Abstract
OBJECTIVES
To evaluate forced expiratory volume in 1 second (FEV1, a measure of overall lung function), long-term average FEV1, and rate of decline in FEV1 in relation to cognition and cognitive decline in older men.
DESIGN
Prospective observational study.
SETTING
Community-based population.
PARTICIPANTS
Eight hundred sixty-four older men from the Normative Aging Study.
MEASUREMENTS
Starting in 1984, participants underwent triennial clinical evaluations. Lung function assessments provided estimates of FEV1. Cognitive assessments entailing tests of several cognitive abilities began in 1993. FEV1 measured approximately 12 years before baseline cognitive testing, average FEV1 over the 12-year period, and rate of change in FEV1 were all evaluated in relation to baseline and change in performance on the cognitive tests.
RESULTS
In multivariable-adjusted analyses, associations between FEV1 and baseline cognitive scores were mixed, although average FEV1 predicted significantly better performance on tests of visuospatial ability (P =.04) and general cognition (P =.03). Higher FEV1 was more consistently associated with slower cognitive decline, but only the association between historical FEV1 and attention was significant (difference per standard deviation in FEV1 = 0.056, P =.05). Rate of FEV1 decline was not consistently associated with cognitive function or decline. Findings were generally similar or stronger in men who had never smoked. To account for potential bias due to selective attrition, inverse probability of censoring weights were applied to the cognitive decline analyses, yielding slightly larger estimates; the inadequate prognostic power of the censoring models limited this approach.
CONCLUSION
Overall, the data provide limited evidence of an inverse association between FEV1 and cognitive aging.
Keywords: lung function, cognition, cognitive decline, epidemiology, aging
Despite the tremendous public health importance of cognitive impairment and decline in older adulthood,1–8 few modifiable risk factors have been identified.
Respiratory function is of particular interest as a potential risk factor because it is potentially modifiable by individual behavior interventions (e.g., smoking cessation9 and pulmonary rehabilitation10) or policy changes (e.g., air pollution reduction11). A large body of evidence suggests that poor lung function is related to impaired cognition12–23 and adverse findings on brain imaging,21,24 but most large-scale studies have been cross-sectional, with only limited evidence indicating that better lung function corresponds to slower cognitive decline.17,20 Moreover, studies of cognitive change have used lung function measured on a single day. Such single assessments may be subject to considerable measurement error or random fluctuation, possibly reducing the ability to identify an association between long-term lung function and cognitive change. Furthermore, no study has examined the association between changes in lung function and cognitive aging, although change in function is arguably more clinically relevant than lung function at a single time point. In a large cohort of older men, this study assessed whether historical, long-term average, or change in forced expiratory volume in 1 second (FEV1), a measure of overall lung function, predicted cognitive function or cognitive change—measures of cognitive aging.
METHODS
Study Population
Participants were from the Normative Aging Study (NAS), a longitudinal study of aging established in 1963 by the Department of Veterans Affairs (VA) and based at the Boston VA Medical Center.25 The study enrolled 2,280 men from the Greater Boston area who were aged 21 to 80 and free of known chronic medical conditions, including chronic lung disease, asthma, coronary heart disease, high blood pressure, diabetes mellitus, and cancer. The men have undergone detailed clinical evaluations at 5-year intervals for those younger than 52 years old and at 3-year intervals for those aged 52 and older; since 1984, evaluations have occurred at 3-year intervals for all participants. These evaluations have entailed the collection of medical history information, physical examinations, laboratory tests, and completion of questionnaires on smoking history, education level, and other factors that may influence health. Annual attrition from all causes has been less than 1%, and more than 80% have responded to mailed questionnaires supplementing on-site examinations. Altogether, 864 men with appropriate data on FEV1 and cognitive function contributed to the analyses.
Assessment of Pulmonary Function
Since 1984, participants have provided information on respiratory symptoms and undergone airway responsiveness tests; 1,296 men are included in this cohort. A trained technician measured each participant’s lung function using spirometry according to the standardized protocols of the American Thoracic Society (ATS).26 FEV1 measurements were obtained using standard techniques.27 Spirometry was performed in the standing position using a nose clip, a 10-L water-filled survey recording spirometer, and an Eagle II minicomputer (Warren E. Collins, Braintree, MA). Spirometry was repeated for up to a maximum of eight spirograms, so that at least three acceptable spirograms were obtained, at least two of which were reproducible with FEV1 measurements within 5% of each spirogram. Acceptability of the spirograms was judged according to the ATS standards.26
Several aspects of FEV1 were evaluated in the analyses. The prospective association between FEV1 and cognitive function was characterized by examining FEV1 measured a median of 12 years (range 7–22 years; ~ 4 study cycles) before baseline cognitive testing, generally the oldest measurement available. This measure is called “historical FEV1.” Annual rate of change in FEV1 up through baseline cognitive testing (up to 2 years after) was also explored. Finally, the possibility that “usual FEV1” may be relevant to cognitive aging was considered, and although historical FEV1 is an indicator of “usual FEV1,” the average of repeated FEV1 measurements is a more-precise indicator, because FEV1 measurements tend to be correlated over time (Spearman correlation = 0.74–0.90 between the first and fifth measurements). Because the number of available measures of FEV1 differed from person to person, a mixed model framework was used to estimate long-term average and rate of change in FEV1 (described further below).
