SUMMARY
Increasing data support a connection between obstructive sleep apnea (OSA) and cognitive impairment but a causal link has yet to be established. Although neuronal loss has been linked to cognitive impairment, emerging theories propose that changes in synaptic plasticity can cause cognitive impairment. Studies demonstrate that disruption to the blood–brain barrier (BBB), which is uniquely structured to tightly maintain homeostasis inside the brain, leads to changes in the brain’s microenvironment and affects synaptic plasticity. Cyclical intermittent hypoxia is a stressor that could disrupt the BBB via molecular responses already known to occur in either OSA patients or animal models of intermittent hypoxia. However, we do not yet know if or how intermittent hypoxia can cause cognitive impairment by mechanisms operating at the BBB. Therefore, we propose that initially, adaptive homeostatic responses at the BBB occur in response to increased oxygen and nutrient demand, specifically through regulation of influx and efflux BBB transporters that alter microvessel permeability. We further hypothesize that although these responses are initially adaptive, these changes in BBB transporters can have long-term consequences that disrupt the brain’s microenvironment and alter synaptic plasticity leading to cognitive impairment.
Keywords: Obstructive sleep apnea, Blood–brain barrier, Cognitive impairment, Oxidative stress, Oxygen sensor, Hif1α, Hif2α, Chronic inflammation, Peroxisome proliferator-activated receptor, Angiogenesis, Microvessel permeability, Leak, Blood–brain barrier transporters, Pore
Introduction
The study of obstructive sleep apnea (OSA) is important because its incidence and prevalence is likely to be increasing and because OSA is associated with many chronic diseases. Obesity is the major risk factor for OSA in middle-aged adults1–4 and with obesity rates increasing, it is likely that the prevalence and incidence rates of OSA in the general population are also increasing.5,6 OSA is associated with cardiovascular and metabolic diseases but its association with cognitive impairment is less well studied. In the last decade, studies have shown an association of OSA to various cardiovascular diseases,7 metabolic disorders8,9 and an increased risk of cancer mortality.10 Studies are showing an association of OSA to cognitive impairment. Surprisingly, 70–80% of patients with Alzheimer’s dementia meet the criteria for OSA (OSA defined as apnea hypopnea index (AHI) >5) with 38–48% of this population having an AHI >2011–14 compared to 5.4% OSA in age-matched controls without Alzheimer’s.13 In elderly women, the prevalence of OSA (AHI > 15) was found to be associated with increased risk of future development of dementia or mild cognitive impairment.15 This association between OSA and seemingly different classes of diseases-cardiovascular, metabolic, cancer and neurodegenerative diseases-raises the possibility of a common underlying pathogenetic mechanism that links OSA primarily or secondarily to these chronic diseases. Common underlying mechanisms include chronic intermittent hypoxia15,16 and sleep fragmentation.17–21
Cognitive impairment can be the consequence of intermittent hypoxia causing neuronal loss in the hippocampus22 and wake-active neurons.23 But frank neuronal loss may not be essential for cognitive impairment to occur. Changes in the brain’s 1014 synaptic connections is what allows us to learn, form memories and respond to environmental stimuli.24 Dendritic spines are the structural basis of these synaptic connections and their structure is regulated in response to synaptic plasticity (the strength of a synapse, or connection, between two neurons that changes in response to its history of use or disuse).25 It has also been argued that toxins (like Aβ accumulation)26 impair structural and functional plasticity of these synapses. Therefore, we propose that intermittent hypoxia causes changes at the BBB, and although this has not yet been described, we know that sustained hypoxia causes changes at the BBB.27 In this review, we identify mechanisms whereby intermittent hypoxia may alter blood–brain barrier permeability, causing changes in synaptic plasticity and consequently, cognitive impairment.
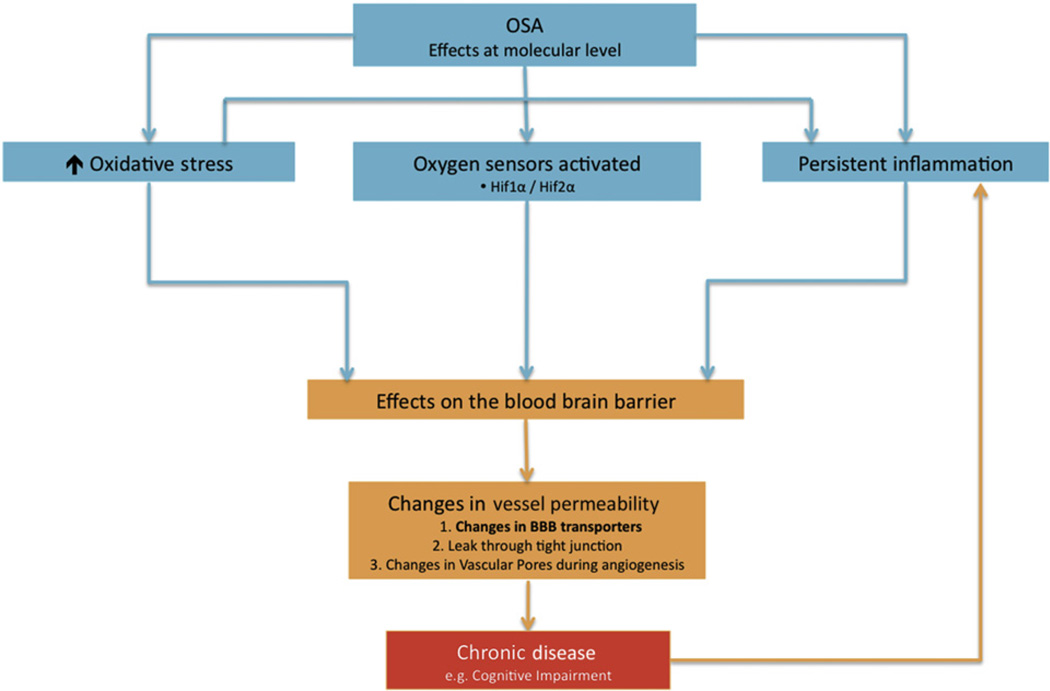
To address these concepts, this review will be divided into three main sections. The first section will outline the structure and function of the blood–brain barrier (BBB) while the second section will review how cyclical intermittent hypoxia can generate reactive oxygen species (ROS), stabilize and activate oxygen sensors and perpetuate the state of chronic inflammation (see Fig. 1). In the third section we discuss how cyclical intermittent hypoxia might alter microvessel permeability by: 1) changing the expression of influx and efflux transporters at the BBB due to increased nutrient and oxygen demand but also possibly through 2) an acute leak through the tight junctions of the BBB or 3) a leak through vascular pores during angiogenesis.
Fig. 1.
Intermittent hypoxia generates increased oxidative stress, can activate molecular oxygen sensors, and induce a chronic inflammatory state at the blood–brain barrier. In OSA patients that are susceptible (e.g., genetic polymorphisms and co-morbidities/epigenetics) this can lead to further effects on the blood–brain barrier, specifically, alterations in microvessel permeability.
The blood–brain barrier
The structure of the BBB gives rise to a uniquely resistant and highly regulated barrier. This unique barrier is able to maintain homeostasis within the brain, different from other organs, all the while dynamically responding to regional increases in metabolic demand within the brain. This first part of this review will be further divided into two sections:1)structure of the BBB; and 2)the normal function of the BBB.
Structure of the BBB: the sum is greater than its parts
The BBB is composed of blood vessel endothelial cells, pericytes and astrocytes. Elegant three-dimensional reconstructions by Mathiisen et al.28 shows the BBB to have a tight overlap of pericytes and astrocytes around capillary endothelial cells in the brain (see Fig. 2). The BBB is unique from other blood–organ barriers due to the presence of astrocytes and the special composition of the tight junction, which, collectively contributes to a high electrical resistance at the blood–brain barrier – as high as 8000 Ω cm2 in certain regions of the brain29 (for comparison, electrical resistance levels of 500–3500 Ω cm2 have been measured in the blood barrier in other tissue but varies widely based on what tissue/organ and condition is studied30,31).
Fig. 2.
Elegant three-dimension reconstruction of the blood–brain barrier28: the sum is greater than its parts. Four endfeet of astrocytes (denoted as pve I–IV), pericyte (pe), and probably a microglial cell process (pvcp) is the tight peripheral sheath surrounding the endothelial tube (not reconstructed in this image). Bar = 1 µm. Reprinted by permission from John Wiley & Sons Inc: Glia, Mathiisen et al. copyright 2010.
