Abstract
Grb7 is a multidomain intracellular signaling protein that links activated tyrosine kinases with downstream signaling targets. Best known for its regulatory role in cell migration and tumor metastasis, Grb7 also regulates inflammation by coupling NF-kappaB-inducing kinase with erbB/EGFR family receptors. The “adaptor” role of Grb7 in these processes depends upon binding to membrane-associated tyrosine kinases through its C-terminal SH2 domain. The Grb7-SH2 domain shares structural and functional similarity with the SH2 domain of Grb2, a constituent of the MAP kinase pathway. Both domains show unusual affinity for cyclic (beta-turn) ligands. The Grb2-SH2 domain also shows distinctive self-association behavior, forming intertwined (“swapped”) dimers. While Grb7 and its SH2 domain are each known to dimerize, the mechanisms and functional significance of this self-association are incompletely understood. Additional residues in the Grb7-SH2 domain effectively lengthen its “EF loop” and render the domain a good candidate for swapped dimerization, through exchange of a C-terminal helix. We propose the existence of a swapped dimeric form of the Grb7-SH2 domain and offer a structural model derived through novel application of nuclear magnetic resonance-derived restraints for homology model refinement.
Keywords: domain swapping, swapped, unswapped, NMR refinement, homology model, Grb2, FAK, EGFR, erbB2, receptor tyrosine kinase
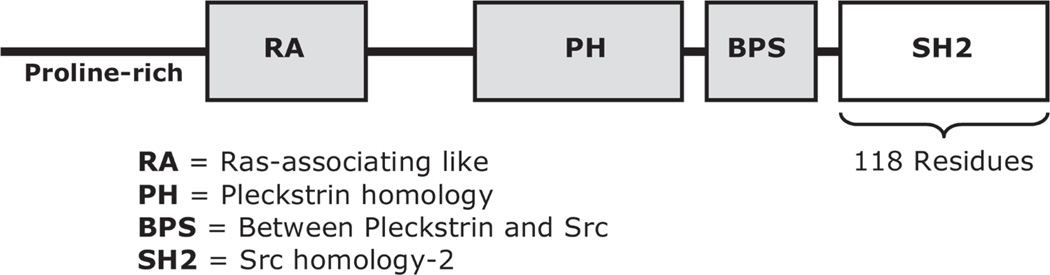
Grb7 is a five-domain intracellular protein that couples membrane-associated tyrosine kinases with downstream signaling partners. The C-terminal SH2 domain (Fig. 1) mediates physical interaction of Grb7 with activated tyrosine kinases through recognition of a phosphorylated tyrosine motif. Grb7 is primarily known as a promoter of tumor progression through regulation of cell migration signaling mediated by the FAK (focal adhesion kinase) and the EphB1 receptor tyrosine kinase.1 As such, its overexpression in tumor tissues is prognostic of high metastatic potential.2–6 Through a distinct pathway, Grb7 overexpression promotes inflammation by coupling NIK (nuclear factor-kappaB-inducing kinase) with growth factor receptors in the erbB/EGFR family, thereby stimulating activation of NF-κB.7 Moreover, improper regulation of Grb7 phosphorylation may promote apoptotic escape, contributing to eosinophilia in patients with atopic dermatitis.8 Grb7 has additionally been identified as a potential component of the T-cell activation pathway9 and as a likely autoimmune target in rheumatoid arthritis.10
FIGURE 1.
Domain topology of the Grb7 protein, highlighting the C-terminal SH2 domain.
The Grb7-SH2 domain shares distinctive structural and functional features with the SH2 domain of the adaptor protein Grb2, known for its role in the MAP kinase/ERK pathway.11 The SH2 domains of Grb7 and Grb2 show similar protein recognition behavior, binding partners in the unusual “beta-turn” (or cyclic) conformation and preferring an unusual phosphotyrosine motif (pYXN, where X is any amino acid).12–15 Both Grb7 and Grb2 self-associate to form dimers. Further, the SH2 domains of both proteins are known to dimerize,16–21 although most SH2 domains are monomeric.17 Crystallographic studies of the Grb2-SH2 domain have shown intertwined (“swapped”) dimers, in which the component monomers exchange a C-terminal helix and form two hybrid “functional monomers.”19–21 We have come to suspect that the Grb7-SH2 domain may also engage in swapped dimerization, and we propose here a structural model constructed through homology modeling coupled with nuclear magnetic resonance (NMR)-derived restraints.