Assessment of Cognitive Function
Cognitive assessments began in 1993. The battery of cognitive tests included measures of sustained attention, perceptual speed, memory, language, visuomotor ability, and global cognition. These tests were taken from several different batteries, including the Neurobehavioral Evaluation System 2 (NES2),28 the Wechsler Adult Intelligence Scale—Revised (WAIS-R),29 the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery,30 and the developmental test of visual-motor integration (VMI).31 The following tests were administered to participants: Mini-Mental State Examination (MMSE);32 continuous performance, pattern memory, and pattern comparison (NES2); vocabulary and digit span backwards (WAIS-R); and the Boston naming test, word list memory, delayed word list recall, and verbal fluency (CERAD); and constructional praxis (copying of figures taken from CERAD, VMI, and MMSE). Cognitive examinations were approximately 3.1 years apart. The composition of the battery changed somewhat over the course of the study (e.g., some tests were administered at only one study cycle), resulting in variable sample sizes and number of repeated assessments for each individual test.
FEV1 was evaluated in relation to baseline cognitive test score and change in cognitive test score over three cycles of testing.
The Human Subjects Committee of the Boston VA Medical Center and the institutional review board of the Brigham and Women’s Hospital approved this research.
Statistical Analysis
Descriptive Analyses
To inform decisions about the approach for modeling exposures and the responses in the association models, the distributions of all FEV1, cognitive function and covariate variables were examined, and means and frequencies of important covariates were computed, testing differences across age- and height-adjusted quartiles of FEV1 using F tests (for continuous variables) and likelihood ratio chi-square tests (for categorical variables). These particular analyses used the data from the analyses of average FEV1 and baseline MMSE, a global cognition test.
Average FEV1 and Annual Rate of Change in FEV1
Each participant’s long-term average FEV1 was estimated using a linear mixed-effects model, regressing the repeated FEV1 measurements (2–5 per person) on an intercept so that average FEV1 was the sum of the fixed intercept (overall average) and the participant’s random intercept coefficient. A linear mixed effects model was also used to estimate annual change in FEV1, regressing the repeated FEV1 measurements on time. The annual rate of change in FEV1 for a given participant was estimated as the sum of the fixed effect for time and his random slope coefficient for time. The use of linear mixed-effects models for both of these estimates accommodated the variability in the timing and number of FEV1 measurements for each participant.33
Association Between FEV1 Measures and Baseline Cognitive Function
Generalized estimating equation (GEE) models were used to estimate the association between each FEV1 measure and each cognitive score when it was first administered. For most models, the identity link was used, a normal response distribution was assumed, and then the mean difference in cognitive score per standard deviation (SD) increment in FEV1 measurement was estimated. Scores on some cognitive tests did not follow a normal distribution. The MMSE and number of correct responses on the pattern comparison test were converted to error counts, and log-link GEE models assuming a negative binomial response distribution were used. The regression coefficients thus estimate percentage difference in error counts per SD increment in FEV1 measurement. Because of an extreme ceiling evident in the distribution of scores on the Boston naming test, the probability of committing at least one error (vs none) on this test was analyzed.
Association Between FEV1 Measures and Change in Cognitive Function
Similar to the approach for analyzing baseline cognitive function, GEE regression models were used to estimate the association between each FEV1 measure and change in cognitive score over up to three testing cycles. Terms were included in these models for time and the cross-product of time and FEV1 measurement. These cross-product terms indicated the difference in cognitive score trajectories across FEV1 level. The timing of the cognitive examinations did not deviate substantially from 3.1-year intervals, so time was modeled as an ordinal variable marking the baseline, second, and third examination cycles. Some tests were introduced to or dropped from the cognitive battery over time, which was accounted for by shifting the “baseline” cycle for some tests to correspond to the first time the participant took the test.
All analyses were adjusted for age at baseline cognitive assessment, time between the lung and baseline cognitive assessment, years of education, computer experience at the time of the cognitive test, smoking status (never, former, or current), pack-years of smoking, and height (shorter persons have smaller lung volumes and, in previous studies, have performed more poorly on cognitive tests in older adulthood34,35). For analyses of cognitive change, cross-products between the time variables and age and education were included. For analyses of average FEV1 and change in FEV1, the span of time covered by the lung assessments was also included in the model.
Reporting Framework
To promote comparability of the findings, they are reported as associations per 6.5-dL increment in FEV1, which is the approximate SD of FEV1 measurements in the analyses of historical FEV1. For annual rate of change in FEV1, associations per SD in decline of FEV1 are reported (0.3 dL/y). The normally distributed cognitive scores were transformed into z scores, using the scores’ respective means and SDs at baseline. This transformation—by homogenizing the scores’ units of measurement—facilitates the comparison of findings from the analyses of these scores.