Endothelial cells
Endothelial cells form an extensive network of cells estimated to have a vascular surface of 117.40 cm2/g of brain tissue.32 An average human adult brain is 1300–1400 g. Thus, the estimated total surface area of endothelial cells in the adult human brain is 16.44 m2 (for reference, a badminton court is = 40.87 m2). Endothelial cells are joined together via tight junctions that are particularly unyielding compared to tight junctions outside of the brain. One reason why the tight junction at the BBB is particularly unyielding is that it has a higher density and more continuous distribution of the protein occludin.33 Interestingly, occludin is also the most redox sensitive protein at the tight junction, meaning it responds readily to a stimulus that changes its oxidation state as a result of free radicals and antioxidants, which are both increased in cyclical intermittent hypoxia, akin to that which occurs in obstructive sleep apnea.34 Therefore the combination of charge and structural barriers, forces water and solutes to normally move from blood to brain via the path of least resistance, specifically, via transcellular transport (e.g., movement through the cell), rather than taking a paracellular pathway (e.g., movement in between cells through the tight junctions).29 Subsequently, transcellular transport itself adds an additional layer of BBB regulation as there are more than 20 transporters and channels at the BBB that can be separated into three major categories: 1) blood to brain influx transporters; 2) ATP binding cassette transporters; and 3) brain-to-blood efflux transporters. Each transporter is responsible for different compounds, e.g., amino acids, neurotransmitters, organic anions, and drugs.35
Pericytes
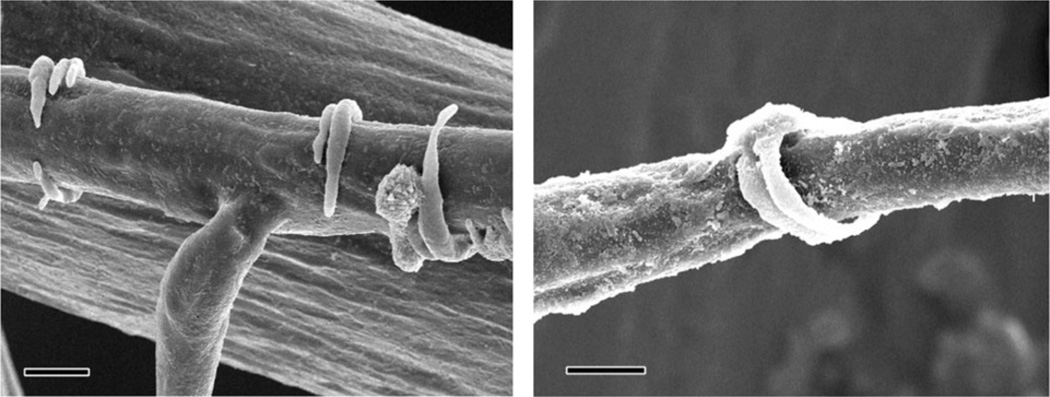
Pericytes are the least understood cells in the BBB but may be the most fascinating cells in the BBB when considering impaired oxygenation.36 Compositionally, they resemble smooth muscle cells37 leading to the theory that pericytes may contract to constrict capillaries and regulate blood flow. Structurally, beautiful work done by Harrison et al.38 visualize pericytes to resemble tendrils that are intermittently spaced on capillaries and post-capillary venules (see Fig. 3), which supports a function for pericytes as regulators of blood flow. In addition to a possible role of regulating blood flow, there are much data describing pericytes to have a prominent role in angiogenesis. Pericytes have three functions in angiogenesis: 1) initiation; 2) sprout extension and connection; and 3) termination or maturation of newly formed vessels.39 Pericytes have a pluripotent nature, and depending on trophic factors, can differentiate along either the mesenchymal or neuronal lineages.40 They also can release vascular endothelial growth factor-A (VEGF-A)41 and urokinase receptor (uPAR),42 both profoundly involved in the remodeling of blood vessels during angiogenesis. Currently it is difficult to directly study the role of pericytes in blood flow regulation, but as technology advances, it will be interesting to see if and how pericytes respond to chronic intermittent hypoxia.
Fig. 3.
Scanning electron micrograph image of resin filled vascular pericytes surrounding pre-capillary arterioles in the cortex of a chinchilla. Bar = 10 µm. Reprinted by permission from Oxford University Press: Cerebral cortex, Harrison et al., University of Toronto, copyright 2002.38
Astrocytes
Astrocytes enjoy an intricate symbiotic relationship with neurons and physically sit between neurons and blood vessels. They relay messages from the neurons to blood vessels to increase blood flow,43 when neurons increase their demand for oxygen and nutrients. Conversely, astrocytes protect neurons by absorbing toxic protein and mineral deposition (e.g., albumin, excessive metals) that may come from the blood that would be detrimental to neurons.44
Function of the blood–brain barrier as a whole
Two main functions of the BBB are: 1) to maintain homeostasis within the brain while concurrently; 2) respond to increased regional metabolic demand (termed angiodynamics) in response to stimuli.
Homeostasis
Maintaining homeostasis in the brain requires controlling and directing many different substances and reactions in the right space at the right time. Therefore the BBB is a busy space45 that is responsible for: i) water balance via aquaporins 1–946 as indiscriminate water movement will lead to brain edema and herniation; ii) ion inward and outward channels, (e.g., potassium, sodium, or calcium) as abnormal ion concentrations in the brain’s extracellular space will adversely affect action potentials,47 the fundamental basis of neural activity; iii) transporting glucose-the primary source of energy for the brain48; iv) transporting essential amino acids49; v) keeping large proteins like albumin,50 excessive iron51 and fibrinogen52 from crossing the BBB as they may create a toxic environment within the brain; vi) preventing most bacteria53 and viruses54 from crossing the BBB; vii) keeping drugs and other substances like chemotherapeutic agents55 and pesticides56 from crossing the BBB and creating a toxic environment, but conversely, may be the basis of drug resistance; viii) regulating leukocytes to cross or not to cross the BBB, a normal process if the brain is fighting an infection, but can be abnormal when there is an uncontrolled response as seen in inflammatory disorders like multiple sclerosis57; ix) maintaining pH within the brain.58
Angiodynamics
Angiodynamics in its simplest terms is the process of supply (e.g., increasing and decreasing delivery via capillary density) and demand (e.g., cellular need for oxygen/glucose).39,59 Blood–oxygen level dependent functional magnetic resonance imaging (BOLD fMRI) reveals the brain to be continuously active-with certain regions of the brain showing activity during wake and other regions showing activity during sleep.60 Activity of different regions of the brain necessitates alterations in blood flow to accommodate increases in metabolic demand (i.e., oxygen and glucose) to that region.61 Pathological processes may lead to unexpected blood flow; for example, patients with OSA have a different blood flow pattern on BOLD-fMRI while performing a cognitive task compared to controls.62 Not only does angiodynamics occur during pathological states like hypoxia, but angiodynamics can occur during normal activities such as motor learning.63 Although it is unknown what pathological processes induce a different blood flow, one plausible explanation could be vascular remodeling of brain capillaries in response to hypoxia,64 including angiogenesis. Angiogenesis (an increase in the number of capillaries) in response to hypoxia has been demonstrated in the cortex of rats65 and arteriogenesis (an increase in diameter of capillaries) in response to hypoxia has been demonstrated in the cerebrum of rats.66 Angiogenesis is discussed in more detail later in this review.
Can obstructive sleep apnea switch an adaptive homeostatic response to one that breaks down and becomes disruptive?
Homeostasis is a fundamental principle of physiology. The editors of Pathologic Basis of Disease eloquently describe homeostasis:
“Just as we live in a constantly changing world, so do the cells and tissues survive in a constantly changing microenvironment. The ‘normal’ or ‘physiologic’ state then is achieved by adaptive responses to the ebb and flow of various stimuli permitting the cells and tissues to adapt and to live in harmony within their microenvironment. Thus, homeostasis is preserved. It is only when the stimuli become more severe, or the response of the organism breaks down, that disease results – a generalization as true for the whole organism as it is for the individual cell.”
S.L Robbins, RS. Cotran, V.K. Kumar67
Obstructive sleep apnea changes the microenvironment of the entire body during sleep but organ systems, tissue, or populations of cells that have the highest oxygen demand will likely be the most sensitive to these changes. OSA creates a subtle injury pattern that must be differentiated from intermittent hypoxia due to ischemia/ reperfusion injury (e.g., trauma, stroke) and high altitude. Intermittent hypoxia seen in OSA is likely to cause molecular changes that are initially an adaptive homeostatic response in the form of altered microvessel permeability (to be reviewed below) that is unlike a response seen in more frank injury. However, in long standing OSA, we argue that this complex adaptive response can, in susceptible patients, result in adverse effects at the BBB. Susceptibility, which will not be discussed in this review, is likely the result of a combination of nature (e.g., relevant genetic polymorphisms) as well as nurture (e.g., co-morbid conditions e.g., obesity/adiposity and epigenetic influences).
Mechanisms by which cyclical intermittent hypoxia could induce changes at the BBB: oxidative stress, oxygen sensors and increased inflammation
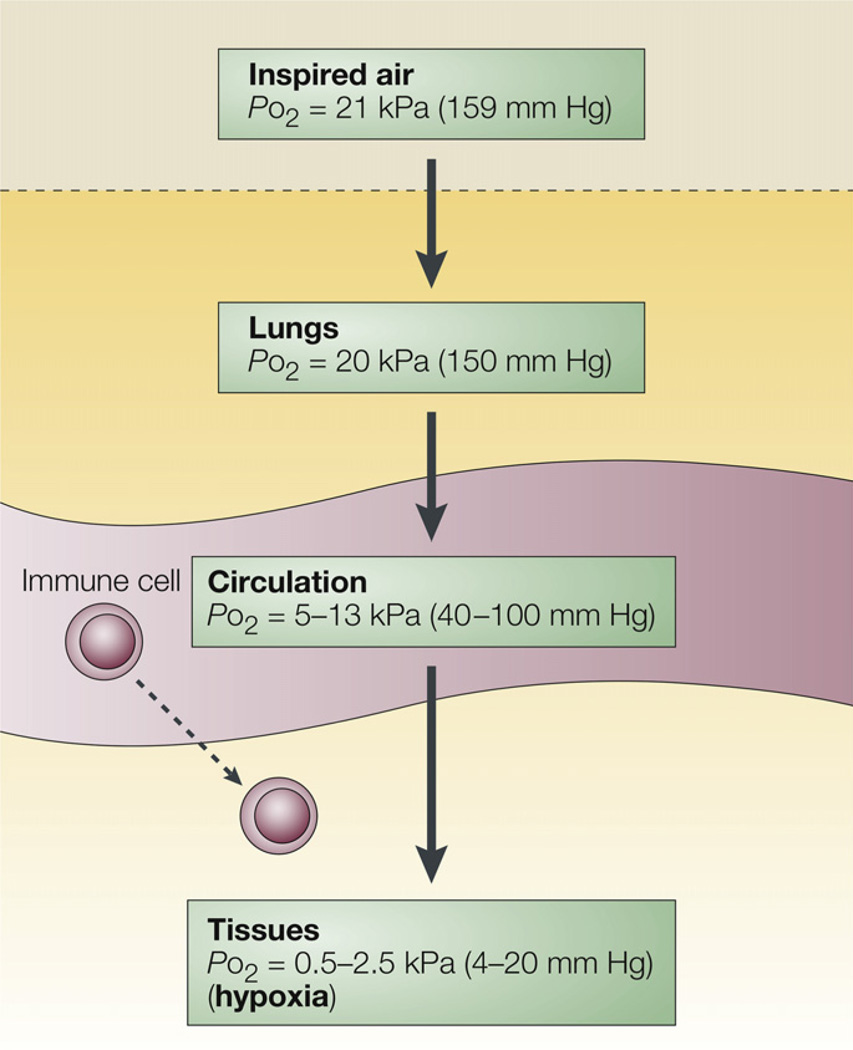
The characteristics of cyclical intermittent hypoxia in OSA can be described in terms of frequency of cycles, (i.e., number of cycles of hypoxia–normoxia), amplitude, nadir and duration (e.g., chronicity of every night during sleep for years to decades). It is important to note that although pulse oximetry is routinely measured during a polysomnogram, this does not tell us about tissue oxygenation (see Fig. 4)68 or the fact that cyclical intermittent hypoxia varies from tissue to tissue69 and is likely related to tissue blood flow. So, a key question is, how do the characteristics of intermittent hypoxia alter downstream events like oxidative stress, changes in Hif levels and inflammatory responses?