Although a recent crystallographic study of the Grb7-SH2 domain found unswapped dimers,18 this finding does not exclude the possibility that a swapped dimer could also exist. Some proteins are known to dimerize in both swapped and unswapped forms, including the well-documented case of bovine seminal ribonuclease.22–23 While the ligand-free Grb7-SH2 domain is predominantly dimeric,18 the relationship of its dimerization to Grb7 function remains unclear. Furthermore, the possible existence of a domain-swapped form has not been explored in the literature.
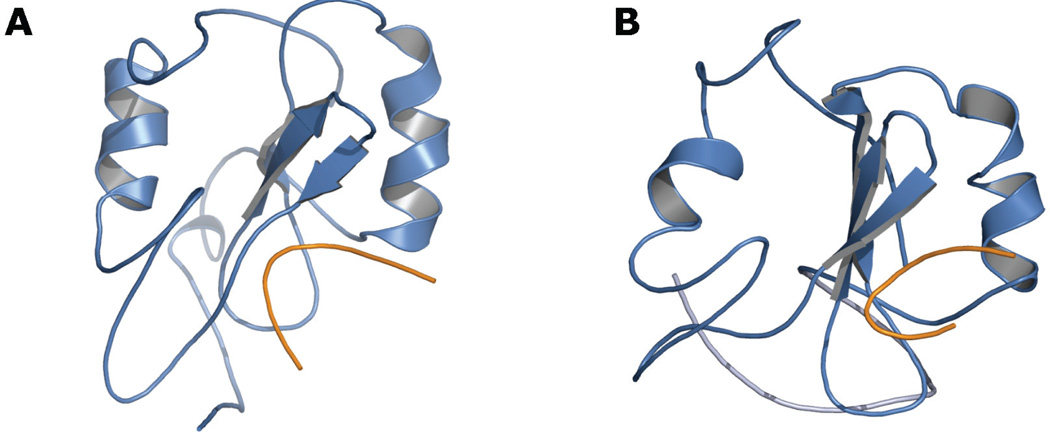
We recently carried out molecular dynamics simulations of the Grb7-SH2 domain monomer, in complex with the erbB2 receptor peptide pY1139. (The solution structure of the Grb7-SH2/pY1139 complex was solved previously by our group.13) Several independent explicit solvent simulations showed destructuring of the domain’s C-terminal tertiary fold (Fig. 2B). Although the secondary structures remained largely intact, they lost the fixed relative positions associated with the canonical SH2 domain architecture and became more dynamic with respect to the well-folded N-terminal region. Affected structures include the D’E and EF beta-sheets, as well as the alpha-B helix and the BG loop (using SH2 domain nomenclature established by Eck et al.24). The destructuring occurred in few-nanosecond simulations at 300 K with a 2-femtosecond time step, but was not seen in a 21-nanosecond simulation at 300 K with a 1-femtosecond time step and with lower average values for total and potential energy (Fig. 2A; energy data not shown). The Grb7-SH2 domain’s propensity for conformational flexibility in the C-terminal region is supported by a corresponding sparsity of NMR-derived distance restraints, particularly in the D’E and EF-loop region.
FIGURE 2.
Molecular dynamics snapshots of the Grb7-SH2 domain (blue) in complex with the erbB2 receptor peptide pY1139 (orange). A, Integrity of the canonical SH2 domain fold following 21 nanoseconds of unrestrained explicit solvent molecular dynamics with a 1-femtosecond time step. B, Destructuring of the domain’s C-terminal region during explicit solvent simulations with a 2-femtosecond time step and with higher average total and potential energy than the simulation represented in panel A. The image shown is a snapshot taken after 11 nanoseconds of unrestrained dynamics at 300 K.