Secondary Analyses
Several secondary analyses were conducted. First, inadequately measured smoking history could influence any observed association between the FEV1 measures and the cognitive outcomes. Thus, all analyses were repeated restricting data to never smokers. Second, as with many studies of aging, there was concern that differential survival to participate in the cognitive study and differential loss to follow-up over the course of the study could bias the findings on cognitive change.36 Analyses restricted to never smokers partially address this problem, because never smokers have lower mortality rates. To further probe the potential effect of differential loss to follow-up over the course of the study, inverse probability of noncensoring weights (IPCW) were applied to the data, in which the weights were based on logit models of not being censored (i.e., continuing in the study) from one cycle to the next.37 These models concern prediction of loss to follow-up, rather than cognitive decline, allowing great flexibility in covariates that they include. The final models of not being censored included diabetes mellitus, fasting blood glucose, white blood cell count, hemoglobin, previous cognitive score, and age-squared, in addition to the variables that were already being used in the analytical models.
For all of the analyses, P<.05 was used as the level of statistical significance.
RESULTS
In the exploration of the men’s characteristics across quartiles of age- and height-adjusted long-term average FEV1, those with higher FEV1 were significantly less likely to have been current or former smokers (Table 1), and in men who had ever smoked, pack-years of smoking corresponded to lower FEV1. In these analyses not adjusted for other covariates, the number of errors on the MMSE, a measure of global cognition, was approximately 12% lower in men in the highest quartile of average FEV1 (P =.08) than in men in the lowest quartile. Of these men who had multiple repeated FEV1 measurements, nearly all experienced a decline in their FEV1 over the course of follow-up (median rate of decline 0.13 dL/y, interquartile range 0.11–0.15 dL/y). Rates of FEV1 decline were significantly faster in men who were older at first measurement. FEV1 also declined significantly faster in former smokers than in never smokers and declined faster still in current smokers; in ever smokers, pack-years of smoking was associated with significantly faster decline. In unadjusted analyses, the number of errors on the MMSE was greater in men in the second, third, and highest quartile of rate of FEV1 decline than in men in the lowest quartile (8.7%, 23.9%, and 27.2%, respectively; P =.003).
Table 1.
Characteristics of Study Participants at Time of Baseline Cognitive Examination,* by Quartile of Age†- and Height-Adjusted Long-Term Average Forced Expiratory Volume in 1 Second (FEV1)
| Characteristic | Quartile of Age†- and Height-Adjusted FEV1 | P-Value from Test of Variation Across FEV1 Quartiles | |||
|---|---|---|---|---|---|
| Lowest 1.28–2.58 n = 217 | Second 2.59–2.87 n = 218 | Third 2.88–3.19 n = 213 | Highest 3.20–4.40 n = 214 | ||
| Age, mean ± SD | 68.1 ± 7.2 | 68.6 ± 7.3 | 69.3 ± 7.2 | 68.3 ± 7.3 | .28 |
| Education, years, mean ± SD | 13.8 ± 2.6 | 14.4 ± 2.6 | 14.3 ± 2.6 | 14.4 ± 2.6 | .07 |
| Previous computer experience, % | 40.6 | 39.0 | 38.0 | 39.7 | >.99 |
| Smoking status, % | <.001 | ||||
| Current | 12.9 | 5.5 | 3.8 | 2.8 | |
| Former | 73.7 | 63.8 | 62.9 | 60.8 | |
| Never | 13.4 | 30.7 | 33.3 | 36.5 | |
| Pack-years (in ever smokers), mean ± SD | 40.5 ± 26.2 | 31.7 ± 21.1 | 26.5 ± 20.8 | 17.3 ± 17.1 | <.001 |
| Baseline MMSE score: relative error count | ref | 6.7% | − 5.1% | − 12.0% | .06 |
Corresponds to analyses of average FEV1 and Mini-Mental State Examination (MMSE).
Age as of participants’ first FEV1 measurement.
SD = standard deviation.
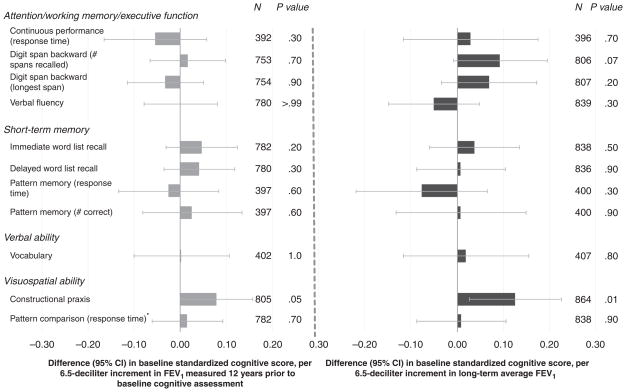
The multivariable-adjusted associations between the FEV1 measures and cognitive test scores at baseline were somewhat mixed, although the largest and most statistically significant findings were those that indicated better cognitive function with higher FEV1 (Figure 1, Table 2). For instance, men with higher average FEV1 performed significantly better on two measures of visuospatial ability: constructional praxis (Figure 1) and errors on the test of pattern comparison (Table 2). A similar albeit weaker pattern was evident for historical FEV1. Errors made on the MMSE were significantly less frequent in men with higher average FEV1 (~ 8%/6.5-dL increment in average FEV1) (Table 2).
