Abstract
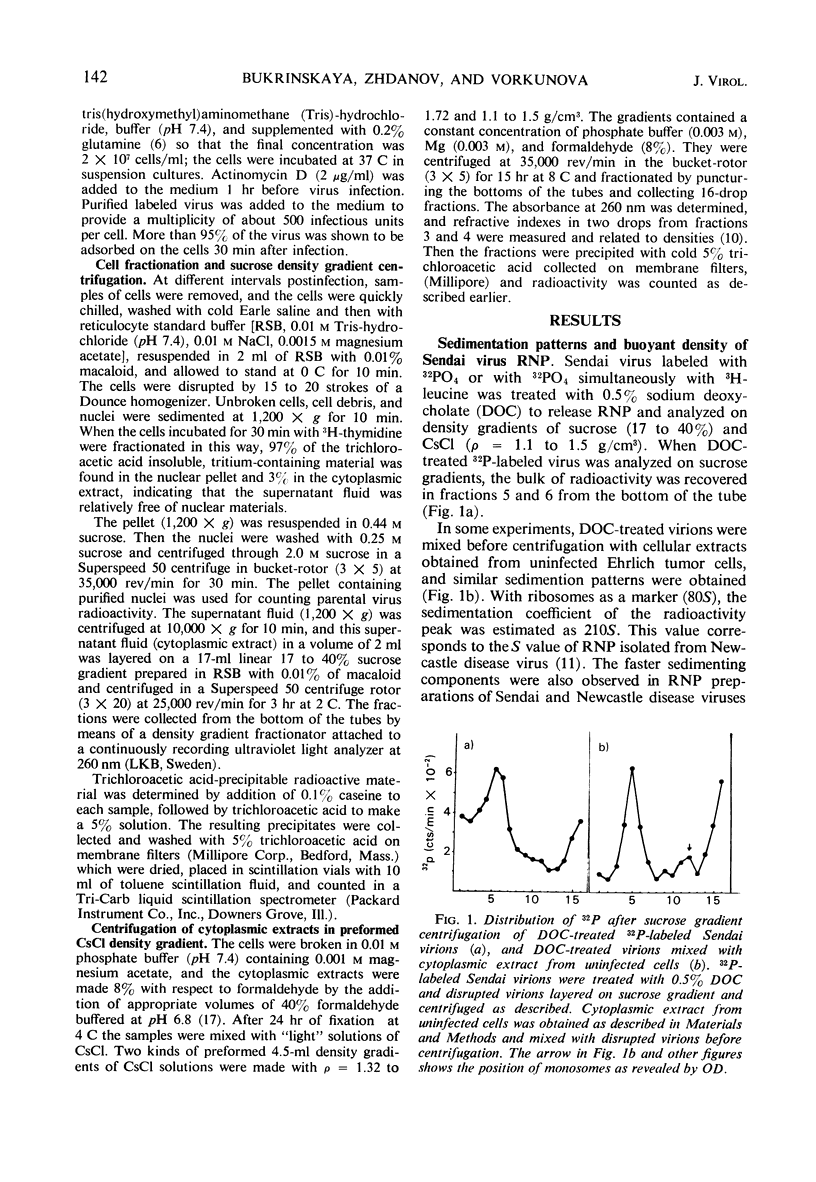
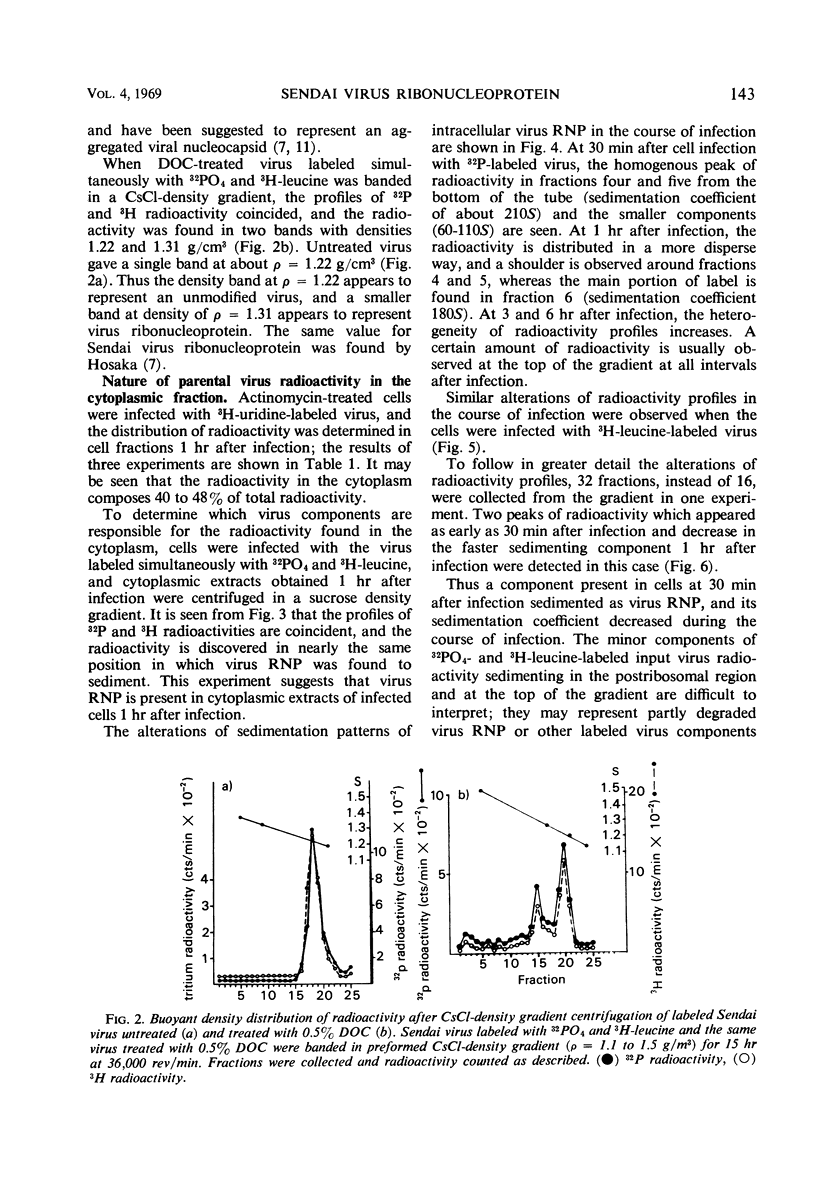
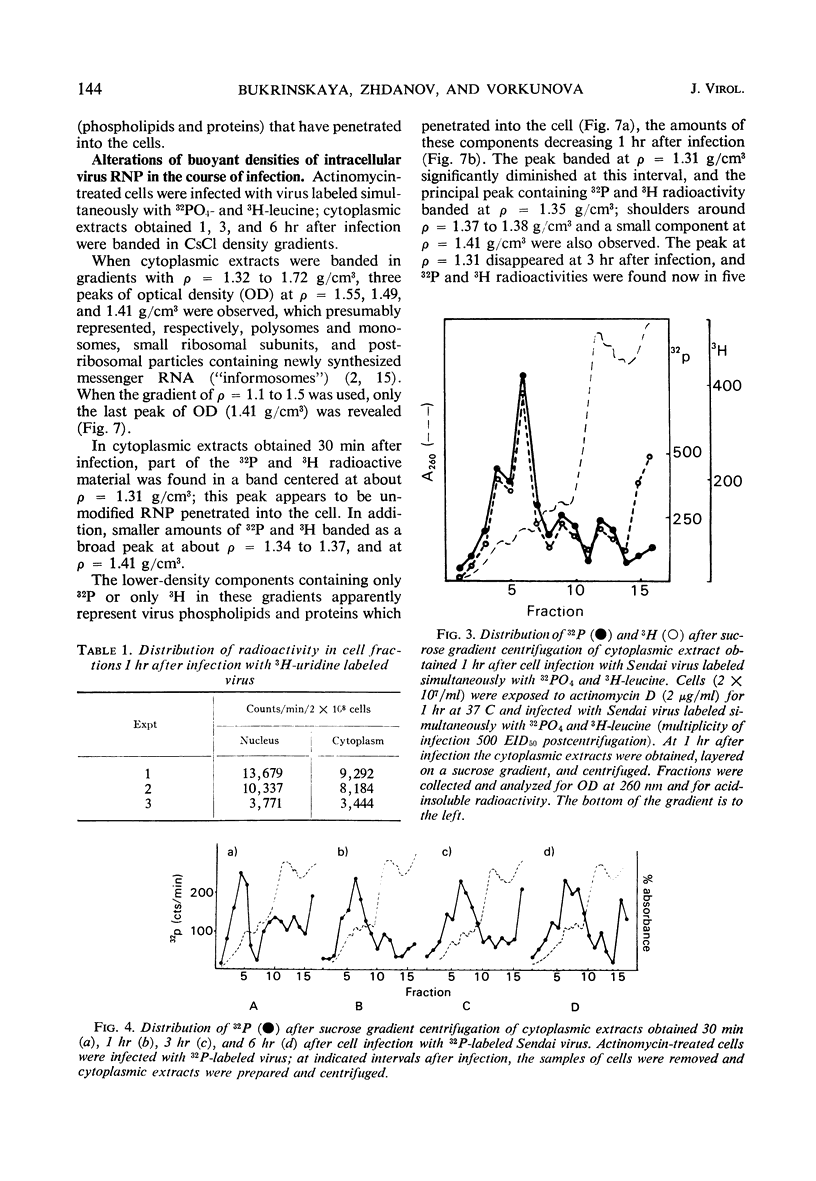
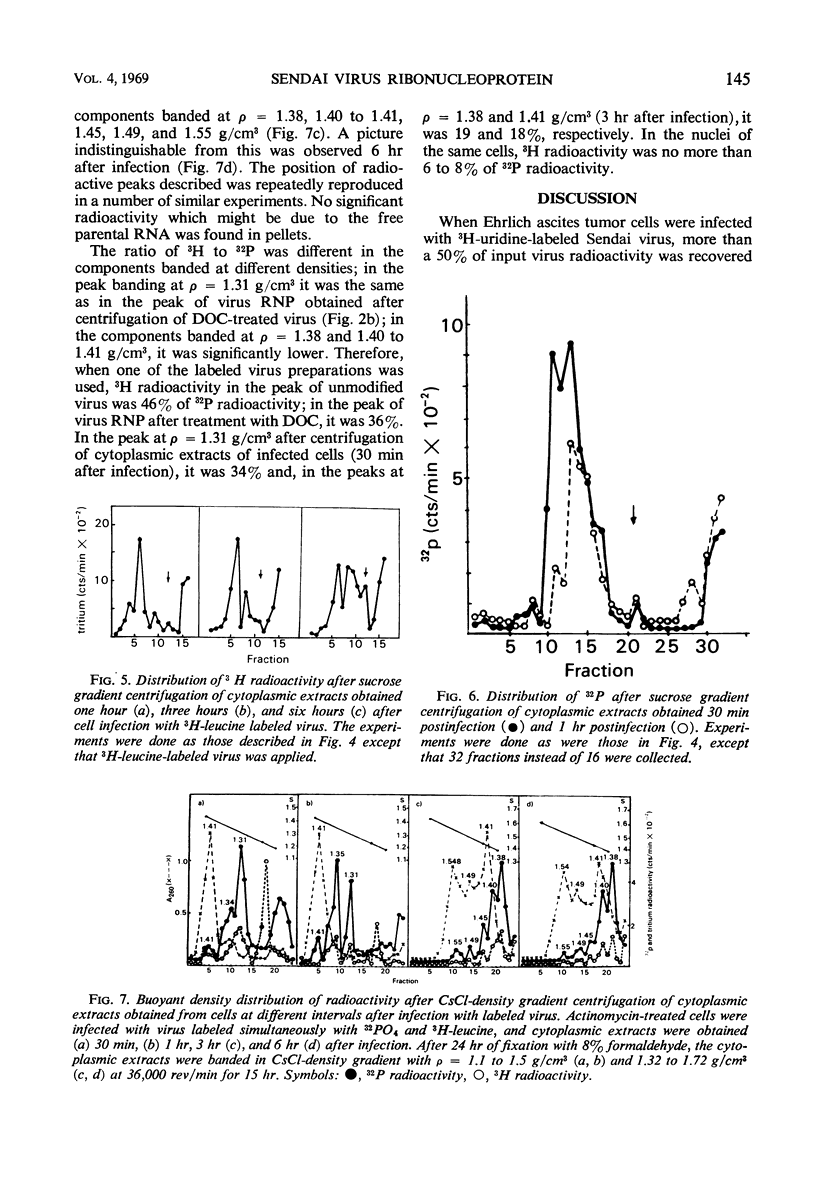
The cytoplasmic extracts of Ehrlich ascites tumor cells infected with 32PO4 and 3H-leucine-labeled Sendai virus have been examined during the course of infection with respect to sedimentation behavior and buoyant densities of input virus radioactivity. It was found that 32P and 3H radioactivities were coincident, and, at 30 min after infection, the bulk of radioactivity was recovered in the polysome region of a sucrose gradient in the position of Sendai virus ribonucleoprotein (210S). The heterogeneity of radioactivity profiles appeared at 1 hr after infection and increased during 6 hr of incubation. The buoyant densities of input virus components were determined by banding in CsCl gradient. Here again the bulk of coincident 32P and 3H radioactivity at 30 min after infection banded at the same density as Sendai virus ribonucleoprotein (1.31 g/cm3.) This component disappeared at 3 