Given the helix-swapping behavior of the Grb2-SH2 domain, we suspect that the partially destructured Grb7-SH2 domain represents an intermediate in the formation of a helix-swapped dimer. Our results seem to conform to a model recently proposed by Malevanets et al. regarding the general mechanism of swapped dimer formation. Namely, our partially destructured monomer could represent a “molten globule-like” state, which poises the monomer to engage in helix swapping with another monomer.25
To generate a structural model of this putative swapped dimeric form of the Grb7-SH2 domain, we have used homology modeling, followed by refinement with NMR-derived distance and angle restraints. The model was initially constructed with SWISS-MODEL software,26 using a swapped Grb2-SH2 domain dimer (2H5K) as a template.21 We manually docked the pY1139 ligand onto each monomer of the model via superposition of the monomeric NMR structure for the Grb7-SH2/pY1139 complex. Subsequently, we refined the model with molecular dynamics simulated annealing using AMBER 9 software27–30 with the ff99SB force field29,31 and with implicit solvent (modified32 generalized Born model33).
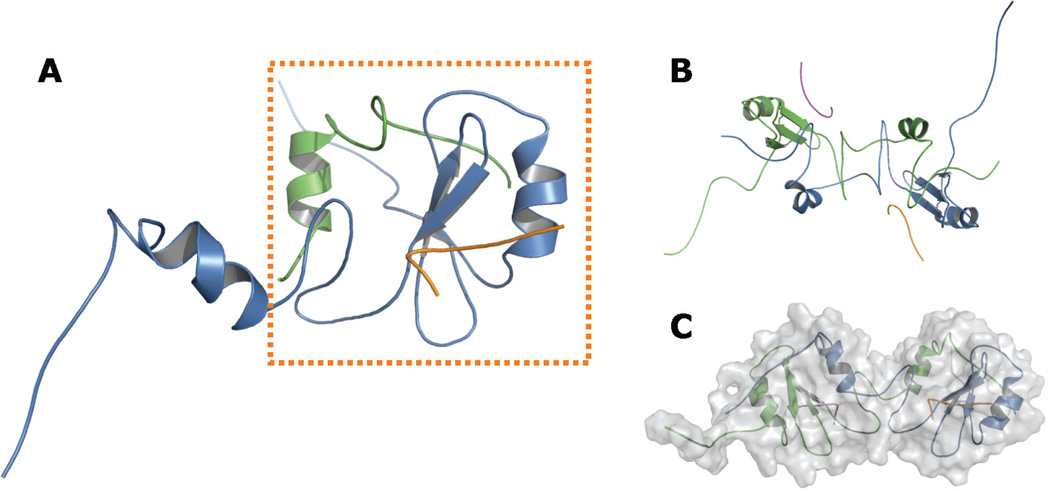
Distance and dihedral angle restraints derived from NMR data for the Grb7-SH2/pY1139 complex13 were applied during the simulated annealing experiments. All distance restraints were defined as ambiguous, with pairs of atoms either within the same monomer or across monomers able to satisfy each restraint. Six iterations of 32-pico-second molecular dynamics simulated annealing from 1200 to 0 K were performed. The resulting refined model of the helix-swapped Grb7-SH2 domain in complex with the pY1139 peptide is shown in Figure 3. Statistics demonstrating the model quality are provided in Table 1.
FIGURE 3.
Proposed helix-swapped Grb7-SH2 domain model. Panels A to C show various views of the Grb7-SH2 domain / pY1139 peptide complex following refinement using NMR-derived restraints. Each monomer and each ligand is shown in a distinct color. A, Side view of a single hybrid Grb7-SH2 domain “functional monomer,” shown in the dashed box. The N-terminal segment of the functional monomer is composed of approximately 85 residues from a single Grb7-SH2 domain chain (blue), ending with the unique Grb7-SH2 domain sequence MD-DGQ. The C-terminal segment is made up of approximately 33 residues from a second Grb7-SH2 domain chain (green), beginning with the sequence T’R’F’T’, which immediately follows M’D’D’G’Q’ in the second chain. The remaining residues of the chain are hidden for clarity. B, View along the axis of symmetry. The model shows C2 symmetry, although no symmetry was imposed in the refinement protocol. C, “Head-to-head” orientation of the two functional monomers, with the molecular surface shaded in gray. Figures rendered with PyMOL.36
TABLE 1.