Figure 1.
Adjusted difference in baseline standardized cognitive score per standard deviation (SD) increment in forced expiratory volume in 1 second (FEV1). 6.5 dL is the SD of historical FEV1. Analyses adjusted for age, height, education, computer experience, smoking status, pack-years of smoking, and time between lung function (most recent for average FEV1) and baseline cognitive assessments. Analyses of average FEV1 additionally adjusted for time span between first and last lung function assessments. *Smaller values for timed tests indicate better performance, but for comparability with the other test scores, the timed scores were reversed so that higher values represent better performance. CI = confidence interval.
Table 2.
Forced Expiratory Volume in 1 Second (FEV1) Measures and Errors Made on the Mini-Mental State Examination (MMSE), the Pattern Comparison Test, and the Boston Naming Test
| Cognitive Test | FEV1 ~ 12 Years Prior
|
Average FEV1
|
Rate of FEV1 Decline
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Observations* | Estimate per SD Increment in FEV1 Measure† (95% CI) | P-Value | N | Observations* | Estimate per SD Increment in FEV1 Measure† (95% CI) | P-Value | N | Observations* | Estimate per SD Increment in FEV1 Measure† (95% CI) | P-Value | |
| Adjusted‡ difference,§ %, in number of errors at baseline cognitive assessment | ||||||||||||
|
| ||||||||||||
| Visuospatial ability: pattern comparison | 782 | − 9.3 (− 18.8–1.4) | .09 | 838 | − 13.6 (− 24.8 to − 0.8) | .04 | 838 | − 0.1 (− 8.3–12.1) | .79 | |||
|
| ||||||||||||
| Global cognition: MMSE | 802 | − 4.7 (− 10.0–1.0) | .11 | 862 | − 8.0 (− 14.6 to − 0.9) | .03 | 862 | 1.2 (− 1.0–10.0) | .12 | |||
|
| ||||||||||||
| Adjusted‡ relative odds of making at least one error at baseline cognitive assessment | ||||||||||||
|
| ||||||||||||
| Verbal and language ability: Boston naming test | 403 | 1.10 (0.84–1.44) | .48 | 408 | 1.06 (0.75–1.50) | .76 | 408 | .89 (0.69–1.14) | .35 | |||
|
| ||||||||||||
| Adjusted‡ percentage difference in percentage change over time§ in number of errors | ||||||||||||
|
| ||||||||||||
| Visuospatial ability: Pattern comparison | 782 | 1,598 | − 0.1 (− 0.3–0.1) | .20 | 838 | 1,695 | 0.0 (− 0.4–0.3) | .85 | 838 | 1,695 | − 0.1 (− 0.4–0.2) | .58 |
|
| ||||||||||||
| Global cognition: MMSE | 802 | 1,777 | 0.4 (− 3.7–4.7) | .86 | 862 | 1,889 | 0.7 (− 4.2–5.9) | .77 | 862 | 1,889 | 1.2 (− 2.6–5.3) | .53 |
Total number of observations included in analyses of repeated cognitive assessments (up to 3 observations per person).
For historical ( ~ 12 years prior) and average FEV1, standard deviation (SD) increment 5= 6.5 dL, based on historical FEV1. For rate of FEV1 decline, SD increment = 0.3 dL/y.
Analyses adjusted for age, height, education, computer experience, smoking status, pack-years of smoking, and time between lung function (most recent for average FEV1) and baseline cognitive assessments. Analyses of average FEV1 and rate of FEV1 decline additionally adjusted for time span between first and last lung function assessments.
Percentage difference in number of errors in a person with a given FEV1, measurement expressed as a percentage of the number of errors of a person with a FEV1 that is 6.5 dL lower. Percentage difference in percentage change in errors is based on percentage change over time in errors in a person with a given FEV1, expressed as a percentage of the percentage change in errors in a person with a FEV1 1 SD lower. Thus, a percentage difference in percentage change that is <0 means that errors increased less over time in those with higher FEV1. Results for rate of FEV1 decline correspond to its SD (0.3 dL/y).
CI = confidence interval.
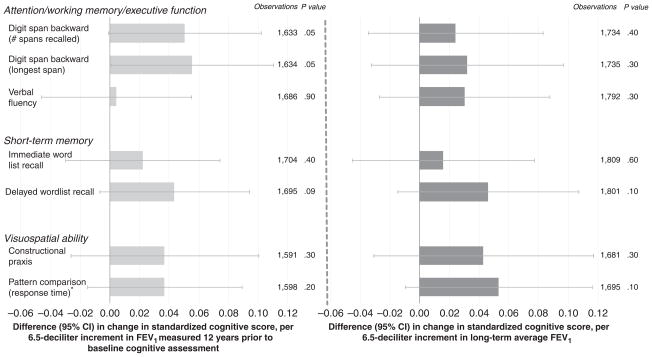
The FEV1 measures were generally associated with slower decline in performance on the cognitive tests (Figure 2 and Table 2), although the only statistically significant association was between historical FEV1 and slower decline in the longest digit span recalled. Modest but not statistically significant associations (.05<P<1.0) were also observed between historical FEV1 and slower decline on the test of delayed word list recall, as well as between average FEV1 and response time on the test of pattern comparison.