hr after infection, and 32P and 3H radioactivities were now found in components banded at densities 1.38, 1.41, 1.45, 1.49, and 1.55 g/cm3. The results presented are consistent with the idea that virus ribonucleoprotein is retained in the cytoplasm of infected cells during at least 6 hr of incubation, being partly deproteinized in the course of infection. The nature of components which banded at ρ = 1.41, 1.45, 1.49, and 1.55 as complexes of partly deproteinized ribonucleoprotein with ribosomes will be described in a separate paper.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. D., Bukrinskaya A. G. The nucleic acid of Sendai virus and ribonucleic acid synthesis in cells infected by Sendai virus. J Gen Virol. 1968 Jan;2(1):71–79. doi: 10.1099/0022-1317-2-1-71. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Klimenko S. M., Smirnov Y. A., Guschin B. V. Infective substructures of Sendai virus from infected Ehrlich ascites tumor cells. J Virol. 1968 Jul;2(7):752–758. doi: 10.1128/jvi.2.7.752-758.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vladimirzeva E. A. Isolation of Sendai virus RNA from infected cells by the method of thermic fractionation. Biochim Biophys Acta. 1966 Apr 18;119(1):219–220. doi: 10.1016/0005-2787(66)90059-1. [DOI] [PubMed] [Google Scholar]
- DALES S., CHOPPIN P. W. Attachment and penetration of influenza virus. Virology. 1962 Nov;18:489–493. doi: 10.1016/0042-6822(62)90041-7. [DOI] [PubMed] [Google Scholar]
- EATON M. D., JEWELL M., SCALA A. R. Formation of noninfectious viral hemaggluinins in ascites tumor cells. Virology. 1960 Jan;10:112–126. doi: 10.1016/0042-6822(60)90010-6. [DOI] [PubMed] [Google Scholar]
- HOYLE L., FINTER N. B. The use of influenza virus labelled with radio-sulphur in studies of the early stages of the interaction of virus with the host cell. J Hyg (Lond) 1957 Jun;55(2):290–297. doi: 10.1017/s0022172400037189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE L. The entry of myxoviruses into the cell. Cold Spring Harb Symp Quant Biol. 1962;27:113–121. doi: 10.1101/sqb.1962.027.001.014. [DOI] [PubMed] [Google Scholar]
- Hosaka Y. Isolation and structure of the nucleocapsid of HVJ. Virology. 1968 Jul;35(3):445–457. doi: 10.1016/0042-6822(68)90223-7. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Darlington R. W. Isolation and properties of Newcastle disease virus nucleocapsid. J Virol. 1968 Mar;2(3):248–255. doi: 10.1128/jvi.2.3.248-255.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Early stages of infection with Newcastle disease virus as revealed by electron microscopy. Virology. 1962 Apr;16:506–509. doi: 10.1016/0042-6822(62)90235-0. [DOI] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN S. C., MARCUS P. I. EARLY STAGES OF NEWCASTLE DISEASE VIRUS-HELA CELL INTERACTION: AN ELECTRON MICROSCOPIC STUDY. Virology. 1964 Jul;23:370–380. doi: 10.1016/0042-6822(64)90259-4. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V., Lerman M. I. Use of formaldehyde fixation for studies of ribonucleoprotein particles by caesium chloride density-gradient centrifugation. J Mol Biol. 1965 Dec;14(2):611–615. doi: 10.1016/s0022-2836(65)80213-3. [DOI] [PubMed] [Google Scholar]