| Violation statisticsa | |
| Per chain average number of | |
| Distance restraint violations > 0.5 Å | 0.5 |
| Distance restraint violations > 0.2 Å | 20 |
| Dihedral angle restraint violations > 5° | 8 |
| Maximum distance restraint violation | 0.54 Å |
| Maximum dihedral angle restraint violation | 10.7° |
| RMSD from idealized covalent geometryb | |
| Covalent bonds | 0.036 Å |
| Angles | 3.6° |
| PROCHECK-NMR Ramachandran plot statisticsb | |
| (excluding glycine, proline, phospho-tyrosine, and end residues) | |
| Residues in most favored regions | 64.0 % |
| Residues in additional allowed regions | 33.2 % |
| Residues in generously allowed regions | 2.8 % |
| Residues in disallowed regions | 0.0 % |
| WHAT IF statisticsc | |
| Z-scores, indicating the number of standard deviations from the expected value for well-refined X-ray structures: | |
| Second-generation packing quality | −3.85 |
| Ramachandran plot appearance | −0.67 |
| chi-1/chi-2 rotamer normality | −0.72 |
| Backbone conformation | −5.35 |
| RMS Z-scores, expected to fall around 1.0: | |
| Bond lengths | 1.93 |
| Bond angles | 1.88 |
| Inside/outside residue distribution | 1.23 |
Violation statistics for the original ensemble as reported previously.1
Statistics acquired using the ADIT validation server of the RCSB protein data bank (http://deposit.pdb.org/validate/).
It is noteworthy that the so-called EF loop of the Grb7 family SH2 domains contains a four-amino acid insertion that is absent in other SH2 domains. The EF loop serves as the “hinge loop” structure34 that mediates domain swapping in the Grb2-SH2 domain.19–21 We suggest that the EF loop insertion may promote swapped dimerization in the Grb7 family SH2 domains, as hinge loop lengthening has been observed to facilitate domain swapping in other proteins.35
In our model, the functional monomers show a “head-to-head” orientation (Fig. 3C), which contrasts with the “head-to-tail” orientation of the crystallographic (unswapped) dimer but resembles the Grb2-SH2 domain dimer orientation.19–21 However, there appears to be significant potential for rotation of the functional monomers with respect to one another. Namely, the application of six rounds of molecular dynamics simulated annealing to a modified homology model, with the functional monomers in a head-to-tail starting orientation, resulted in a head-to-tail model with only slightly less favorable restraint energy than the head-to-head model featured here (alternate model data not shown).
Although our group previously found the Grb7-SH2 domain to be largely monomeric when bound to the pY1139 peptide (based on measured molecular tumbling times),16 we have used NMR-derived restraints obtained through study of the Grb7-SH2/pY1139 complex to help us create an approximate swapped dimer model. We should expect the functional monomers of a swapped Grb7-SH2 domain dimer to be highly similar in structure to the monomeric domain, as is the case for the Grb2-SH2 domain and for many other proteins.21,32 The functional monomers of our model overlay well (RMSD around 1.5 Å) with the Grb7-SH2 domain monomer refined from the previously published Grb7-SH2/pY1139 NMR structure using molecular dynamics simulated annealing in generalized Born solvent (unpublished data). Our success in producing a model that fits the NMR-derived restraints strengthens our hypothesis that the Grb7-SH2 domain can exist in a swapped dimer form. Further computational modeling has enabled us to suggest elsewhere (publication forthcoming) a thermodynamic argument as to why swapped dimers are not observed in a previously described dimerization-deficient Grb7-SH2 domain mutant.17
ACKNOWLEDGMENTS
We gratefully acknowledge computational resources provided by the New Mexico State University Department of Computer Science (Bioinformatics Cluster) and the National Center for Genome Resources (NCGR, Santa Fe, NM), as well as research training provided by NCGR (Dr. Jim Huntley). This work was supported by National Science Foundation awards 420-40-50 (IGERT training grant) and HRD-0420407 (CREST Center for Research Excellence), as well as National Institutes of Health grant P20RR016480 (New Mexico INBRE network).