Figure 2.
Adjusted difference in baseline standardized cognitive score per standard deviation (SD) increment in forced expiratory volume in 1 second (FEV1). 6.5 dL is the SD of historical FEV1. Analyses adjusted for age, height, education, computer experience, smoking status, pack-years of smoking, and time between lung function (most recent for average FEV1) and baseline cognitive assessments. Analyses of average FEV1 additionally adjusted for time span between first and last lung function assessments. *Smaller values for timed tests indicate better performance, but for comparability with the other test scores, the timed scores were reversed so that higher values represent better performance. CI = confidence interval.
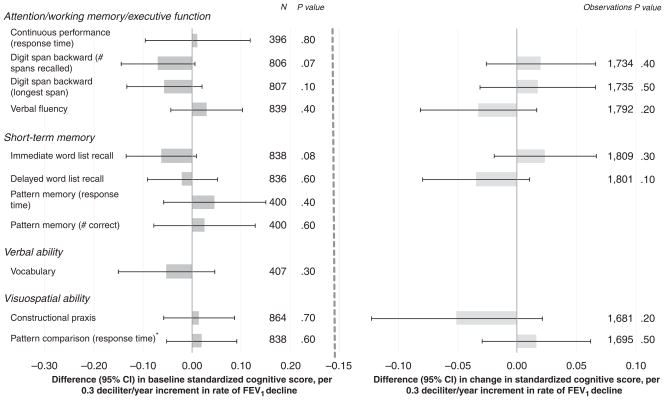
Annual rate of decline in FEV1 was not consistently associated with baseline or change in performance on the cognitive tests (Figure 3 and Table 2). The largest associations, observed between rate of decline in FEV1 and worse performance on the number of digit spans recalled (P =.07) and immediate word list recall (P =.08) at baseline testing, were modest and not statistically significant.
Figure 3.
Adjusted difference in baseline standardized cognitive score per standard deviation (SD) increment in rate of decline in forced expiratory volume in 1 second (FEV1). 0.3 dL/y is the SD of the rate of FEV1 decline. Analyses adjusted for age, height, education, computer experience, smoking status, pack-years of smoking, time between lung function (most recent) and baseline cognitive assessment, and time span between first and last lung function assessments. *Smaller values for timed tests indicate better performance, but for comparability with the other test scores, the timed scores were reversed so that higher values represent better performance. CI = confidence interval.
The secondary analyses provided some support for the findings. In never smokers, associations between average FEV1 and cognitive decline were generally much larger in magnitude than in the overall study population (Supplementary Table S1). In particular, never smokers with higher FEV1 had significantly slower decline than those with lower FEV1 in the constructional praxis test and errors committed on the pattern comparison test, both of which assess visuospatial ability (P =.05 for both outcomes; error count data not shown in Table). In never smokers, rate of FEV1 decline was more uniformly associated with worse cognitive decline than in the overall study population, but none of these findings was significant. By contrast, in former smokers, average FEV1 and rate of FEV1 decline did not predict worsening cognitive decline.
For the IPCW models of censoring, it was possible to identify variables that were strongly associated with censoring, although the models themselves did not fit the data well (e.g., a typical pseudo-R-squared for a model was 0.05). Weighted analyses produced results indicating slightly stronger associations between higher FEV1 and slower cognitive decline, particularly with respect to FEV1 measures in association with decline on delayed word list recall and pattern comparison response time (e.g., estimate shifted by 25% for average FEV1 and pattern comparison; data not shown).
DISCUSSION
The results from this study of 864 men, many who were middle-aged at the time of their initial lung function assessments, suggest that lung function, as indicated by prospectively measured and long-term average FEV1, may be modestly associated with slower decline in some cognitive functions, including working memory and visuospatial ability. Some significant associations were found between FEV1 and baseline cognitive performance—including performance on a global test of cognition—but these results were less directionally consistent than the results for cognitive change. This study is the first, to the knowledge of the authors, to report on decline in FEV1 in relation to cognitive aging; little evidence was found of an association between rate of decline in FEV1 over the past 12 years and subsequent cognitive performance and decline in cognitive function.
Although some of these findings are suggestive, they are not inconsistent with a null association between FEV1 and cognitive decline. The associations were generally not statistically significant. Moreover, results for long-term average FEV1 were not consistently stronger than those for historical FEV1. If usual FEV1 and cognitive decline were related, then the use of long-term average FEV1—a more-precise measure of usual FEV1 than the single historical measurement of FEV1—would yield, in theory, somewhat “deattenuated” estimates of association.38 Nonetheless, results were often stronger in men who had never smoked, suggesting that cigarette smoking history may account for some of the link between FEV1 cognitive decline by confounding the association or, as described below, as a determinant of selection into the study. The results from the IPCW analyses provided suggestive yet weak evidence in this regard.
