholar]
- 138.Xie Z, Liu SJ, Bleris L, Benenson Y. 2010. Logic integration of mRNA signals by an RNAi-based molecular computer. Nucleic Acids Res. 38, 2692–2701. ( 10.1093/nar/gkq117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lipton RJ. 1995. DNA solution of hard computational problems. Science 268, 542–545. ( 10.1126/science.7725098) [DOI] [PubMed] [Google Scholar]
- 140.Faulhammer D, Cukras AR, Lipton RJ, Landweber LF. 2000. Molecular computation: RNA solutions to chess problems. Proc. Natl Acad. Sci. USA 97, 1385–1389. ( 10.1073/pnas.97.4.1385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shapiro E, Gil B. 2008. Cell biology. RNA computing in a living cell. Science 322, 387–388. ( 10.1126/science.1165665) [DOI] [PubMed] [Google Scholar]
- 142.Watts JK, Deleavey GF, Damha MJ. 2008. Chemically modified siRNA: tools and applications. Drug Discov. Today 13, 842–855. ( 10.1016/j.drudis.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 143.Madhuri V, Kumar VA. 2010. Design, synthesis and DNA/RNA binding studies of nucleic acids comprising stereoregular and acyclic polycarbamate backbone: polycarbamate nucleic acids (PCNA). Org. Biomol. Chem. 8, 3734–3741. ( 10.1039/c003405n) [DOI] [PubMed] [Google Scholar]
- 144.Mathe C, Perigaud C. 2008. Recent approaches in the synthesis of conformationally restricted nucleoside analogues. Eur. J. Org. Chem. 2008, 1489–1505. ( 10.1002/ejoc.200700946) [DOI] [Google Scholar]
- 145.Shlyakhtenko LS, Gall AA, Filonov A, Cerovac Z, Lushnikov A, Lyubchenko YL. 2003. Silatrane-based surface chemistry for immobilization of DNA, protein-DNA complexes and other biological materials. Ultramicroscopy 97, 279–287. ( 10.1016/S0304-3991(03)00053-6) [DOI] [PubMed] [Google Scholar]
- 146.Solomatin S, Herschlag D. 2009. Methods of site-specific labeling of RNA with fluorescent dyes methods in enzymology. In Biophysical, chemical, and functional probes of RNA structure, interactions and folding (ed. Herschlag D.), pp. 47–68. Methods in Enzymology, vol. 469 San Diego, CA: Academic Press; ( 10.1016/S0076-6879(09)69003-0) [DOI] [PubMed] [Google Scholar]
- 147.Lavergne T, Bertrand JR, Vasseur JJ, Debart F. 2008. A base-labile group for 2′-OH protection of ribonucleosides: a major challenge for RNA synthesis. Chemistry 14, 9135–9138. ( 10.1002/chem.200801392) [DOI] [PubMed] [Google Scholar]
- 148.Hoeprich S, Guo P. 2002. Computer modeling of three-dimensional structure of DNA-packaging RNA(pRNA) monomer, dimer, and hexamer of ϕ29 DNA packaging motor. J. Biol. Chem. 277, 20 ( 10.1074/jbc.M112061200) [DOI] [PubMed] [Google Scholar]
- 149.Ponchon L, Beauvais G, Nonin-Lecomte S, Dardel F. 2009. A generic protocol for the expression and purification of recombinant RNA in Escherichia coli using a tRNA scaffold. Nat. Protoc. 4, 947–959. ( 10.1038/nprot.2009.67) [DOI] [PubMed] [Google Scholar]
- 150.Kuwabara T, Warashina M, Orita M, Koseki S, Ohkawa J, Taira K. 1998. Formation of a catalytically active dimer by tRNA-driven short ribozymes. Nat. Biotechnol. 16, 961–965. ( 10.1038/nbt1098-961) [DOI] [PubMed] [Google Scholar]