REFERENCES
- 1.Shen TL, Guan JL. Grb7 in intracellular signaling and its role in cell regulation. Front Biosci. 2004 Jan 1;9:192–200. doi: 10.2741/1229. [DOI] [PubMed] [Google Scholar]
- 2.Dahlberg PS, Jacobson BA, Dahal G, Fink JM, Kratzke RA, Maddaus MA, Ferrin LJ. ERBB2 amplifications in esophageal adenocarcinoma. Ann Thorac Surg. 2004 Nov;78(5):1790–1800. doi: 10.1016/j.athoracsur.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 3.Haran M, Chebatco S, Flaishon L, Lantner F, Harpaz N, Valinsky L, Berrebi A, Shachar I. Grb7 expression and cellular migration in chronic lymphocytic leukemia: a comparative study of early and advanced stage disease. Leukemia. 2004 Dec;18(12):1948–1950. doi: 10.1038/sj.leu.2403512. [DOI] [PubMed] [Google Scholar]
- 4.Walch A, Specht K, Braselmann H, Stein H, Siewert JR, Hopt U, Höfler H, Werner M. Coamplification and coexpression of GRB7 and ERBB2 is found in high grade intraepithelial neoplasia and in invasive Barrett’s carcinoma. Int J Cancer. 2004 Dec 10;112(5):747–753. doi: 10.1002/ijc.20411. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Pero SC, Taguchi K, Shimada M, Mori M, Krag DN, Arii S. Specific peptide ligand for Grb7 signal transduction protein and pancreatic cancer metastasis. J Natl Cancer Inst. 2006 Apr 5;98(7):491–498. doi: 10.1093/jnci/djj105. [DOI] [PubMed] [Google Scholar]
- 6.Itoh S, Taketomi A, Tanaka S, Harimoto N, Yamashita Y, Aishima S, Maeda T, Shirabe K, Shimada M, Maehara Y. Role of growth factor receptor bound protein 7 in hepatocellular carcinoma. Mol Cancer Res. 2007 Jul;5(7):667–673. doi: 10.1158/1541-7786.MCR-06-0282. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Xu LG, Chen L, Li L, Zhai Z, Shu HB. NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene. 2003 Jul 10;22(28):4348–4355. doi: 10.1038/sj.onc.1206532. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SW, Kim TY, Sung MH, Kim CJ, Poo H. Comparative proteomic analysis of peripheral blood eosinophils from healthy donors and atopic dermatitis patients with eosinophilia. Proteomics. 2005 May;5(7):1987–1995. doi: 10.1002/pmic.200401086. [DOI] [PubMed] [Google Scholar]
- 9.Chu P, Pardo J, Zhao H, Li CC, Pali E, Shen MM, Qu K, Yu SX, Huang BC, Yu P, Masuda ES, Molineaux SM, Kolbinger F, Aversa G, de Vries J, Payan DG, Liao XC. Systematic identification of regulatory proteins critical for T-cell activation. J Biol. 2003;2(3):21. doi: 10.1186/1475-4924-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CW, Cho EH, Lee YJ, Kim YH, Hah YS, Kim DR. Disease-specific proteins from rheumatoid arthritis patients. J Korean Med Sci. 2006 Jun;21(3):478–484. doi: 10.3346/jkms.2006.21.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis RJ. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995 Dec;42(4):459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 12.Rahuel J, Gay B, Erdmann D, Strauss A, Garcia-Echeverría C, Furet P, Caravatti G, Fretz H, Schoepfer J, Grütter MG. Structural basis for specificity of Grb2-SH2 revealed by a novel ligand binding mode. Nat Struct Biol. 1996 Jul;3(7):586–589. doi: 10.1038/nsb0796-586. [DOI] [PubMed] [Google Scholar]
- 13.Ivancic M, Daly RJ, Lyons BA. Solution structure of the human Grb7-SH2 domain/erbB2 peptide complex and structural basis for Grb7 binding to ErbB2. J Biomol NMR. 2003 Nov;27(3):205–219. doi: 10.1023/a:1025498409113. [DOI] [PubMed] [Google Scholar]
- 14.Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pero SC, Oligino L, Daly RJ, Soden AL, Liu C, Roller PP, Li P, Krag DN. Identification of novel non-phosphorylated ligands, which bind selectively to the SH2 domain of Grb7. J Biol Chem. 2002 Apr 5;277(14):11918–11926. doi: 10.1074/jbc.M111816200. [DOI] [PubMed] [Google Scholar]
- 16.Ivancic M, Spuches AM, Guth EC, Daugherty MA, Wilcox DE, Lyons BA. Backbone nuclear relaxation characteristics and calorimetric investigation of the human Grb7-SH2/erbB2 peptide complex. Protein Sci. 2005 Jun;14(6):1556–1569. doi: 10.1110/ps.041102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter CJ, Wilce MC, Mackay JP, Leedman P, Wilce JA. Grb7-SH2 domain dimerisation is affected by a single point mutation. Eur Biophys J. 2005 Jul;34(5):454–460. doi: 10.1007/s00249-005-0480-1. [DOI] [PubMed] [Google Scholar]