The hypothesized relationship between lung function and cognitive aging has several compelling biological mechanisms. Poor lung function and lung injury, or their triggers, may initiate an inflammatory response.39,40 The ensuing inflammation may evolve into low-grade systemic inflammation, which in turn may invoke an adverse vascular response and additional oxidative stress.41 Although evidence of the neurocognitive effects of vascular inflammation remains limited, inflammation and oxidative stress in the central nervous system (CNS) appear to have roles in the pathogenesis of dementia.42 Impaired lung function is also associated with a procoagulant state,43,44 which increases the risk of cerebrovascular injury and stroke. Several epidemiological studies have found prospective associations between low FEV1 and greater stroke risk.45–47 Nonetheless, mixed findings have emerged from studies that have evaluated FEV1 in relation to imaging-based measures of cerebrovascular injury.21,24,48 In one study, lower FEV1 was associated with significantly higher risks of high white matter lesion grade and cerebral infarcts, even in nonsmokers.24 By contrast, comparable findings from other studies diminished substantially upon further adjustments for smoking and other covariates.21,48 Finally, it is possible that impairment in lung function may cause episodes of mild hypoxia, resulting in transient deficits in the metabolism of central nervous system neurotransmitters,49 but ventilatory function measures such as FEV1 are not good indices of gas exchange and vascular oxygenation, so studies using these measures are not well suited for addressing this mechanism.
Numerous cross-sectional studies of FEV1 and cognitive performance have been conducted, many of which found strong associations between the two,12–21,23 but some of these studies did not account in detail for potentially important sources of confounding, including age, education, height, and smoking history, which may have resulted in higher estimates. Fewer studies, by contrast, have examined FEV1 in prospective relation to cognitive function, although they have observed similarly protective associations.19,20,22 For example, in 3,036 men participating in the Honolulu Heart Program, higher FEV1, measured when they were aged 53 on average, was associated with significantly better performance on a test of general cognition administered 23 years later.22 Likewise, in the Medical Research Council (MRC) National Survey of Health and Development (NSHD), FEV1, measured in 1,778 43-year-old adults, was associated with better performance 10 years later on a test of verbal ability.20 A smaller study of twins (N=444) aged 40 to 84 found that higher FEV1 predicted better performance 6 years later on tests of perceptual speed and visuospatial ability but not on a test of attention, even though FEV1 was cross-sectionally associated with performance on all tests.19 In the current study, measures of cognitive function were not consistently associated with FEV1 measured 12 years earlier; only performance on a test of visuospatial ability was markedly better with higher historical FEV1.
Studies of lung function in relation to cognitive decline have been even rarer, and their findings have been mixed.17,20 Of 1,011 participants aged 70 to 80 in the Established Populations for Epidemiologic Studies of the Elderly (EPESE), higher baseline peak expiratory flow rate (PEFR) corresponded to less decline over a 2.5-year period in a global measure of cognition,17 yet in the MRC NSHD study, baseline FEV1 predicted significantly less decline over the ensuing 10 years on a test of perceptual speed but not on a test of verbal memory.20 Although the current study found less cognitive decline with higher levels of FEV1, the association was significant only with respect to FEV1 measured 12 years before baseline cognitive testing and 9-year decline in working memory and attention.
No consistent association was found between rate of decline in FEV1 and cognitive function or decline. It may be that absolute FEV1 is more important than decline or that the rates of FEV1 decline that the men in this study experienced were slow enough to permit adequate neurocognitive adaptation.
This study has limitations that warrant mention. First, factors that were not assessed or were mismeasured may have confounded the estimates that suggest cognitive benefit with higher FEV1. In particular, this study did not account for participants’ engagement in physical activity, an independent predictor of FEV1 in some studies (e.g., Jakes et al.50) and of cognitive function and decline,51 although the findings were adjusted for several other potential confounding variables, including some that typically correlate strongly with engagement in physical activity. By contrast, this study offers careful consideration of smoking history. In particular, sensitivity analyses restricted to never smokers circumvented some of the challenges associated with measuring smoking exposure dose and with the complex relationship between smoking history, lung function, and cognitive decline.
The suggestive but generally statistically nonsignificant findings related to FEV1 and cognitive decline were consistent with those from the EPESE and MRC NSHD cohorts,17,20 but the current analyses may have been underpowered, given that they included fewer individuals. Nevertheless, many of this study’s cross-sectional analyses entailed sample sizes that were at least as large as those from other cross-sectional studies that found significant associations. Moreover, previous work in the NAS has identified risk factors significantly associated with cognition and cognitive decline (e.g., Weisskopf et al.52, Tucker et al.53), supporting the ability to identify predictors of cognitive aging in these data.