Fig. 4.
Although we breathe room air, by the time oxygen diffuses to the tissue, there is a relative hypoxia in the tissue, and the amount of hypoxia varies from tissue to tissue as well as the distance of the cells from capillaries. Reproduced by permission from Macmillan Publishers Ltd: Nature Reviews Immunology, Sitkovsky and Lukashev copyright 2005.176
Much of our current knowledge of consequences of cyclical intermittent hypoxia, specifically its effects on oxidative stress, changes in Hif proteins, and chronic inflammation, come from extreme conditions in in vitro and animal models that are not applicable to the majority of patients with OSA. Also, routinely used measures of OSA poorly assess the precise nature of cyclical intermittent hypoxia in individual patients. Currently, severity of OSA is based on the AHI, which does not take into account tissue oxygenation. On one end of the spectrum, there are patients with moderate–severe OSA whose oxygen desaturation to less than 90% are minimal. On the other end are moderate–severe OSA patients whose oxygen saturation nadir reaches 60–80% every 30–60 s. Based on what we know about how oxidative stress is generated, it would seem that the degree of oxidative stress is associated with the re-oxygenation to normoxia. However, whether using an oxygen desaturation index (ODI) of 3% or 4% is adequate to determine severity of oxidative stress generation is debatable since oxygen dissociation curves suggest that oxygen desaturations from 98% to 94% are not the same as oxygen desaturations of 91% to87% from the perspective of tissue oxygenation. And, an oxygen desaturation of 10–20% is most likely to affect tissue oxygenation differently than an oxygen desaturation of 4%. Also, based on what we know about how oxygen sensors like hypoxia inducible factors are activated,70 the speed of oxygen desaturation in OSA patients may be a factor with further studies needed to understand how changes in oxygen desaturation and oxygen resaturation correlates to oxidative stress. Thus, oxygen saturation nadir and percentage of time with oxygen saturation less than 90% seem inadequate to describe the nature of cyclical intermittent hypoxia in individual patients. If we believe cyclical changes in tissue oxygenation seen in our patients with OSA are an important link to metabolic, cancer, cardiac and neurodegenerative diseases, then does the AHI adequately convey the severity of tissue hypoxia? And without a more accurate representation of tissue hypoxia severity in OSA patients, it is difficult to simulate a more accurate animal model to study the pathological effects of cyclical intermittent hypoxia. Collectively, this is a major reason why there is difficulty in translating results from animal studies to clinical practice.
Oxidative stress
Oxidative stress is the result of either increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) or inadequate clearance of the ROS/RNS by anti-oxidants like glutathione, superoxide dismutase or catalases or a combination of both. In stroke and traumatic brain injury patients, where hypoxia and reperfusion injury is severe, the immense production of ROS/RNS is largely responsible for ensuing brain pathology, as the brain is unable to produce enough antioxidants to clear the oxidative stress burden. Although patients with OSA also have a process akin to hypoxia-reperfusion injury it is to a more subtle degree (e.g., smaller amplitude of hypoxia–normoxia), characteristically has a much higher frequency, and is of longer duration. Nevertheless, there is evidence that chronic intermittent hypoxia, as occurs in OSA, results in increases in ROS and RNS and overall oxidative stress.71,72
What is less clear are the specific downstream effects of oxidative stress, and why some organs seem to be affected while some are protected. And even within one organ like the brain, why are some brain regions protected while other regions are more susceptible? A review by Manukhina et al.73 discusses studies that demonstrate the favorable and beneficial effects of intermittent hypoxia and how the cardiovascular, immune and other systems adapt. In short, when the production of ROS and RNS are increased, antioxidants like superoxide dismutase (SOD), catalase and glutathione are also upregulated. Therefore, an OSA patient may experience cyclical intermittent hypoxia in tissues just enough to generate protective antioxidants or be severe enough to acquire the detrimental effects of intermittent hypoxia. If there is an increase in ROS generation then it reacts instantly and indiscriminately with whatever molecules they come in contact, making it difficult to predict the specific downstream effects of oxidative stress with any accuracy. ROS not only produce direct effects on protein function but can also do the following: 1) activate the redox sensitive transcription factor, nuclear factor kappa B (NFkB) as well as activator protein-1 (AP-1)74; 2) activate the unfolded protein response due to aberrations in protein folding, processing or degradation from the oxidative stress75; and 3) can lead to apoptosis (e.g., programmed cell death) or autophagy (e.g., degradation of cell’s own components through lysosomal machinery). These mechanisms can lead to cognitive impairment (see9 for details regarding the unfolded protein response, apoptosis and autophagy).
Molecular oxygen sensor, the hypoxia inducible factors (Hifs)
Besides oxidative stress, another way intermittent hypoxia can alter cellular function is via molecular oxygen sensors. Although there are many cellular oxygen sensors, we will limit this review to hypoxia inducible factors – a family of transcription factors that respond to cellular levels of oxygen. Currently six members of this family have been identified- Hif1α, Hif1β, Hif2α, Hif2β, Hif3α, and Hif3β. Hif1α and Hif2α (also known as EPAS-1- endothelial PAS domain-containing protein 1) are known to heterodimerize with Hif1β (also known as ARNT – aryl hydrocarbon receptor nuclear translocator) although little is known about Hif3α, Hif2β and Hif3β. The half life of Hif1α protein is about 4–6 min76 making it difficult to measure outside an in vitro model. The half-life of Hif1α mRNA, however, is about 7.5 h (http://lgsun.grc.nia.nih.gov/mRNA/index.html) making it much easier to measure, although this does not necessarily translate to protein stabilization and function.
Hifs are oxygen sensors77 in that they normally degrade in the presence of normal levels of oxygen,78 but under hypoxic conditions, the Hif protein stabilizes79 and is then able to move to the nucleus and function as a transcription factor. More specifically, during normoxia the Hif alpha subunits are hydroxylated at proline residues by Hif prolyl-hydroxylases. Then these sites are ubiquitinated by the VHL E3 ubiquitin ligase leading to degradation by the proteasome.80 However, during sustained hypoxic conditions, oxygen is utilized as a cosubstrate whereby Hif alpha subunits become stabilized81 and can then act as a transcription factor at hypoxia responsive elements (HRE) in promoters that contain the sequence RCGTG.82 Although Hif1α and Hif2α have many similarities, there are notable differences between Hif1α and Hif2α. First, during sustained hypoxia (e.g., as seen in solid cancers), both Hif1α and Hif2α are stabilized.83 However, during intermittent hypoxia in rodent models alternating from 15 s of sustained 5% inspired O2 and 5 min of room air, 8 h/d for 10 d, Hif1α protein is stabilized in carotid bodies and adrenal glands, but Hif2α protein undergoes calpain-dependent degradation.84
Although we know how Hif proteins are stabilized in the context of sustained hypoxia, it has not been well studied in the context of intermittent hypoxia. Furthermore, the actual mechanism of how cells sense oxygen levels is still not understood. Data from a cell culture model of sustained hypoxia demonstrates Hif1α stabilizes acutely to changes in decreased oxygen, but this effect is relatively short lived (how “short” may depend on how fast or how slow the rate of oxygen decreases, as well as how low the oxygen decreases to).70,85 This is in comparison to Hif2 α that demonstrates a slower response to sustained hypoxia but one that persists for a longer period of time.86 Also, variants in the Hif2α haplotype (and specifically not Hif1α) in Tibetans (when compared to Han Chinese) are associated with a tolerance to high altitude, revealed by a genome-wide study.87 Therefore, Hif1α may be considered an “acute” responder to sustained hypoxia and Hif2α a “chronic” responder to sustained hypoxia. It will be important to determine how different frequencies, amplitudes, nadirs and durations of cyclical intermittent hypoxia (i.e., “dose response curve”) affect levels of Hif1α and Hif2α’s both short- and long-term.
