Abstract
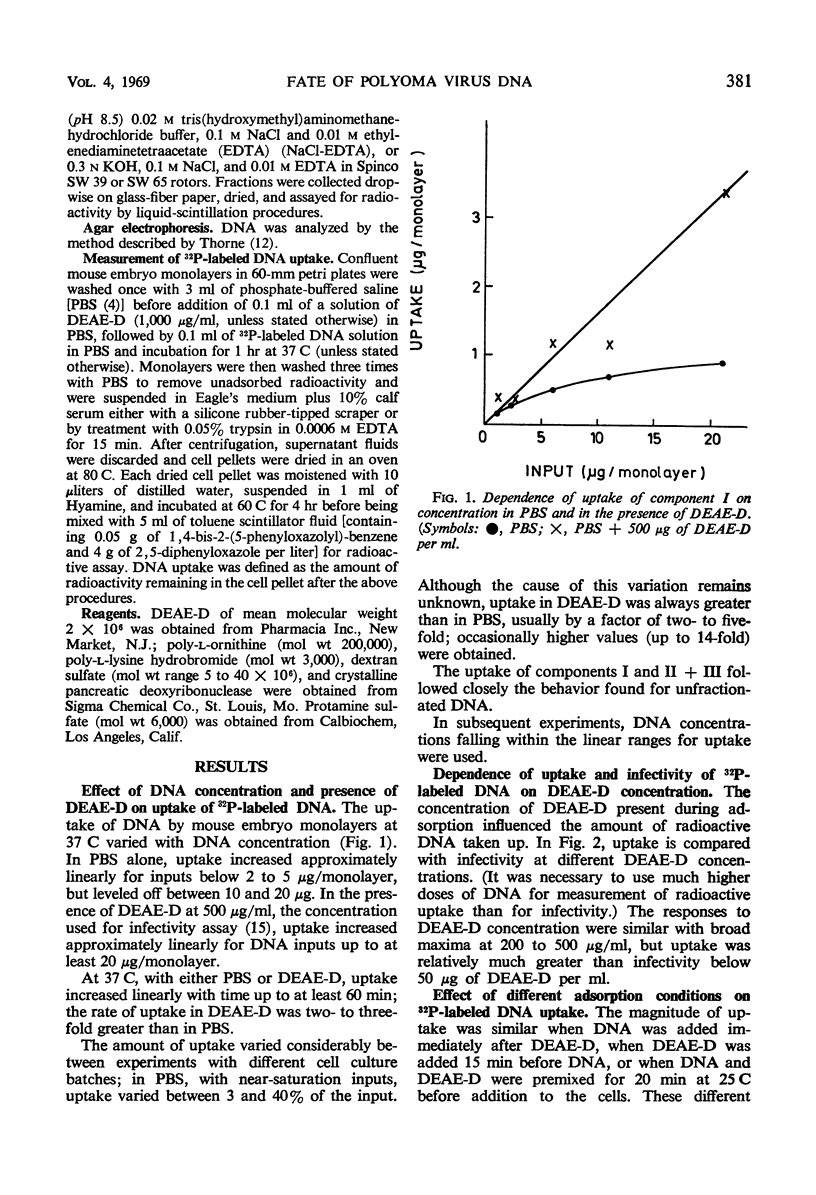
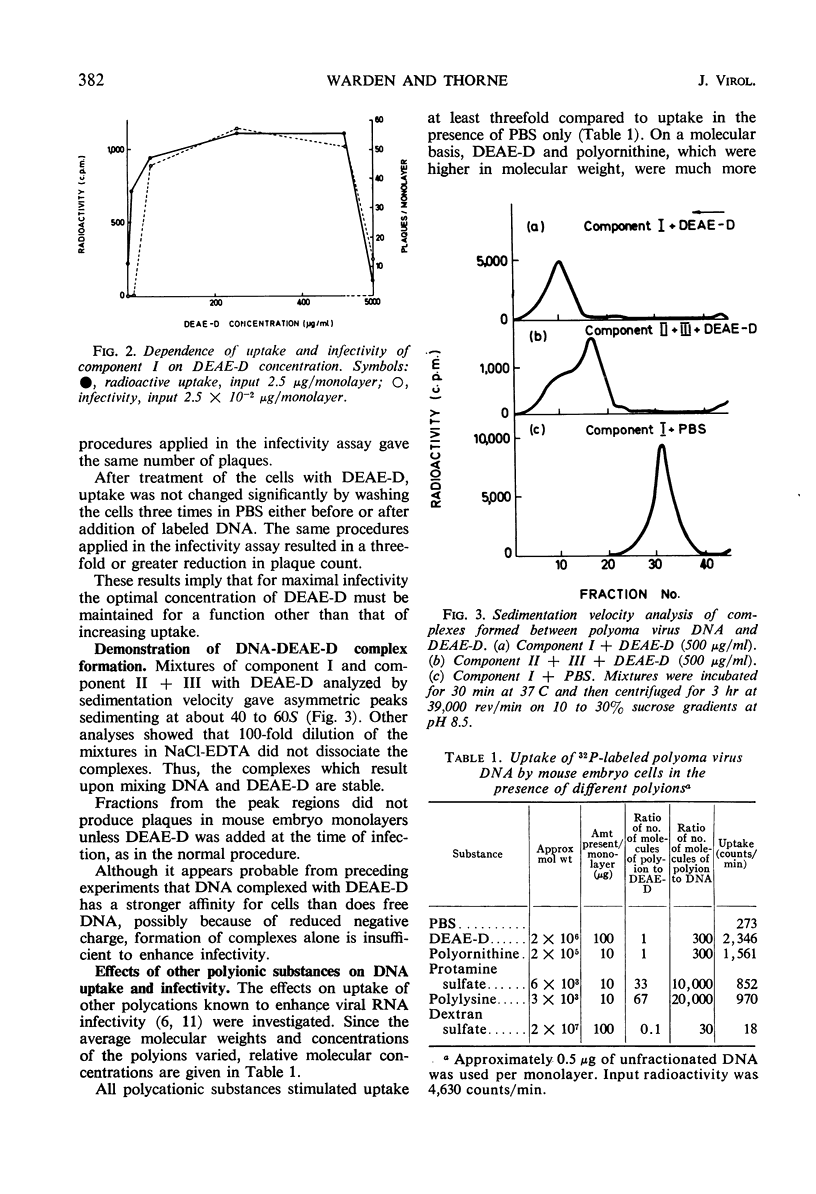
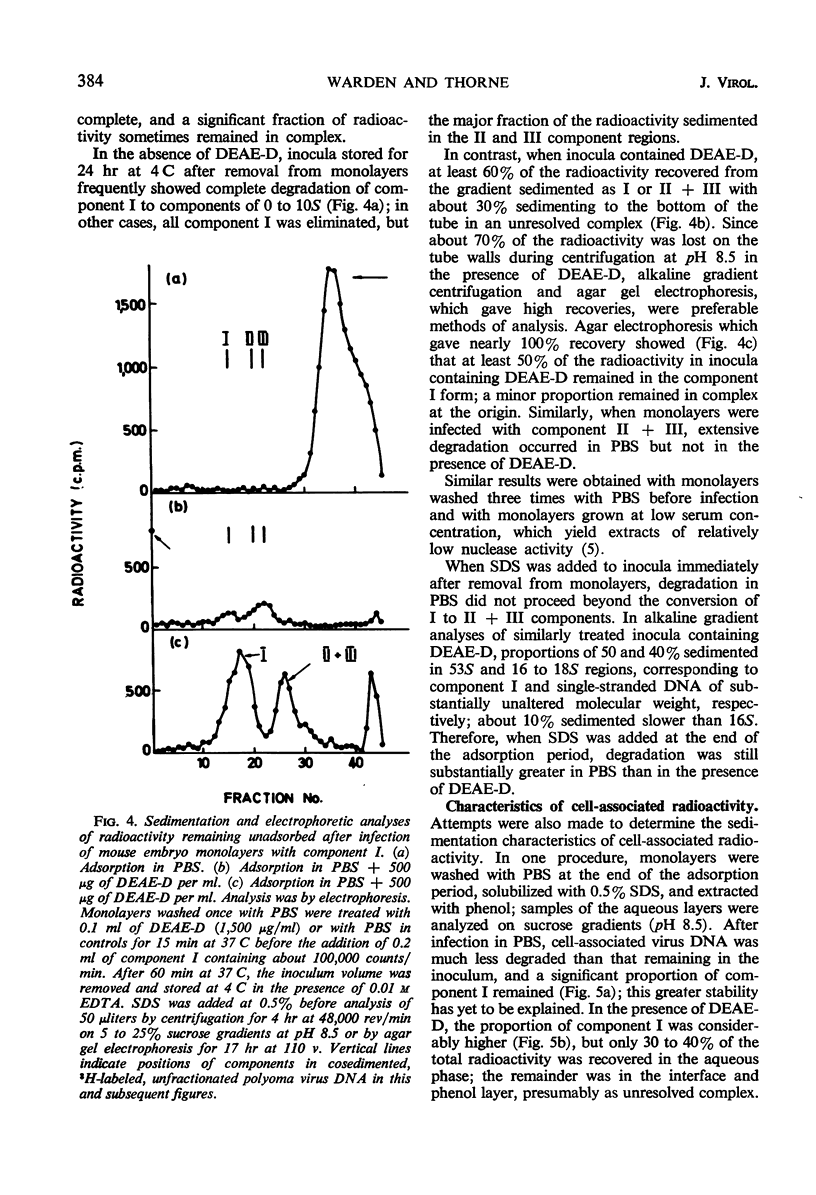
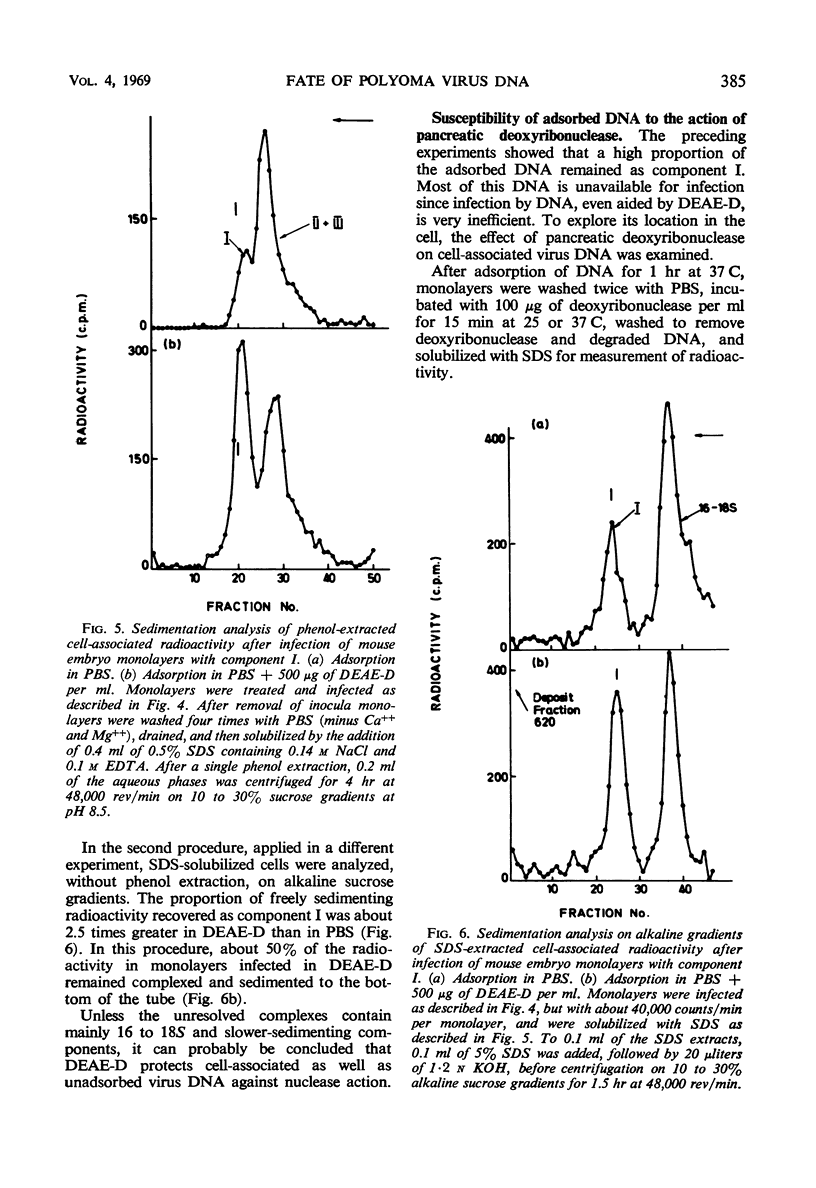
The uptake of 32P-labeled polyoma virus deoxyribonucleic acid (DNA) (I and II + III) by mouse embryo cells was increased from two- to fivefold in the presence of 500 μg of diethylaminoethyl-dextran (DEAE-D) per ml. This concentration of DEAE-D gives maximal enhancement of infectivity; however, the increase is many thousand-fold. As the DEAE-D concentration was increased from 0 μg/ml, uptake and infectivity increased to flat maxima and then decreased in a similar manner, except that at low DEAE-D concentrations uptake was relatively much greater than infectivity. Several other polycations also increased DNA uptake but did not enhance infectivity, and uptake of viral DNA was unaffected by the presence of mouse DNA, although infectivity was reduced. Thus, increased uptake is not the sole basis for the enhancement