It is possible that continuation in the study, from initial lung function testing to subsequent baseline cognitive testing and then to follow-up cognitive testing, varied according FEV1 and cognitive trajectory. Specifically, there was concern that participants with higher FEV1, better baseline cognitive function, and less cognitive decline would be less likely to drop out of the study for any reason. The ensuing bias in the estimates would be downward, in that any benefit of FEV1 on cognitive aging would be underestimated. This possibility was evaluated by constructing predictive models of continuation and computing analytical weights based on these models. FEV1 and prior cognitive function were generally weak predictors of continuation in the study after baseline cognitive assessment. These continuation models, overall, did not provide good fits to the patterns of continuation in the data, and thus, with a few exceptions, the results from the IPCW analyses were only slightly stronger than those from the conventional analyses. Moreover, this IPCW approach did not address loss to follow-up from first FEV1 assessment to baseline cognitive assessment. By contrast, the findings for cognitive decline in never smokers, in whom it was believed that these differential patterns of continuation would be less problematic, were generally stronger in magnitude than those in the complete cohort.
In summary, these findings provide limited support for an inverse association between FEV1 and cognitive aging in men. In contrast, in this first evaluation of long-term decline in FEV1 and cognitive aging, no clear association was found between the two.
Supplementary Material
Acknowledgments
We thank Hongshu Guan (Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts) for his critical help with data management for this project.
The project was funded in part by National Institute on Aging Grant R01AG027014-04. Dr. Spiro received support from a VA Merit Review and VA Research Career Scientist award. The Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs supported the VA Normative Aging Study, which is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Sponsor’s Role: The sponsors had no role in the design, methods, recruitment, data collection, analysis, or preparation of this manuscript.
Footnotes
A portion of this work was presented as a poster at the 2010 meeting of the Society for Epidemiologic Research. (See Weuve J et al. Lung function and cognitive aging in men. Am J Epidemiol 2010;171 (Suppl 11):S114)
Conflicts of Interest: The authors have no financial conflicts of interest to report.
Author Contributions: As first and lead author, Weuve was primarily responsible for the data analysis and manuscript preparation. Weuve and Litonjua: study concept and design. Glymour and Litonjua: data analysis. Hu, Sparrow, Spiro, Vokonas, and Litonjua: participant recruitment and data collection. Litonjua: acquiring funding. All authors contributed to the manuscript preparation.
Additional Supporting Information may be found in the online version of this article:
Table S1. Adjusted association between FEV1 measures and change in cognitive function, by smoking status.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Raji MA, Al Snih S, Ray LA, et al. Cognitive status and incident disability in older Mexican Americans: Findings from the Hispanic established population for the Epidemiological Study of the Elderly. Ethn Dis. 2004;14:26–31. [PubMed] [Google Scholar]
- 2.Greiner PA, Snowdon DA, Schmitt FA. The loss of independence in activities of daily living: The role of low normal cognitive function in elderly nuns. Am J Public Health. 1996;86:62–66. doi: 10.2105/ajph.86.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chodosh J, Seeman TE, Keeler E, et al. Cognitive decline in high-functioning older persons is associated with an increased risk of hospitalization. J Am Geriatr Soc. 2004;52:1456–1462. doi: 10.1111/j.1532-5415.2004.52407.x. [DOI] [PubMed] [Google Scholar]
- 4.Welmerink DB, Longstreth WT, Jr, Lyles MF, et al. Cognition and the risk of hospitalization for serious falls in the elderly: Results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:1242–1249. doi: 10.1093/gerona/glq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 6.Small BJ, Fratiglioni L, Viitanen M, et al. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 7.Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 9.Willemse BW, Postma DS, Timens W, et al. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23:464–476. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 10.Cooper CB. Exercise in chronic pulmonary disease: Aerobic exercise prescription. Med Sci Sports Exerc. 2001;33:S671–S679. doi: 10.1097/00005768-200107001-00005. [DOI] [PubMed] [Google Scholar]
- 11.Menzies D, Nair A, Williamson PA, et al. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296:1742–1748. doi: 10.1001/jama.296.14.1742. [DOI] [PubMed] [Google Scholar]
- 12.Emery CF, Huppert FA, Schein RL. Do pulmonary function and smoking behavior predict cognitive function? Findings from a British sample. Psychol Health. 1997;12:265–275. [Google Scholar]
- 13.Anstey KJ, Windsor TD, Jorm AF, et al. Association of pulmonary function with cognitive performance in early, middle and late adulthood. Gerontology. 2004;50:230–234. doi: 10.1159/000078352. [DOI] [PubMed] [Google Scholar]
- 14.Min JY, Min KB, Paek D, et al. The association between neurobehavioral performance and lung function. Neurotoxicology. 2007;28:441–444. doi: 10.1016/j.neuro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study. Investigators Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate in an elderly population. Am J Epidemiol. 1989;130:66–78. doi: 10.1093/oxfordjournals.aje.a115324. [DOI] [PubMed] [Google Scholar]