Once Hif1α is stabilized, it acts as a transcription factor that activates hundreds of target genes that contain hypoxic response elements,88 ranging from glucose metabolism to stimulating erythropoietin89 and angiogenesis,90 and can be seen as adaptive responses to hypoxia. Although downstream gene targets of Hif1α overlap with downstream gene targets of Hif2α, there are gene targets that are unique to Hif2α (for a partial list comparing downstream targets of Hif1α and Hif2α, see Fig. 583). Another notable difference is that Hif1α is ubiquitous and found in almost every cell type whereas Hif2α is largely found in endothelial cells and is not equally distributed from organ to organ91 but is expressed in brain endothelial cells.92 Therefore, the distribution of Hif1α and Hif2α , different RNA expression levels,93 as well as varying protein degradation rates, all contribute to Hif1α and Hif2α having different physiological roles.83 The specific roles of these transcription factors in OSA patients are still undetermined. This is an area of research opportunity.
Fig. 5.
Downstream target genes of Hif1α and Hif2α. This is a representation of shared and unique target genes regulated by Hif1α and Hif2α. Reproduced by permission from Macmillan Publishers Lt: Nature Reviews Cancer, Keith et al. copyright 2011.83
Chronic inflammation
Lastly, we will address how chronic inflammation may occur at the BBB in response to cyclical intermittent hypoxia and how its persistence can act as another mechanism leading to cognitive impairment. Cyclical intermittent hypoxia leads to activation of NFkB, AP-174 and other transcription factors involved in inflammation in monocytes,94 and neutrophils.95 Once these transcription factors are activated in various cells, there is an up-regulation of downstream targets including: cytokines, chemokines, immune receptors, adhesion molecules and other mediators of inflammation.74 Whether intermittent hypoxia activates these inflammatory pathways in endothelial cells of the BBB remain to be seen.
In whatever way inflammation is initiated there are two major outcomes – it can either resolve, or it can persist, and it is the persistent chronic inflammatory state that is associated with cognitive impairment. Although there are increasing data supporting the view that chronic inflammation is part of the pathophysiology of dementia and Alzheimer’s disorder,96 how chronic inflammation causes cognitive impairment is unclear. Chronic inflammation may lead to cognitive impairment by the following mechanisms: 1) cytokines affect gene expression of growth factors, important in synaptic plasticity97 and critical to memory; 2) persistent microglial activation (the resident macrophage of the brain)97 leads to neuronal damage98; 3) inflammation changes neuronal morphology, specifically by re-organizing neuronal dendritic spines in susceptible regions of the brain.97 Milatovic et al. proposes that changes in neuronal morphology are the predominant mechanism, not cell death, that explains behavioral changes.99 Although the literature well substantiates chronic inflammation to be a major participant in cognitive impairment, study of post-mortem brains is needed to link chronic inflammation to cognitive impairment in OSA patients.
While there is evidence of a chronic inflammatory state in patients with OSA,100 it is difficult to determine how much obesity, a major risk factor for OSA, contributes to this inflammatory state. Reinke et al. provide insight into this issue when they measured tissue oxygen partial pressure in liver, skeletal muscle and epididymal fat, then measured many markers including tumor necrosis factor- α (TNF-α).69 They report that not only did each tissue have different swings in oxygenation when exposed to sustained or intermittent hypoxia, but that obesity overwhelmed the effects of hypoxia when assessing TNF-α levels in epididymal fat. Levels of TNF-α in fat were not augmented by cyclical intermittent hypoxia in obese animals, who had very high levels even in controls, but intermittent hypoxia did increase TNF-α in fat tissue in lean animals. Drager et al.,101 from the same lab, reported a higher baseline of TNF-α protein and macrophage inflammatory protein-2 (MIP-2) in the liver of obese mice compared to lean mice and interestingly, the liver in obese mice did demonstrate an increase in TNF-α and MIP-2 proteins when exposed to IH that did not occur in lean mice. These two studies highlight that obesity can play a more significant role than intermittent hypoxia, but only in certain tissues. In other tissues, the effect of intermittent hypoxia on pro-inflammatory cytokines can be augmented by obesity. In human studies, Arnardottir et al. reported that in OSA patients, severity of sleep disordered breathing as assessed by oxygen desaturation index, hypoxia time, and minimum oxygen saturation, is an independent predictor of inflammation (IL-6 and C-reactive protein), but this was found only in obese patients with OSA (not in those with BMI <30 kg/m2).102 Collectively, these studies demonstrate an undeniable interaction between obesity and OSA when measuring inflammation that seems to be quite complex.
Although the expression of pro-inflammatory cytokines in the serum of OSA patients has been extensively studied, there are studies showing that in the setting of intermittent hypoxia there is an increase in leukotrienes, and this has been implicated in vascular remodeling as well as transforming monocytes to activated macrophages.103 Another interesting perpetrator of the chronic inflammatory state is likely to be prostaglandins (PG), also found to be increased in the setting of intermittent hypoxia and implicated in memory impairment.104 Interestingly, leukotrienes and PG can also perpetuate an inflammatory state by serving as ligands for the subfamily of nuclear factor receptor family called the peroxisome-proliferator-activated receptors (PPAR). PPARs form a heterodimer with retinoid X receptor (RXR) and may collectively bind to either a co-activating ligand or co-repressor ligand.105 The PPAR subfamily is made up PPAR-α, PPAR-β/δ and PPAR-ϒ, all of which can bind to the peroxisome proliferator response element (PPRE),106 and activate many downstream genes involved in chronic inflammation, metabolism and angiogenesis.105 Interestingly, PPAR-γ was significantly decreased in alveolar macrophages of obese patients with OSA compared to obese patients without OSA,107 although the reason is unclear. What we do know is that transcription factors from the CCAAT/enhancer binding proteins (C/EBP) family and early B-cell factor (EBF) family108 regulate expression of PPAR. Also, recent data implicate diet (e.g., dietary ω-3 polyunsaturated fatty acids (PUFAs)) can increase or decrease PPAR). However, most PPAR regulation studies focus on how different ligands repress or activate PPAR complexes,109 and there are many different natural ligands, including free fatty acids,110 inflammatory markers (e.g., leukotrienes and PG)111 and hormones like estrogen112 not to mention many synthetic ligands (e.g., drugs).113
Effects of intermittent hypoxia on BBB microvessel permeability – could this disrupt brain homeostasis enough to change neuronal morphology and/or synaptic function?
Oxidative stress, oxygen sensors and chronic inflammation have a plethora of downstream effects, but we will focus on mechanisms specific to altered microvessel permeability at the BBB. We will further break down altered microvessel permeability into three subsections– the first deals with a discriminating microvessel permeability via transporters/channels at the BBB. The second subsection discusses a somewhat indiscriminate leak through the tight junction via a paracellular mechanism, which is frequently studied in trauma, ischemia-reperfusion injury and stroke. The third subsection discusses altered regulation of vascular pores in the setting of angiogenesis.
Alterations in BBB transporters may alter microvessel permeability
Although the human brain is only 2% of the total body mass, it utilizes 25% of total body glucose and consumes 20% of total body oxygen114 and is very dependent on cerebral blood flow to deliver a constant supply of glucose and oxygen.115 This massive energy requirement is largely used to maintain ionic gradients.116 We know that sudden drops in glucose and oxygen leads to failure of ATP-dependent pumps, uncontrolled depolarization and a release of excitatory neurotransmitters like glutamate all leading to neurotoxicity and neuronal death within minutes.116 Thus even during homeostasis, the brain’s rigid, continuous need for high levels of energy to maintain these ionic gradients requires equally rigid control of microvessel permeability of ions and water, nutrients and cells moving into the brain and toxins moving or staying out of the brain.117 With over 20 transporters and channels at the BBB, chronic changes in the overall concentration and regulation of BBB transporters may have significant clinical implications such as increasing disease risk of Alzheimer’s.118 Although a subtle insult like chronic intermittent hypoxia would not cause a sudden drop in blood flow, it does alter blood flow as seen with BOLD-fMRI, and by its very nature creates an environment that is not normoxic thus increasing the demand for more oxygen and most likely, glucose.
Intermittent hypoxia and transporters
Several studies address changes in transporters/channels in response to intermittent hypoxia. Dopp et al. demonstrated an increase in Abcb1a mRNA (Abcb1 is the gene that codes for p-glycoprotein) expression in the heart and liver of mice exposed to 2 wks of cyclical intermittent hypoxia.119 Gong et al. showed cerebral ischemic preconditioning with intermittent hypobaric hypoxia increases glutamate transporter 1 in neurons of the hippocampus of rats.120 But expression of other transporters is decreased. Wang et al. reported monocarboxylate transporter 2 (MCT2) to decrease by RT-PCR in cortical neurons in a rat model of sleep apnea.58 Baronio et al. report higher overall brain water and lower levels of aquaporin 1 (by ELISA) in the hippocampus and cerebellum of mice exposed to chronic intermittent hypoxia.121 Although no studies have specifically addressed the effect of intermittent hypoxia on transporters in endothelial cells of the BBB, it is reasonable to propose this because oxidative stress and/or Hifs regulate several BBB transporters including: i) p-glycoprotein, a glycoprotein that is part of the ATP-binding cassette transporter family, that effluxes substances like glutamate and xenobiotics122; ii) multidrug resistance protein 1 (MRP1) an ABC transporter that effluxes glutathione, leukotrienes and Aβ 123,124; iii) aquaporin-1, a channel for water, but recently implicated in gaseous diffusion including oxygen and carbon dioxide125; iv) sulfonylurea receptor 1 (SUR1), an ABC transporter that couples a cell’s metabolic state to membrane potential by assisting in potassium influx126,127; v) the breast cancer resistance protein (BCRP1) an ABC transporter that effluxes urate and Aβ 128 ;vi) glucose transporters, such as GLUT1 that influxes glucose129; vii) monocarboxylate transporters (MCT), that influxes lactate when glucose is in short supply but can also efflux out lactate130; viii) norepinephrine transporter (NET) that effluxes out norepinephrine131; ix) gamma-aminobutyric acid transporter 2 (GAT2) that effluxes out GABA, a chief inhibitory neurotransmitter132; x) excitatory amino acid transporters (EAAT) that effluxes out anionic amino acids like glutamate133; xi) serotonin transporter (SERT) that effluxes out serotonin134; and xii) l-cystine/l-glutamic acid exchange transporter that influxes in amino acids cystine and glutamic acid.135 Some of these transporters are also downstream targets of PPARs including: ATP-binding cassette transporter A1 (ABCA1)136 important in cholesterol efflux and breast cancer resistance protein (BCRP1).137 While cyclical intermittent hypoxia could alter expression of transporters at the BBB, this is not known and would be an important area for future research.