- 18.Porter CJ, Matthews JM, Mackay JP, Pursglove SE, Schmidberger JW, Leedman PJ, Pero SC, Krag DN, Wilce MC, Wilce JA. Grb7 SH2 domain structure and interactions with a cyclic peptide inhibitor of cancer cell migration and proliferation. BMC Struct Biol. 2007;7:58. doi: 10.1186/1472-6807-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiering N, Casale E, Caccia P, Giordano P, Battistini C. Dimer formation through domain swapping in the crystal structure of the Grb2-SH2-Ac-pYVNV complex. Biochemistry. 2000 Nov 7;39(44):13376–13382. doi: 10.1021/bi0012336. [DOI] [PubMed] [Google Scholar]
- 20.Nioche P, Liu WQ, Broutin I, Charbonnier F, Latreille MT, Vidal M, Roques B, Garbay C, Ducruix A. Crystal structures of the SH2 domain of Grb2: highlight on the binding of a new high-affinity inhibitor. J Mol Biol. 2002 Feb 1;315(5):1167–1177. doi: 10.1006/jmbi.2001.5299. [DOI] [PubMed] [Google Scholar]
- 21.Benfield AP, Whiddon BB, Clements JH, Martin SF. Structural and energetic aspects of Grb2-SH2 domain-swapping. Arch Biochem Biophys. 2007 Jun 1;462(1):47–53. doi: 10.1016/j.abb.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccoli R, Tamburrini M, Piccialli G, Di Donato A, Parente A, D’Alessio G. The dual-mode quaternary structure of seminal RNase. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1870–1874. doi: 10.1073/pnas.89.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merlino A, Ercole C, Picone D, Pizzo E, Mazzarella L, Sica F. The buried diversity of bovine seminal ribonuclease: shape and cytotoxicity of the swapped non-covalent form of the enzyme. J Mol Biol. 2008 Feb 15;376(2):427–437. doi: 10.1016/j.jmb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Eck MJ, Shoelson SE, Harrison SC. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- 25.Malevanets A, Sirota FL, Wodak SJ. Mechanism and energy landscape of domain swapping in the B1 domain of protein G. J Mol Biol. 2008 Sep 26;382(1):223–235. doi: 10.1016/j.jmb.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006 Jan 15;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 27.Case DA, Darden TA, T E, Cheatham I, Simmerling CL, Wang J, Duke RE, et al. AMBER 9. San Francisco: University of California; 2006. [Google Scholar]
- 28.Wang J, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- 29.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006 Nov 15;65(3):712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsui V, Case DA. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers. 2000;56(4):275–291. doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Cieplak P, Kollman P. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? Journal of Computational Chemistry. 2000;21(12):1049–1074. [Google Scholar]
- 32.Onufriev A, Bashford D, Case DA. Modification of the generalized Born model suitable for macromolecules. J Phys Chem B. 2000;104(15):3712–3720. [Google Scholar]
- 33.Still WC, Tempczyk A, Hawley RC, Hendrickson T. Semianalytical treatment of solvation for molecular mechanics and dynamics. J Am Chem Soc. 1990;112(16):6127–6129. [Google Scholar]
- 34.Bennett MJ, Schlunegger MP, Eisenberg D. 3D domain swapping: a mechanism for oligomer assembly. Protein Sci. 1995 Dec;4(12):2455–2468. doi: 10.1002/pro.5560041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousseau F, Schymkowitz JW, Itzhaki LS. The unfolding story of three-dimensional domain swapping. Structure. 2003 Mar;11(3):243–251. doi: 10.1016/s0969-2126(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 36.DeLano WL. The PyMOL molecular graphics system. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- 37.Hooft RW, Vriend G, Sander C, Abola EE. Errors in protein structures. Nature. 1996;381(6580):272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]