- 17.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur Studies of Successful Aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 18.Aleman A, Muller M, de Haan EH, et al. Vascular risk factors and cognitive function in a sample of independently living men. Neurobiol Aging. 2005;26:485–490. doi: 10.1016/j.neurobiolaging.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Emery CF, Pedersen NL, Svartengren M, et al. Longitudinal and genetic effects in the relationship between pulmonary function and cognitive performance. J Gerontol B Psychol Sci Soc Sci. 1998;53B:311–317. doi: 10.1093/geronb/53b.5.p311. [DOI] [PubMed] [Google Scholar]
- 20.Richards M, Strachan D, Hardy R, et al. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005;67:602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord. 2006;21:300–308. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- 22.Chyou PH, White LR, Yano K, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 23.Singh-Manoux A, Dugravot A, Kauffman F, et al. Association of lung function with physical, mental and cognitive function in early old age. Age (Dordr) 2010 Sep 29; doi: 10.1007/s11357-010-9189-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Higgins M, Bryan NR, et al. Lower pulmonary function and cerebral subclinical abnormalities detected by MRI: The Atherosclerosis Risk in Communities study. Chest. 1999;116:150–156. doi: 10.1378/chest.116.1.150. [DOI] [PubMed] [Google Scholar]
- 25.Bell B, Rose CL, Damon A. The Veterans Administration Longitudinal Study of Healthy Aging. Gerontologist. 1966;6:179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 26.ATS statement—Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 27.Kory RC, Callahan R, Boren HG, et al. The Veterans Administration-Army cooperative study of pulmonary function. I. Clinical spirometry in normal men. Am J Med. 1961;30:243–258. doi: 10.1016/0002-9343(61)90096-1. [DOI] [PubMed] [Google Scholar]
- 28.Letz R. NES2 User’s Manual (version 4. 4) Winchester, MA: Neurobehavioral Systems, Inc; 1991. [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York, NY: Brace Janovich; 1981. [Google Scholar]
- 30.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31.Beery KE, Buktenica NA. Developmental Test of Visual-Motor Integration. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 32.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 34.Zhang ZX, Plassman BL, Xu Q, et al. Lifespan influences on mid- to late-life cognitive function in a Chinese birth cohort. Neurology. 2009;73:186–194. doi: 10.1212/WNL.0b013e3181ae7c90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott RD, White LR, Ross GW, et al. Height as a marker of childhood development and late-life cognitive function: The Honolulu-Asia Aging Study. Pediatrics. 1998;102:602–609. doi: 10.1542/peds.102.3.602. [DOI] [PubMed] [Google Scholar]
- 36.Glymour MM, Weuve J, Chen JT. Methodological challenges in causal research on racial and ethnic patterns of cognitive trajectories: Measurement, selection, and bias. Neuropsychol Rev. 2008;18:194–213. doi: 10.1007/s11065-008-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55:651–656. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds HY. Lung inflammation: Normal host defense or a complication of some diseases? Annu Rev Med. 1987;38:295–323. doi: 10.1146/annurev.me.38.020187.001455. [DOI] [PubMed] [Google Scholar]
- 40.Nel A. Enhanced: Atmosphere. Air pollution-related illness: Effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 41.Han MK, McLaughlin VV, Criner GJ, et al. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 42.Maccioni RB, Rojo LE, Fernandez JA, et al. The role of neuroimmunomodulation in Alzheimer’s disease. Ann N Y Acad Sci. 2009;1153:240–246. doi: 10.1111/j.1749-6632.2008.03972.x. [DOI] [PubMed] [Google Scholar]
- 43.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168:602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thyagarajan B, Jacobs DR, Apostol GG, et al. Plasma fibrinogen and lung function: The CARDIA Study. Int J Epidemiol. 2006;35:1001–1008. doi: 10.1093/ije/dyl049. [DOI] [PubMed] [Google Scholar]
- 45.Agnarsson U, Thorgeirsson G, Sigvaldason H, et al. Effects of leisure-time physical activity and ventilatory function on risk for stroke in men: The Reykjavik Study. Ann Intern Med. 1999;130:987–990. doi: 10.7326/0003-4819-130-12-199906150-00006. [DOI] [PubMed] [Google Scholar]
- 46.Truelsen T, Prescott E, Lange P, et al. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol. 2001;30:145–151. doi: 10.1093/ije/30.1.145. [DOI] [PubMed] [Google Scholar]
- 47.Hozawa A, Billings JL, Shahar E, et al. Lung function and ischemic stroke incidence: The Atherosclerosis Risk in Communities study. Chest. 2006;130:1642–1649. doi: 10.1378/chest.130.6.1642. [DOI] [PubMed] [Google Scholar]
- 48.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 49.Gibson GE, Pulsinelli W, Blass JP, et al. Brain dysfunction in mild to moderate hypoxia. Am J Med. 1981;70:1247–1254. doi: 10.1016/0002-9343(81)90834-2. [DOI] [PubMed] [Google Scholar]
- 50.Jakes RW, Day NE, Patel B, et al. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am J Epidemiol. 2002;156:139–147. doi: 10.1093/aje/kwf021. [DOI] [PubMed] [Google Scholar]
- 51.Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 52.Weisskopf MG, Proctor SP, Wright RO, et al. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology. 2007;18:59–66. doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 53.Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.