Increases in influx and efflux transporters – short-term benefit but long-term adverse consequences?
Increases in influx transporters like GLUT1 produce short-term benefits by increasing energy delivery. But are there adverse long-term consequences? Increases in GLUT1 have been associated with increased inflammation138 which has been associated with cognitive impairment. Increases in efflux transporters (moving molecules from brain to blood) also has obvious short term benefits as they function to clear the brain of toxic molecules like amyloid-beta peptides,139 excitatory amino acids,140–142 neurotransmitter metabolites such as homovanillic acid, a major metabolite of dopamine,143,144 and keep toxic molecules like amyloid-beta peptides145 and xenobiotics35 out of the brain. On the other hand, a long-term consequence of increases in efflux transporters may result in therapeutic drug resistance, seen in OSA patients with hypertension.146 This phenomenon of drug resistance has been extensively studied by pharmaceutical companies and more than 200 xenobiotics have been associated with specific efflux transporters. Whether cyclical intermittent hypoxia influences drug resistance would be clinically very significant but remains to be determined.
With respect to adverse consequences, there is an emerging body of literature examining neuronal behavior after BBB disruption with the area of epilepsy being well studied.147 The underlying concept is that a compromised BBB alters the microenvironment around neurons and glia by allowing an “infiltration of cells, ions or molecules that initiate, amplify, procrastinate repair or further disrupt a CNS response.” One way a disrupted BBB affects cell metabolism, resting membrane potential and membrane conductance is by altered ion homeostasis. For example, potassium is maintained within a very narrow range because the brain cannot tolerate surges of potassium from the blood as it not only causes persistent depolarization (with toxic build up) but itself, causes large reductions in blood flow further affecting homeostasis by decreasing metabolic support of neurons and glia. Thus, disruption of ion homeostasis has a direct impact on synaptic function and plasticity. Other ways a disrupted BBB affects brain function is that it cannot deliver enough products to assist in function, or it cannot clear toxic metabolites fast enough.
Leak through the tight junctions
Another way that the BBB can be altered is by a paracellular leak, an indiscriminate BBB leak of solutes, cells and water (e.g., movement of solutes paracellularly through the tight junctions). Leakage that does occur through the tight junctions would mean a breakdown in the unique structural (e.g., occludin) and charge barriers as well as the loss of protection from astrocytes and pericytes. Therefore, a BBB leak is generally a direct result of an insult that opens the tight junction. Such insults include: traumatic brain injury (e.g., forceful impact or penetrating trauma that sever capillaries); sustained ischemia (e.g., ligation or embolus of major blood vessels); high altitude cerebral edema148 and huge shifts in osmotic pressure (e.g., supratherapeutic doses of mannitol that shrink endothelial cells and “rips” open the tight junctions). Currently it is unknown whether a paracellular leak through the tight junction occurs in response to cyclical intermittent hypoxia.
Leaking through vascular pores during angiogenesis
Another effect of cyclical intermittent hypoxia on the BBB is angiogenesis, i.e., the process of growing new blood vessels from pre-existing vessels in response to increased oxygen demand. On one hand, angiogenesis allows an increase in delivery of oxygen and nutrients, a critical response for normal development and wound healing. On the other hand, angiogenesis itself can consume large amounts of oxygen and nutrients causing metabolic shifts to occur as well as allow leakage through vascular pores.
In human subjects with OSA, Wahlin–Larsson et al. demonstrated increased angiogenesis (i.e., proliferating capillaries) in skeletal muscles compared to healthy, age-matched (but not BMI-matched) controls.149 They demonstrated that the increases in angiogenesis correlated with increased immunofluorescence staining of VEGF-A in skeletal muscle.150 In rodent models of cyclical intermittent hypoxia, there has been demonstration of both angiogenesis in the cerebral cortex and hippocampus151 as well as arteriogenesis (enlargement of the vascular size of the blood vessels) in the carotid body.152
The pathological significance of angiogenesis is largely unknown. Since angiogenesis coexists with an increased chronic inflammatory state (e.g., activated microglia), it is difficult to tease out which of these processes is detrimental and which is the innocent bystander. The evidence supports the co-occurrence of angio-genesis and chronic inflammation.153 When cells are exposed to hypoxia, endothelial cells release angiogenic factors such as cytokines and growth factors that orchestrates and controls angiogenesis; but the same signals concurrently recruit monocytes/ macrophages to the same site.154 Thus, there are common mechanisms.
Examples of uncontrolled angiogenesis linked to disease progression include the following: angiogenesis in pannus formation as in rheumatoid arthritis and psoriasis153; cancers155; cardiovascular disease156; and Alzheimer’s disease.157 Although there is evidence of angiogenesis in patients with OSA, the clinical significance of this remains to be revealed. For example, in susceptible individuals with OSA, can cyclical intermittent hypoxia influence the aggressiveness of cancer progression?
Role of VEGF in angiogenesis
Sustained hypoxia (e.g., as in the environment of tumors)158 as well as intermittent hypoxia159,160 stabilizes Hif1α and stimulates downstream gene targets, one particular target being VEGF,158 a ligand protein of VEGF receptors, important in angiogenesis. VEGF binds to VEGF receptors on endothelial cells, triggering a tyrosine kinase pathway leading to angiogenesis. Angiogenesis, when stimulated by hypoxia, is largely mediated by growth factors including basic fibroblast growth factor (b-FGF) and VEGF, both of which are downstream targets of Hif1α. The subsequent complex interaction of VEGF with a host of other molecules involved in angiogenesis is well worked out.161 In support of this role of VEGF, Elson et al. demonstrated in transgenic mice with overexpression of Hif1α a 13-fold increase in VEGF and a 66% increase in dermal capillaries.162
In human subjects with OSA, changes in molecular oxygen sensors have not yet been measured, but their downstream target, VEGF, has been used as an endpoint in many studies. VEGF-A protein (by ELISA) has been demonstrated to be elevated in patients with OSA by Lavie et al. (in plasma),163 Gozal et al. (in serum)164 and Schulz et al. (in serum).165 It is notable that Lavie et al.163 demonstrated that patients with OSA have an increased VEGF-A protein before falling asleep, with increasing VEGF levels throughout the night, and VEGF levels returning back to pre-sleep values by the morning. Thus, VEGF-A protein levels remain elevated during the day despite being exposed to intermittent hypoxia only at night. Several investigators have demonstrated that normalization of OSA by continuous positive airway pressure (CPAP) results in a decrease of VEGF-A protein in blood.163,166 Lavie et al.163 demonstrated that 90 d of CPAP resulted in a decrease in VEGF-A levels, while Cifti et al.166 demonstrated a similar decrease after 12 wks of CPAP (although values did not reach levels seen in normal controls). Interestingly, Teramoto et al. demonstrated that one night of supplemental oxygen decreased VEGF-A levels in patients with OSA.167
Studies with cyclical intermittent hypoxia in rodents have measured levels of molecular oxygen sensors Hif1α and Hif2α as well as some of their downstream targets including VEGF-A. Peng et al.168 demonstrated a two-fold increase in Hif1α protein in cerebral cortex (relative to Hif2α protein) in mice in response to 10 d of chronic intermittent hypoxia (CIH). Li et al. demonstrated a 4-fold increase in VEGF mRNA in liver of wild type (WT) mice after 5 d of CIH and no increase in VEGF in Hif1α heterozygous mice (low levels of Hif1α as Hif1α knock-out mice are lethal).169 Nanduri et al. used a rodent model of intermittent hypoxia and found that Hif1α protein stabilizes and increases in response to CIH in the carotid body and adrenal glands, but Hif2α protein, which has a higher baseline level in normoxia, surprisingly decreases significantly in response to CIH.84 They determined the decrease in Hif2α was due to increased degradation – interestingly, not by prolyl hydroxylases and proteosomal degradation, but by calpain degradation. It is unclear why Hif2α protein is targeted by calpains during intermittent hypoxia and Hif1α is not. Further exploration of the interactions between the Hifs and their downstream targets in intermittent hypoxia is another area of research opportunity.
Role of DLL4 in angiogenesis
Sustained and intermittent hypoxia also influences Hif2α and its downstream gene targets, one particular target being DLL4,170 a notch ligand, also important in angiogenesis.91 Work by Skuli et al. compared a conditional Hif2α VE cadherin-Cre mouse endothelial cells (i.e., a mouse that has conditionally knocked down levels of Hif2α in only endothelial cells) to control mouse endothelial cells, and demonstrated Hif2α mRNA and protein to be increased in control mice when exposed to hypoxia but decreased in the conditional mouse with knock-down of Hif2α91 When exposed to hypoxia, control mice show an induction of DLL4 expression but in conditional Hif2α endothelial cells DLL4 is not significantly increased. In an ischemia model, Skuli et al. went on to further demonstrate that Hif2α is required for angiogenesis.171 They also provide clear evidence that DLL4/Notch signaling is a key target of Hif2α171 by rescuing the phenotype of Hif2α deficient endothelial cells with DLL4 (using DLL4 viral transgene expression) and restoring angiogenesis. The effect of intermittent hypoxia on Hif1α influencing VEGF and Hif2α influencing DLL4 remains to be investigated in patients with OSA.