of infectivity produced by DEAE-D. The possibilities that DNA complexed with DEAE-D penetrates more rapidly or is stabilized against degradation do not completely account for enhancement since complexes formed in mixtures of DNA and DEAE-D, which sedimented heterogeneously from 40 to 60S, were infectious only for monolayers treated with DEAE-D. A more likely factor in enhancement is inhibition of the cellular nuclease activity detected, since virus DNA exposed to cells was much more degraded in the absence than in the presence of DEAE-D. The nuclease activity produced single-strand breaks in double-stranded DNA. Treatment of monolayers with deoxyribonuclease after adsorption of DNA in the presence of DEAE-D reduced cell-associated radioactivity by about 70%, although the number of plaques formed was not affected. In the absence of DEAE-D, 90 to 100% was removed by deoxyribonuclease. Thus, in both cases most of the DNA was adsorbed extracellularly. The greater deoxyribonuclease-resistant fraction in the presence of DEAE-D would be consistent with another possibility: that enhancement results from an increase in DNA penetration rate due to some action of DEAE-D on the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach H. L. Ribonucleic acid of foot-and-mouth disease virus: an ultrasensitive plaque assay. Proc Soc Exp Biol Med. 1966 Dec;123(3):939–945. doi: 10.3181/00379727-123-31644. [DOI] [PubMed] [Google Scholar]
- Bases R. E., Huppert J. Impairment of EMC viral infectivity by cell-associated ribonuclease. Virology. 1966 Aug;29(4):596–604. doi: 10.1016/0042-6822(66)90283-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Pitts J. D. Replication of polyoma virus DNA. I. A resting cell system for biochemical studies on polyoma virus. Virology. 1968 Apr;34(4):761–770. doi: 10.1016/0042-6822(68)90097-4. [DOI] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- SMULL C. E., LUDWIG E. H. Enhancement of the plaque-forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol. 1962 Nov;84:1035–1040. doi: 10.1128/jb.84.5.1035-1040.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H. V. Electrophoretic characterization and fractionation of polyoma virus DNA. J Mol Biol. 1967 Mar 14;24(2):203–211. doi: 10.1016/0022-2836(67)90326-9. [DOI] [PubMed] [Google Scholar]
- Thorne H. V., Evans J., Warden D. Detection of biologically defective molecules in component I of polyoma virus DNA. Nature. 1968 Aug 17;219(5155):728–730. doi: 10.1038/219728a0. [DOI] [PubMed] [Google Scholar]
- Tovell D. R., Colter J. S. Observations on the assay of infectious viral ribonucleic acid: effects of DMSO and DEAE-dextran. Virology. 1967 May;32(1):84–92. doi: 10.1016/0042-6822(67)90255-3. [DOI] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. The infectivity of polyoma virus DNA for mouse embryo cells in the presence of diethylaminoethyl-dextran. J Gen Virol. 1968 Dec;3(3):371–377. doi: 10.1099/0022-1317-3-3-371. [DOI] [PubMed] [Google Scholar]