Leak through vascular pore during angiogenesis
Angiogenesis can lead to microvessel permeability changes through vascular pores. Our understanding of vascular pores has come from seminal studies done by the laboratories of Dvorak and Dvorak. Specifically, they demonstrated VEGF-A causing a vascular leak172 through vascular pores, during a specific phase of angiogenesis called the Mother Vessel Phase. This is when the basement membrane undergoes the most amount of degradation and is at its thinnest and is therefore the most permeable. This may be relevant to OSA because angiogenesis does occur in response to intermittent hypoxia. Dvorak and Dvorak used 3-D reconstructions to describe vesicle-vacuolar organelles (VVO), which are small vesicles conjoined together (i.e., about 150–300 of them) to form a pore. This pore transects the endothelial cell providing a path for substances to cross in a transcellular manner and is regulated by small diaphragms172 that may be regulated by VEGF.173 Although their work is done largely in skin, work done by other labs have shown that sustained hypoxia27 as well as intracranial injections of VEGF-A174 also increase BBB “leak.” Whether these vascular pores appear in response to cyclical intermittent hypoxia is currently unknown. But since intermittent hypoxia upregulates VEGF and promote angiogenesis, it is conceivable that over weeks and months a small subtle “leak” through the pores during the mother vessel phase may occur at the BBB. But whether the rate of angiogenesis is significant enough to cause changes in the brain’s microenvironment remains to be determined.
Conclusion
In conclusion, our understanding of how obstructive sleep apnea affects cardiovascular, metabolic and neurodegenerative disease has evolved rapidly over recent years. We have presented data demonstrating that chronic intermittent hypoxia during sleep not only increases oxidative stress, but may activate oxygen sensors Hif1α and Hif2α, and contribute to a persistent, chronic inflammatory state. We have emphasized the possibility of these mechanisms at the blood–brain barrier. Although an adaptive response like altered microvessel permeability at the blood–brain barrier may be initiated to maintain homeostasis in response to intermittent hypoxia, in susceptible individuals, chronic intermittent hypoxia may lead to adverse consequences. Specifically, altered microvessel permeability can change the concentration of solutes, cells and water thereby altering neuronal morphology and synaptic plasticity and causing cognitive impairment. Another unintended consequence of altered microvessel permeability is brain-related drug resistance, with more than 200 drugs already associated with BBB transporters. Although we know there is a high prevalence of OSA in patients with drug-resistant hypertension,175 whether intermittent hypoxia alters the regulation of transporters at the BBB or elsewhere is unknown.
Our review raises many questions about changes that may take place in the blood–brain barrier of OSA patients and what role this might play in cognitive impairment. This is a new and exciting area of inquiry and one where further studies are needed.
Practice points.
A subset of patients with OSA is at increased risk for cognitive impairment.
Intermittent hypoxia seen in OSA may do the following: 1) increase oxidative stress; 2) activate molecular oxygen sensors Hif1α and Hif2α; 3) induce a chronic inflammatory state.
Research agenda.
Further basic and clinical experiments are required to:
Determine whether BBB permeability is altered in rodent models and in patients with OSA.
Identify which BBB transporters are increased or decreased in response to chronic intermittent hypoxia and whether polymorphisms of their genes modify this response.
Determine whether biomarkers exist on monocytes and macrophages that reflect changes in BBB transporters in both rodent models and patients with OSA and assess whether these correlate with cognitive impairment.
Identify the presence or absence of transcription factors like Hifs and PPARs, and their downstream targets at the BBB in rodent models of cyclical intermittent hypoxia and patients with OSA.
Determine if intermittent hypoxia induced angiogenesis at the BBB correlates with cognitive impairment.
Acknowledgment
We would like to gratefully acknowledge NIH grants T32HL07713, and K12 HL090021 and the Sleep Foundation for funding Diane C. Lim, and NIH grant HL094307 for supporting Allan I. Pack. The authors declare no conflicts of interest. We would like to thank Dr. Nicolas Skuli (Institut National de la Santé Et de la Recherche Médicale) for his assistance in manuscript review as well as Dr. Stephen Robinson (Royal Melbourne Institute of Technology, Australia) for his assistance in manuscript review and for suggesting that studies of the blood–brain barrier in obstructive sleep apnea are needed.
List of abbreviations
- ABC
transporter ATP binding cassette transporter
- AHI
apnea-hypopnea index
- AP-1
activator protein-1
- ARNT
aryl hydrocarbon receptor nuclear translocator
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- BCRP1
breast cancer resistance protein
- b-FGF
basic fibroblast growth factor
- BMI
body mass index
- BOLD fMRI
blood-oxygen level dependent functional magnetic resonance imaging
- C/EBP
CCAAT/enhancer binding proteins
- CIH
chronic intermittent hypoxia
- CNS
central nervous system
- CPAP
continuous positive airway pressure
- DLL4
delta like ligand 4
- EAAT
excitatory amino-acid transporter
- EBF
early B-cell factor
- ELISA
enzyme-linked immunosorbent assay
- EPAS-1
endothelial PAS domain-containing protein 1
- GABA
gamma-aminobutyric acid
- GAT2
gamma-aminobutyric acid transporter 2
- GLUT1
glucose transporter 1
- Hif
hypoxia inducible factors
- HRE
hypoxia responsive elements
- IL-6
interleukin-6
- MCT
monocarboxylate transporter
- MIP-2
macrophage inflammatory protein-2
- MRP1
multidrug resistance protein 1
- NET
norepinephrine transporter
- NFκB
nuclear factor kappa B
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PG
prostaglandins
- PPAR
peroxisome-proliferator activated receptors
- PPRE
peroxisome proliferator response element
- PUFA
polyunsaturated fatty acids
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RT-PCR
real-time polymerase chain reaction
- RXR
retinoid X receptor
- SERT
serotonin transporter
- SOD
superoxide dismutase
- SUR1
sulfonylurea receptor 1
- TNF-α
tumor necrosis factor-alpha
- uPAR
urokinase receptor
- VEGF
vascular endothelial growth factor
- VVO
vesicle-vacuolar organelles
Glossary of terms
- Mild cognitive impairment
definition from the National Institute of Ageing – a condition in which people have memory or other thinking problems greater than normal for their age and education, enough to be noticed and measured, but not compromising a person’s independence
- Homeostasis
the ability for a biological system to maintain its internal environment while continuously interacting with and adjusting to stimuli originating from within or outside the system
- Oxidative stress
a net overproduction of reactive species as well as non-radical species (e.g., hydrogen peroxide, lipid peroxide) that leads to damage of specific molecules and consequential injury to cells and/or tissue
- Cellular oxygen sensor
molecules that respond to the mismatch of oxygen demand and supply and attempts to maintain an optimal oxygen partial pressure
- Chronic inflammation
inflammation that is characterized by its persistence and lack of resolution
- Angiogenesis
formation of new blood vessels from pre-existing vessels to supply oxygen and nutrients;
- Leak
increased passage of molecules (e.g., ions, water, glucose) in a pericellular manner (through tight junctions)
- Microvessel permeability
the ability of blood vessels to allow small molecules (e.g., ions, water, glucose) and cells (e.g., leukocytes) to pass in a transcellular manner (through transporters, pores and channels)
Contributor Information
Diane C. Lim, Email: limdiane@mail.med.upenn.edu.
Allan I. Pack, Email: pack@mail.med.upenn.edu.
References
* The most important references are denoted by an asterisk.
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 3.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 5.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2:349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24:703–715. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apneahypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49–64. doi: 10.1055/s-0032-1306426. [DOI] [PubMed] [Google Scholar]
- 10.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Klauber MR, Butters N, Parker L, Kripke DF. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39:258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch CC, Reynolds CF, 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry. 1986;47:499–503. [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–433. [PubMed] [Google Scholar]
- 17.Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184:1305–1312. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O‘Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep. 2004;27:279–282. doi: 10.1093/sleep/27.2.279. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh V, Nair D, Zhang SX, Hakim F, Kaushal N, Kayali F, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-alpha pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri R, Drago V, Arico D, Bruni O, Remington RW, Stamatakis K, et al. The effects of experimental sleep fragmentation on cognitive processing. Sleep Med. 2010;11:378–385. doi: 10.1016/j.sleep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, et al. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003;122:585–590. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, et al. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci. 2007;27:10060–10071. doi: 10.1523/JNEUROSCI.0857-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee KF, Soares C, Beique JC. Examining form and function of dendritic spines. Neural Plast. 2012;2012:704103. doi: 10.1155/2012/704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spires-Jones T, Knafo S. Spines, plasticity, and cognition in Alzheimer’s model mice. Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125:2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 28.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 29.Nag S. Morphology and molecular properties of cellular components of normal cerebral vessels. Methods Mol Med. 2003;89:3–36. doi: 10.1385/1-59259-419-0:3. [DOI] [PubMed] [Google Scholar]
- 30.Utech M, Bruwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–195. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- 31.Meyle J, Gultig K, Rascher G, Wolburg H. Transepithelial electrical resistance and tight junctions of human gingival keratinocytes. J Periodontal Res. 1999;34:214–222. doi: 10.1111/j.1600-0765.1999.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 32.Lauwers F, Cassot F, Lauwers-Cances V, Puwanarajah P, Duvernoy H. Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles. Neuroimage. 2008;39:936–948. doi: 10.1016/j.neuroimage.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 34.Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 36.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarthy U, Gardiner TA. Endothelium-derived agents in pericyte function/dysfunction. Prog Retin Eye Res. 1999;18:511–527. doi: 10.1016/s1350-9462(98)00034-2. [DOI] [PubMed] [Google Scholar]
- 38.Harrison RV, Harel N, Panesar J, Mount RJ. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex. 2002;12:225–233. doi: 10.1093/cercor/12.3.225. [DOI] [PubMed] [Google Scholar]
- 39.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 40.Dore-Duffy P, Mehedi A, Wang X, Bradley M, Trotter R, Gow A. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripotent. Microvasc Res. 2011;82:18–27. doi: 10.1016/j.mvr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 43.Bennett MR, Farnell L, Gibson WG. Origins of the BOLD changes due to synaptic activity at astrocytes abutting arteriolar smooth muscle. J Theor Biol. 2008;252:123–130. doi: 10.1016/j.jtbi.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Nag S. Morphology and properties of astrocytes. Methods Mol Biol. 2011;686:69–100. doi: 10.1007/978-1-60761-938-3_3. [DOI] [PubMed] [Google Scholar]
- 45.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Zador Z, Bloch O, Yao X, Manley GT. Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res. 2007;161:185–194. doi: 10.1016/S0079-6123(06)61012-1. [DOI] [PubMed] [Google Scholar]
- 47.Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8:254–267. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- 48.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 50.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter AJ. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 51.Gorter JA, Mesquita AR, van Vliet EA, da Silva FH, Aronica E. Increased expression of ferritin, an iron-storage protein, in specific regions of the parahippocampal cortex of epileptic rats. Epilepsia. 2005;46:1371–1379. doi: 10.1111/j.1528-1167.2005.11505.x. [DOI] [PubMed] [Google Scholar]
- 52.Piers TM, Heales SJ, Pocock JM. Positive allosteric modulation of metabotropic glutamate receptor 5 down-regulates fibrinogen-activated microglia providing neuronal protection. Neurosci Lett. 2011;505:140–145. doi: 10.1016/j.neulet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 53.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7:383–394. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salinas S, Schiavo G, Kremer EJ. A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol. 2010;8:645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 55.Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011;50:209–232. doi: 10.1042/bse0500209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macdonald N, Gledhill A. Potential impact of ABCB1 (p-glycoprotein) polymorphisms on avermectin toxicity in humans. Arch Toxicol. 2007;81:553–563. doi: 10.1007/s00204-007-0193-6. [DOI] [PubMed] [Google Scholar]
- 57.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Guo SZ, Bonen A, Li RC, Kheirandish-Gozal L, Zhang SX, et al. Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J Neurosci. 2011;31:10241–10248. doi: 10.1523/JNEUROSCI.1462-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto YL, Phillips KM, Hodge CP, Feindel W. Microregional blood flow changes in experimental cerebral ischemia Effects of arterial carbon dioxide studied by fluorescein angiography and xenon 133 clearance. J Neurosurg. 1971;35:155–166. doi: 10.3171/jns.1971.35.2.0155. [DOI] [PubMed] [Google Scholar]
- 60.Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukunaga M, Horovitz SG, de Zwart JA, van Gelderen P, Balkin TJ, Braun AR, et al. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab. 2008;28:1377–1387. doi: 10.1038/jcbfm.2008.25. [DOI] [PubMed] [Google Scholar]
- 62.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–2234. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 63.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 64.Harik SI, Hritz MA, LaManna JC. Hypoxia-induced brain angiogenesis in the adult rat0. J Physiol. 1995;485(Pt 2):525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mironov V, Hritz MA, LaManna JC, Hudetz AG, Harik SI. Architectural alterations in rat cerebral microvessels after hypobaric hypoxia. Brain Res. 1994;660:73–80. doi: 10.1016/0006-8993(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 66.Stewart PA, Isaacs H, LaManna JC, Harik SI. Ultrastructural concomitants of hypoxia-induced angiogenesis. Acta Neuropathol. 1997;93:579–584. doi: 10.1007/s004010050654. [DOI] [PubMed] [Google Scholar]
- 67.Robbins SL, Cotran RS, Kumar V. Pathologic basis of disease. 3rd ed. Philadelphia: Saunders; 1984. [Google Scholar]
- 68.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millonig G, Hegedusch S, Becker L, Seitz HK, Schuppan D, Mueller S. Hypoxia-inducible factor 1 alpha under rapid enzymatic hypoxia: cells sense decrements of oxygen but not hypoxia per se. Free Radic Biol Med. 2009;46:182–191. doi: 10.1016/j.freeradbiomed.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–16. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavie L. Obstructive sleep apnoea syndrome-an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 73.Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 2006;231:343–365. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- 74.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 2005;23:1059–1075. doi: 10.1016/j.ncl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Brown MK, Naidoo N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol. 2010;42:103–113. doi: 10.1007/s12035-010-8114-8. [DOI] [PubMed] [Google Scholar]
- 76.Moroz E, Carlin S, Dyomina K, Burke S, Thaler HT, Blasberg R, et al. Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One. 2009;4:e5077. doi: 10.1371/journal.pone.0005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 79.Freeman RS, Hasbani DM, Lipscomb EA, Straub JA, Xie L. SM-20, EGL-9, and the EGLN family of hypoxia-inducible factor prolyl hydroxylases. Mol Cells. 2003;16:1–12. [PubMed] [Google Scholar]
- 80.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 81.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 82.Xia X, Kung AL. Preferential binding of HIF-1 to transcriptionally active loci determines cell-type specific response to hypoxia. Genome Biol. 2009;10:R113. doi: 10.1186/gb-2009-10-10-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, et al. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lofstedt T, Fredlund E, Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L, et al. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 86.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 87.Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, et al. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol. 2011;28:1003–1011. doi: 10.1093/molbev/msq277. [DOI] [PubMed] [Google Scholar]
- 88.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 89.Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, Gozal D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci. 2012;13:77. doi: 10.1186/1471-2202-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011;1389:50–60. doi: 10.1016/j.brainres.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, et al. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 93.Ignacak ML, Harbaugh SV, Dayyat E, Row BW, Gozal D, Czyzyk-Krzeska MF. Intermittent hypoxia regulates RNA polymerase II in hippocampus and prefrontal cortex. Neuroscience. 2009;158:1436–1445. doi: 10.1016/j.neuroscience.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamauchi M, Tamaki S, Tomoda K, Yoshikawa M, Fukuoka A, Makinodan K, et al. Evidence for activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath. 2006;10:189–193. doi: 10.1007/s11325-006-0074-x. [DOI] [PubMed] [Google Scholar]
- 95.Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 96.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 99.Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem. 2003;87:1518–1526. doi: 10.1046/j.1471-4159.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- 100.Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008;18:253–260. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19:2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic sleep apnea cohort. Sleep. 2012;35:921–932. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184:124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, et al. Cyclo-oxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med. 2003;168:469–475. doi: 10.1164/rccm.200211-1264OC. [DOI] [PubMed] [Google Scholar]
- 105.Choi JM, Bothwell AL. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cells. 2012;33:217–222. doi: 10.1007/s10059-012-2297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5’ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S, Malur A, Marshall I, Huizar I, Barna BP, Pories W, et al. Alveolar macrophage activation in obese patients with obstructive sleep apnea. Surgery. 2012;151:107–112. doi: 10.1016/j.surg.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 108.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bunger M, Hooiveld GJ, Kersten S, Muller M. Exploration of PPAR functions by microarray technology-a paradigm for nutrigenomics. Biochim Biophys Acta. 2007;1771:1046–1064. doi: 10.1016/j.bbalip.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 110.Guri AJ, Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptors: bridging metabolic syndrome with molecular nutrition. Clin Nutr. 2006;25:871–8785. doi: 10.1016/j.clnu.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, Reddy RC. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem. 2010;285:22067–22074. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tiyerili V, Muller CF, Fung S, Panek D, Nickenig G, Becher UM. Estrogen improves vascular function via peroxisome-proliferator-activated-receptor-gamma. J Mol Cell Cardiol. 2012;53:268–276. doi: 10.1016/j.yjmcc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 113.Burris TP, Busby SA, Griffin PR. Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem Biol. 2012;19:51–59. doi: 10.1016/j.chembiol.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 115.Sokoloff L. The physiological and biochemical bases of functional brain imaging. Cogn Neurodyn. 2008;2:1–5. doi: 10.1007/s11571-007-9033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 117.Hermann DM, Kilic E, Spudich A, Kramer SD, Wunderli-Allenspach H, Bassetti CL. Role of drug efflux carriers in the healthy and diseased brain. Ann Neurol. 2006;60:489–498. doi: 10.1002/ana.21012. [DOI] [PubMed] [Google Scholar]
- 118.Pahnke J, Wolkenhauer O, Krohn M, Walker LC. Clinico-pathologic function of cerebral ABC transporters - implications for the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2008;5:396–405. doi: 10.2174/156720508785132262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dopp JM, Moran JJ, Abel NJ, Wiegert NA, Cowgill JB, Olson EB, et al. Influence of intermittent hypoxia on myocardial and hepatic P-glycoprotein expression in a rodent model. Pharmacotherapy. 2009;29:365–372. doi: 10.1592/phco.29.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gong SJ, Chen LY, Zhang M, Gong JX, Ma YX, Zhang JM, et al. Intermittent hypobaric hypoxia preconditioning induced brain ischemic tolerance by up-regulating glial glutamate transporter-1 in rats. Neurochem Res. 2012;37:527–537. doi: 10.1007/s11064-011-0639-3. [DOI] [PubMed] [Google Scholar]
- 121.Baronio D, Martinez D, Fiori CZ, Bambini-Junior V, Forgiarini LF, Pase da Rosa D, et al. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir Physiol Neurobiol. 2012 doi: 10.1016/j.resp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 122.Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17:503–505. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- 123.Ji Z, Long H, Hu Y, Qiu X, Chen X, Li Z, et al. Expression of MDR1, HIF-1alpha and MRP1 in sacral chordoma and chordoma cell line CM-319. J Exp Clin Cancer Res. 2010;29:158. doi: 10.1186/1756-9966-29-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ding Z, Yang L, Xie X, Xie F, Pan F, Li J, et al. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol. 2010;136:1697–1707. doi: 10.1007/s00432-010-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abreu-Rodriguez I, Sanchez Silva R, Martins AP, Soveral G, Toledo-Aral JJ, Lopez-Barneo J, et al. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha. PLoS One. 2011;6:e28385. doi: 10.1371/journal.pone.0028385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab. 2012;32:525–536. doi: 10.1038/jcbfm.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seino S, Miki T. Gene targeting approach to clarification of ion channel function: studies of Kir6.x null mice. J Physiol. 2004;554:295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 129.Yasuda M, Miyazawa M, Fujita M, Kajiwara H, Iida T, Hirasawa T, et al. Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in hypoxic status depending on histological character. Oncol Rep. 2008;19:111–116. [PubMed] [Google Scholar]
- 130.Perez de Heredia F, Wood IS, Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch. 2010;459:509–518. doi: 10.1007/s00424-009-0750-3. [DOI] [PubMed] [Google Scholar]
- 131.Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, et al. Distinct gene expression profiles in norepinephrine- and epinephrineproducing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr. Relat Cancer. 2004;11:897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- 132.Miwa M, Tsuboi M, Noguchi Y, Enokishima A, Nabeshima T, Hiramatsu M. Effects of be taine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation. 2011;8:153. doi: 10.1186/1742-2094-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dallas M, Boycott HE, Atkinson L, Miller A, Boyle JP, Pearson HA, et al. Hypoxia suppresses glutamate transport in astrocytes. J Neurosci. 2007;27:3946–3955. doi: 10.1523/JNEUROSCI.5030-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mossner R, Dringen R, Persico AM, Janetzky B, Okladnova O, Albert D, et al. Increased hippocampal DNA oxidation in serotonin transporter deficient mice. J Neural Transm. 2002;109:557–565. doi: 10.1007/s007020200046. [DOI] [PubMed] [Google Scholar]
- 135.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−): cystine supplier and beyond. Amino Acids. 2012;42:231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- 136.Nakaya K, Tohyama J, Naik SU, Tanigawa H, MacPhee C, Billheimer JT, et al. Peroxisome proliferator-activated receptor-alpha activation promotes macrophage reverse cholesterol transport through a liver X receptor-dependent pathway. Arterioscler Thromb Vasc Biol. 2011;31:1276–1282. doi: 10.1161/ATVBAHA.111.225383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoque MT, Robillard KR, Bendayan R. Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor alpha in human brain microvessel endothelial cells. Mol Pharmacol. 2012;81:598–609. doi: 10.1124/mol.111.076745. [DOI] [PubMed] [Google Scholar]
- 138.Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z, Lehman U, et al. Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2011;31:86–94. doi: 10.1161/ATVBAHA.110.215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, et al. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hosoya K, Sugawara M, Asaba H, Terasaki T. Blood-brain barrier produces significant efflux of L-aspartic acid but not D-aspartic acid: in vivo evidence using the brain efflux index method. J Neurochem. 1999;73:1206–1211. doi: 10.1046/j.1471-4159.1999.0731206.x. [DOI] [PubMed] [Google Scholar]
- 141.Tetsuka K, Takanaga H, Ohtsuki S, Hosoya K, Terasaki T. The l-isomer-selective transport of aspartic acid is mediated by ASCT2 at the blood-brain barrier. J Neurochem. 2003;87:891–901. doi: 10.1046/j.1471-4159.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- 142.O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA. Na(+)- dependent glutamate transporters (EAAT1 EAAT2 EAAT3) of the blood-brain barrier A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–31895. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 143.Mori S, Takanaga H, Ohtsuki S, Deguchi T, Kang YS, Hosoya K, et al. Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J Cereb Blood Flow Metab. 2003;23:432–440. doi: 10.1097/01.WCB.0000050062.57184.75. [DOI] [PubMed] [Google Scholar]
- 144.Ohtsuki S, Kikkawa T, Mori S, Hori S, Takanaga H, Otagiri M, et al. Mouse reduced in osteosclerosis transporter functions as an organic anion transporter 3 and is localized at abluminal membrane of blood-brain barrier. J Pharmacol Exp Ther. 2004;309:1273–1281. doi: 10.1124/jpet.103.063370. [DOI] [PubMed] [Google Scholar]
- 145.Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, et al. Apical-to-basolateral transport of amyloid-beta peptides through blood- brain barrier cells is mediated by the receptor for advanced glycation endproducts and is restricted by P-glycoprotein. J Alzheimers Dis. 2010;22:849–859. doi: 10.3233/JAD-2010-100462. [DOI] [PubMed] [Google Scholar]
- 146.Delou JM, Lopes AG, Capella MA. Unveiling the role of multidrug resistance proteins in hypertension. Hypertension. 2009;54:210–216. doi: 10.1161/HYPERTENSIONAHA.109.129742. [DOI] [PubMed] [Google Scholar]
- 147.Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl. 1):26–34. doi: 10.1111/j.1528-1167.2012.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol. 2004;5:136–146. doi: 10.1089/1527029041352054. [DOI] [PubMed] [Google Scholar]
- 149.Wahlin Larsson B, Kadi F, Ulfberg J, Piehl Aulin K. Skeletal muscle morphology and aerobic capacity in patients with obstructive sleep apnoea syndrome. Respiration. 2008;76:21–27. doi: 10.1159/000126492. [DOI] [PubMed] [Google Scholar]
- 150.Wahlin-Larsson B, Ulfberg J, Aulin KP, Kadi F. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve. 2009;40:556–561. doi: 10.1002/mus.21357. [DOI] [PubMed] [Google Scholar]
- 151.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–R1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- 152.Del Rio R, Munoz C, Arias P, Court FA, Moya EA, Iturriaga R. Chronic intermittent hypoxia-induced vascular enlargement and VEGF upregulation in the rat carotid body is not prevented by antioxidant treatment. Am J Physiol Lung Cell Mol Physiol. 2011;301:L702–L711. doi: 10.1152/ajplung.00128.2011. [DOI] [PubMed] [Google Scholar]
- 153.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 154.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 155.Bikfalvi A, Moenner M, Javerzat S, North S, Hagedorn M. Inhibition of angiogenesis and the angiogenesis/invasion shift. Biochem Soc Trans. 2011;39:1560–1564. doi: 10.1042/BST20110710. [DOI] [PubMed] [Google Scholar]
- 156.Slevin M, Krupinski J. A role for monomeric C-reactive protein in regulation of angiogenesis, endothelial cell inflammation and thrombus formation in cardiovascular/cerebrovascular disease? Histol Histopathol. 2009;24:1473–1478. doi: 10.14670/HH-24.1473. [DOI] [PubMed] [Google Scholar]
- 157.Vagnucci AH, Jr, Li WW. Alzheimer’s disease and angiogenesis. Lancet. 2003;361:605–608. doi: 10.1016/S0140-6736(03)12521-4. [DOI] [PubMed] [Google Scholar]
- 158.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reinke C, Bevans-Fonti S, Grigoryev DN, Drager LF, Myers AC, Wise RA, et al. Chronic intermittent hypoxia induces lung growth in adult mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L266–L273. doi: 10.1152/ajplung.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Toffoli S, Roegiers A, Feron O, Van Steenbrugge M, Ninane N, Raes M, et al. Intermittent hypoxia is an angiogenic inducer for endothelial cells: role of HIF-1. Angiogenesis. 2009;12:47–67. doi: 10.1007/s10456-009-9131-y. [DOI] [PubMed] [Google Scholar]
- 161.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 162.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1 alpha. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med. 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 164.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25:59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 165.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165:67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 166.Ciftci TU, Kokturk O, Demirtas S, Gulbahar O, Bukan N. Consequences of hypoxia-reoxygenation phenomena in patients with obstructive sleep apnea syndrome. Ann Saudi Med. 2011;31:14–18. doi: 10.4103/0256-4947.75772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Teramoto S, Kume H, Matsuse T, Ishii T, Miyashita A, Akishita M, et al. Oxygen administration improves the serum level of nitric oxide metabolites in patients with obstructive sleep apnea syndrome. Sleep Med. 2003;4:403–417. doi: 10.1016/s1389-9457(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 168.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, et al. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics. 2006;25:450–457. doi: 10.1152/physiolgenomics.00293.2005. [DOI] [PubMed] [Google Scholar]
- 170.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, et al. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 171.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dobrogowska DH, Lossinsky AS, Tarnawski M, Vorbrodt AW. Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol. 1998;27:163–173. doi: 10.1023/a:1006907608230. [DOI] [PubMed] [Google Scholar]
- 175.Ruttanaumpawan P, Nopmaneejumruslers C, Logan AG, Lazarescu A, Qian I, Bradley TD. Association between refractory hypertension and obstructive sleep apnea. J Hypertens. 2009;27:1439–1445. doi: 10.1097/HJH.0b013e32832af679. [DOI] [PubMed] [Google Scholar]
- 176.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]